Introduction
Angiostrongylus malaysiensis (Bhaibulaya & Cross, Reference Bhaibulaya and Cross1971) is a metastrongyloid nematode parasite of the family Angiostrongylidae (Eamsobhana, Reference Eamsobhana2014; Spratt, Reference Spratt2015). When first documented in Malaysia it was referred to as A. cantonensis (Lim et al., Reference Lim, Ow Yang and Lie1965; Schacher & Cheong, Reference Schacher and Cheong1960). It was subsequently recognized as a valid species and named as A. malaysiensis (Bhaibulaya & Cross, Reference Bhaibulaya and Cross1971). Its species status was confirmed by cross-breeding experiments with A. cantonensis (Cross & Bhaibulaya, Reference Cross and Bhaibulaya1974). In addition to Malaysia, A. malaysiensis has been reported to occur in Thailand (Bhaibulaya & Techasoponmani, Reference Bhaibulaya and Techasoponmani1972), Indonesia (Carney & Stafford, Reference Carney, Stafford and Cross1979), Japan (Sawabe & Makiya, Reference Sawabe and Makiya1995), and Laos and Myanmar (Rodpai et al., Reference Rodpai, Intapan, Thanchomnang and Sanpoolt2016).
Like A. cantonensis, A. malaysiensis has a relatively simple life cycle, involving rodent definitive hosts and mollusc intermediate hosts (Lim & Ramachandran, Reference Lim, Ramachandran and Cross1979). The adult worms live in the pulmonary arteries of rats. The infectivity of its first-stage larvae in the snail Biomphalaria glabrata is much lower than that of the congener A. cantonensis, due to greater sensitivity to pH and component proteases in the snail body (Sawabe & Makiya, Reference Sawabe and Makiya1995).
Unlike A. cantonensis, which causes eosinophilic encephalitis in humans, A. malaysiensis has not been indisputably incriminated as a zoonotic parasite (Spratt, Reference Spratt2015). However, the potential exists, as it occurs widely in Thailand (an endemic country of human angiostrongyliasis) (Yong et al., Reference Yong, Eamsobhana, Song, Prasartvit and Lim2015; Eamsobhana et al., Reference Eamsobhana, Yong, Prasartvit, Wanachiwanawin and Boonyong2016; Rodpai et al., Reference Rodpai, Intapan, Thanchomnang and Sanpoolt2016) and mixed infection has been reported in rats (Bhaibulaya & Techasophonmani, Reference Bhaibulaya and Techasoponmani1972).
Mitochondrial DNA is reported to be effective in uncovering potential cryptic species when sequence data of small sample sizes are used (Blouin, Reference Blouin2002). Among the mitochondrial loci, cytochrome c oxidase subunit I (COI) is one of the most sequenced loci of nematodes, including Angiostrongylus. Until recently there have been far fewer genomics studies on A. malaysiensis compared to A. cantonensis. The earlier studies on genetic diversity of Angiostrongylus nematodes have used single or very few specimens (Fontanilla & Wade, Reference Fontanilla and Wade2008; Jefferies et al., Reference Jefferies, Shaw, Viney and Morgan2009, Reference Jefferies, Shaw, Willesen, Viney and Morgan2010; Eamsobhana et al., Reference Eamsobhana, Lim, Zhang, Gan and Yong2010a, Reference Eamsobhana, Lim, Solano, Zhang, Gan and Yongb). Recent studies have included the complete mitochondrial genome (Yong et al., Reference Yong, Song, Eamsobhana and Lim2016) and molecular phylogeography (Yong et al., Reference Yong, Eamsobhana, Song, Prasartvit and Lim2015; Rodpai et al., Reference Rodpai, Intapan, Thanchomnang and Sanpoolt2016). In the present study, we report our findings on high cytochrome c oxidase subunit I (COI) haplotype diversity of A. malaysiensis from various localities in Thailand.
Materials and methods
Angiostrongylus worms
Angiostrongylus malaysiensis adult male and female worms were collected from the pulmonary arteries of wild-caught rodents in different provinces around Thailand (Eamsobhana et al., Reference Eamsobhana, Yong, Prasartvit, Wanachiwanawin and Boonyong2016). After morphological identification (Bhaibulaya & Cross, Reference Bhaibulaya and Cross1971; Bhaibulaya, Reference Bhaibulaya and Cross1979; Eamsobhana, Reference Eamsobhana2014), the worms were preserved in absolute alcohol and kept at –70°C. Table 1 summarizes the locality and rodent hosts of the five representative specimens used for the present study.
Table 1. Locality, rodent host, COI haplotype and GenBank accession number of Angiostrongylus malaysiensis from Thailand.

a M, male; F, female.
DNA extraction
Genomic DNA extraction from individual adult female and male worms of A. malaysiensis was carried out using the FTA (fast technology for analysis of nucleic acid) classic card method (Whatman BioScience, Newton Center, Massachusetts, USA) (Eamsobhana et al., Reference Eamsobhana, Lim, Zhang, Gan and Yong2010a, Reference Eamsobhana, Lim, Solano, Zhang, Gan and Yongb).
PCR amplification and DNA sequencing
The primers COI_F 5′–TAAAGAAAGAACATAATG-AAAATG–3′ and COI_R 5′–TTTTTTGGGCATCCTGAG-GTTTAT–3′ for a partial region of the COI gene (Bowles et al., Reference Bowles, Hope, Tiu, Liu and McManus1993; Hu et al., Reference Hu, Chilton and Gasser2002; Jefferies et al., Reference Jefferies, Shaw, Viney and Morgan2009; Eamsobhana et al., Reference Eamsobhana, Lim, Solano, Zhang, Gan and Yong2010b) were used for DNA amplification by polymerase chain reaction. The amplication and sequencing procedure followed that described in Eamsobhana et al. (Reference Eamsobhana, Lim, Solano, Zhang, Gan and Yong2010b).
Cytochrome c oxidase subunit I nucleotide sequences from GenBank
Representative COI nucleotide sequences of A. malaysiensis (from Thailand, Myanmar, Laos and Malaysia) and its congener A. cantonensis were obtained from GenBank for comparison (see fig. 1). Metastrongylus pudendotectus and Metastrongylus salmi were included as outgroup taxa.
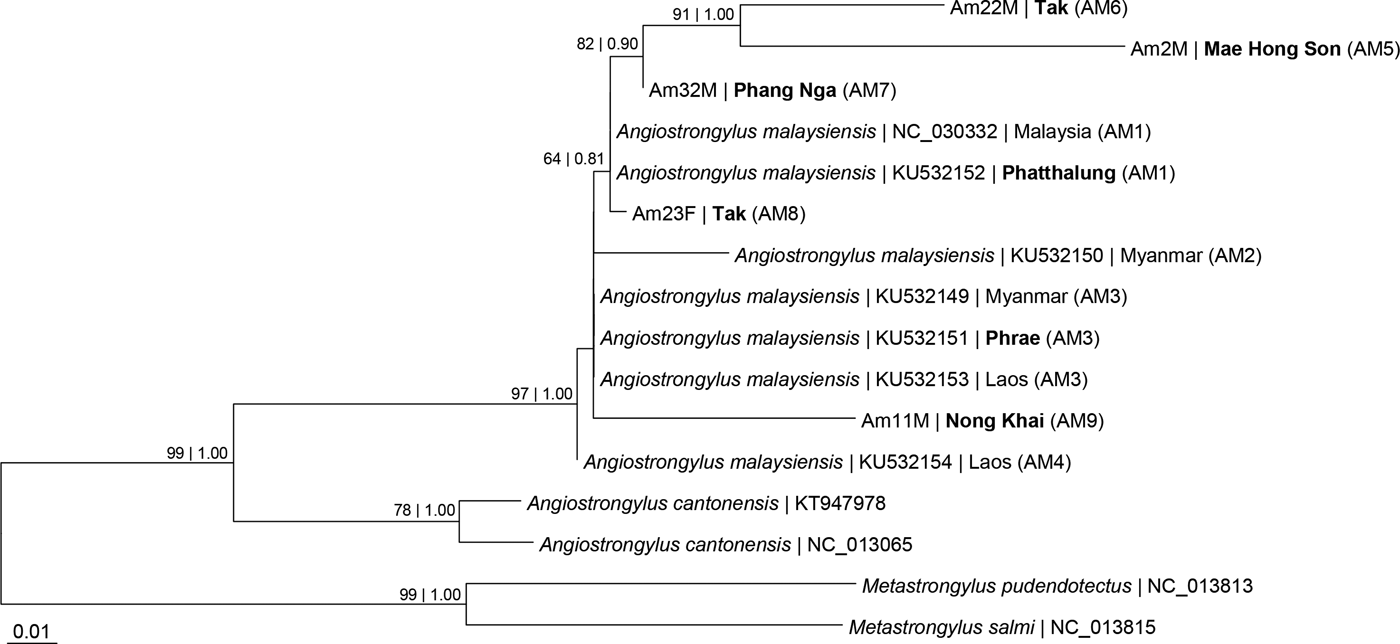
Fig. 1. Maximum likelihood (ML) tree based on the cytochrome c oxidase subunit I (COI) gene of Angiostrongylus malaysiensis and congener A. cantonensis, with Metastrongylus spp. as outgroup taxa. Numeric values at the nodes are Bayesian posterior probabilities/ML bootstrap. Bold type indicates localities in Thailand. Haplotypes in parentheses.
Sequence alignment and phylogenetic analysis
ChromasPro v.1.5 (Technelysium Pty Ltd., Tewantin, Queensland, Australia) software was used for editing and assembling the COI sequences. This was followed by multiple sequence alignment with MAFFT version 7 (Katoh & Standley, Reference Katoh and Standley2013). BioEdit v.7.0.5.3 (Hall, Reference Hall1999) was used to trim the resulting alignment. The best-fit nucleotide substitution models for maximum likelihood (ML) and Bayesian (BI) analyses, determined by Kakusan v.3 (Tanabe, Reference Tanabe2007), were selected using the corrected Akaike Information Criterion (AIC; Akaike, Reference Akaike, Petrov and Csaki1973) and the Bayesian Information Criterion (Schwarz, Reference Schwarz1978), respectively. Phylogenetic trees were reconstructed using TreeFinder (Jobb et al., Reference Jobb, von Haeseler and Strimmer2004) prior to the annotations of bootstrap values (BP) generated via 1000 ML bootstrap replicates. Bayesian analyses were conducted using the Markov chain Monte Carlo (MCMC) method via Mr. Bayes v.3.1.2 (Huelsenback et al., Reference Huelsenback, Ronquist, Nielsen and Bollback2001), with two independent runs of 2 × 106 generations with four chains, and with trees sampled every 200th generation. The likelihood values for all post-analysis trees and parameters were evaluated for convergence and burn-in using the ‘sump’ command in MrBayes and the computer program Tracer v.1.5 (http://tree.bio.ed.ac.uk/software/tracer/). The first 200 trees from each run were discarded as burn-in (where the likelihood values were stabilized prior to the burn-in), and the remaining trees were used for the construction of a 50% majority-rule consensus tree. FigTree v.1.4 (Rambaut, Reference Rambaut2012) was used to view and edit the final phylogenetic trees.
Haplotype network reconstruction
The genealogical relationships of the haplotypes were estimated by the median joining (MJ) network (Bandelt et al., Reference Bandelt, Forster and Röhl1999). The MJ network was calculated using NETWORK v.5.0.0.1 (http://www.fluxus-engineering.com).
Gene/haplotype and nucleotide diversity
Arlequin v.3.5.2.1 (Excoffier & Lischer, Reference Excoffier and Lischer2010) was used to estimate the haplotype/gene and nucleotide diversity by analysis of molecular variance (AMOVA). The statistical significance of AMOVA was tested from 10,000 permutations.
Results
Phylogenetic relationships
The aligned COI sequences of A. malaysiensis consisted of 316 sites, of which 204 were invariable and 59 were parsimony informative. The best-fit nucleotide substitution model selected based on AIC was TN + Gamma, and that selected based on BIC was TN93 + Gamma. The phylogenetic trees reconstructed using the BI and ML methods had similar topology for the geographical isolates of A. malaysiensis (fig. 1). The A. malaysiensis sequences formed a monophyletic clade distinct from the closely related congener A. cantonensis.
Haplotype diversity and nucleotide diversity
Each of the five A. malaysiensis COI sequences from four geographical localities/provinces in Thailand was represented by a distinct haplotype (table 1, figs 1 and 2). Four of these five haplotypes – one from Mae Hong Song (northern region), two from Tak (western region), and one from Phang Nga (southern region) – formed a distinct clade with those from Phatthalung (southern region) and Malaysia. The haplotype from Malaysia (NC_030332) was identical to that of Phatthalung (AM1) from southern Thailand. The haplotype/gene diversity for the 12 COI sequences (two were from GenBank) of the Thailand A. malaysiensis was 0.9394 ± 0.0577, and the nucleotide diversity was 0.038694 ± 0.021190.
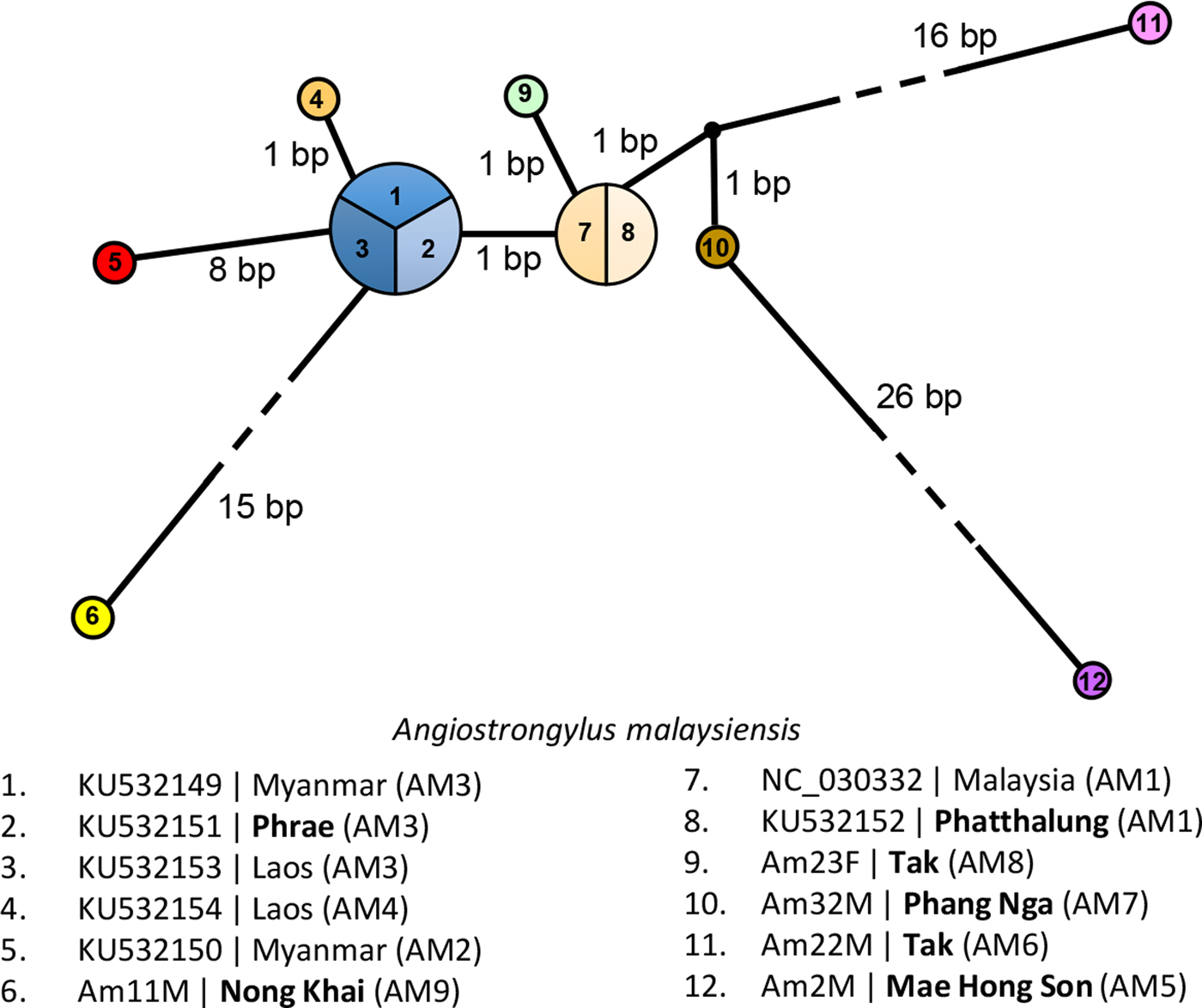
Fig. 2. Haplotype network of Angiostrongylus malaysiensis based on cytochrome c oxidase subunit I (COI) sequences generated by NETWORK software. Circles represent haplotypes and numbers represent individuals sharing the specific haplotype. Bold type indicates localities in Thailand.
Discussion
Cytochrome c oxidase subunit I (COI) is one of the most commonly sequenced mitochondrial loci for differentiating closely related Angiostrongylus species, as well as geographical isolates of A. cantonensis and A. vasorum (Jefferies et al., Reference Jefferies, Shaw, Viney and Morgan2009, Reference Jefferies, Shaw, Willesen, Viney and Morgan2010; Eamsobhana et al., Reference Eamsobhana, Lim, Solano, Zhang, Gan and Yong2010b; Monte et al., Reference Monte, Simões, Oliveira, Novaes, Thiengo, Silva, Estrela and Maldonado2012; Tokiwa et al., Reference Tokiwa, Harunari, Tanikawa, Komatsu, Koizumi, Tung, Suzuki, Kadosaka, Takada, Kumagai, Akao and Ohta2012; Lee et al., Reference Lee, Chung, Wang, Lin, Wang, Tu, Wu and Yen2014; Rodpai et al., Reference Rodpai, Intapan, Thanchomnang and Sanpoolt2016; Vitta et al., Reference Vitta, Srisongcram, Thiproaj, Wongma, Polsut, Fukruksa, Yimthin, Mangkit, Thanwisai and Dekumyoy2016). In contrast to A. cantonensis, COI has not been used extensively to study A. malaysiensis.
Apart from COI, several other molecular markers have been reported for studies involving A. malaysiensis – 18S (small subunit, SSU) rDNA (Fontanilla & Wade, Reference Fontanilla and Wade2008; Eamsobhana et al., Reference Eamsobhana, Lim and Yong2015; Rodpai et al., Reference Rodpai, Intapan, Thanchomnang and Sanpoolt2016), 66-kDa protein gene (Eamsobhana et al., Reference Eamsobhana, Lim, Zhang, Gan and Yong2010a), internal transcribed spacer 2 (ITS2) region (Rodpai et al., Reference Rodpai, Intapan, Thanchomnang and Sanpoolt2016), and cytochrome b (CYTB) gene (Yong et al., Reference Yong, Eamsobhana, Song, Prasartvit and Lim2015). Other than the CYTB gene, the numbers of specimens studied were very small.
In our earlier study, three COI haplotypes were represented in four A. malaysiensis specimens from Malaysia (Eamsobhana et al., Reference Eamsobhana, Lim, Solano, Zhang, Gan and Yong2010b). A recent study reported four COI haplotypes for A. malaysiensis – haplotype AM1 in three specimens from Thailand, haplotype AM2 in two specimens from Myanmar, haplotype AM3 in two specimens from Thailand and one from Myanmar, and haplotype AM4 in eight specimens from Laos (Rodpai et al., Reference Rodpai, Intapan, Thanchomnang and Sanpoolt2016). The present study has added five COI haplotypes for A. malaysiensis to those reported in the literature (table 2).
Table 2. Summary of the reported COI haplotypes and GenBank accession numbers of Angiostrongylus malaysiensis from Malaysia, Myanmar, Laos and Thailand.

The high COI haplotype diversity observed in A. malaysiensis has also been observed in A. cantonensis, with 13 reported COI haplotypes (Monte et al., Reference Monte, Simões, Oliveira, Novaes, Thiengo, Silva, Estrela and Maldonado2012; Tokiwa et al., Reference Tokiwa, Harunari, Tanikawa, Komatsu, Koizumi, Tung, Suzuki, Kadosaka, Takada, Kumagai, Akao and Ohta2012; Rodpai et al., Reference Rodpai, Intapan, Thanchomnang and Sanpoolt2016). This high COI genetic diversity in A. malaysiensis worms from Thailand is also reflected by 15 CYTB haplotypes from 15 geographical localities in Thailand (Yong et al., Reference Yong, Eamsobhana, Song, Prasartvit and Lim2015). The results indicate the occurrence of an elevated mutation rate in mitochondrial genes and restricted gene flow between localities. In Thailand, COI sequences of the third-stage larvae of the congener A. cantonensis in 19 provinces revealed two independent origins of the parasite – Group 1: Phetchabun, Tak, Kamphaeng Phet and Thailand ac4; and Group 2: Kalasin, Tak, Phitsanuluk, Kamphaeng Phet and AC Thai (Vitta et al., Reference Vitta, Srisongcram, Thiproaj, Wongma, Polsut, Fukruksa, Yimthin, Mangkit, Thanwisai and Dekumyoy2016). A high mitochondrial mutation rate and probability of parallel mutations have been documented in the nematode Caenorhabditis elegans (Denver et al., Reference Denver, Morris, Lynch, Larissa, Vassilieva and Thomas2000).
In the present study, the COI sequences for A. malaysiensis from a particular locality may belong to different sister lineages, for example, from Tak and Laos (fig. 1). Also, they may not differentiate the various geographical isolates of A. malaysiensis; for example, haplotype AM1 was recorded in Malaysia and Thailand, and haplotype AM3 in Thailand, Laos and Myanmar. This condition has also been reported for the geographical isolates of the congeners A. cantonensis (Rodpai et al., Reference Rodpai, Intapan, Thanchomnang and Sanpoolt2016) and A. vasorum (Jefferies et al., Reference Jefferies, Shaw, Viney and Morgan2009). Studies on convergent and parallel mutations of the COI gene (and other mitochondrial genes), genetic drift and gene flow among populations are needed.
This study has confirmed the presence of high COI haplotype diversity in various geographical isolates of A. malaysiensis. As indicated in earlier studies, COI will be a suitable marker for studying genetic diversity, population structure and phylogeography.
Acknowledgements
We thank the Faculty of Medicine Siriraj Hospital, Mahidol University and University of Malaya for providing various research facilities and other support. The constructive comments and valuable suggestions of the editor and two anonymous reviewers have helped to improve this manuscript.
Financial support
This work was supported in part by the Department of Disease Control, Ministry of Public Health, Thailand, and University of Malaya research grant H-5620009 to H.S.Y.
Conflict of interest
None.
Ethical standards
All procedures involving animals were conducted under animal use protocols approved by the Animal Ethical Committee of the Ministry of Public Health, Thailand (approval no. FWA00013622).






