Published online by Cambridge University Press: 10 June 2004
This paper investigates a cohort of 2187 laboratory reared Culex quinquefasciatus fed on 69 human volunteers, including 59 persons with different levels of Wuchereria bancrofti microfilariae and 10 without microfilaria. Mosquitoes were followed until death. Mosquito survival was analysed in relation to the level of microfilaria in the human and larval count in the dead mosquito. Vector mortality during the extrinsic incubation period (12 days post-engorgement) was significantly higher in mosquitoes fed on microfilaraemic volunteers (50%) than in those fed on amicrofilaraemics (29%). Both the percentage infected and the geometric mean parasite density was significantly higher among mosquitoes which died before 13 days (45% infected and 10 larvae per infected mosquito) than those surviving beyond 13 days (39% and 2·2), suggesting a parasite loss of more than 80% during the extrinsic incubation period. A large proportion (62%) of the mosquitoes that died during the early of phase of parasite development were infected (36% in low, 26% in medium and 90% in high human Mf-density). Survival analysis showed that the parasite load in mosquitoes and the human Mf-density for a given parasite load are independent risk factors of vector survival. Overall, the hazard of dying was found to be 11–15 times higher among mosquitoes fed on microfilaraemic volunteers than those fed on amicrofilaraemics. The hazard doubles for every increase of about 60–70 parasites in the vector. As a consequence of the parasite-induced reduction in vector survival, the transmission success of the parasite is reduced. The implication of the results on control/elimination of lymphatic filariasis using mass-drug administration is discussed.
The survival of the vector Culex quinquefasciatus is an important determinant of the transmission dynamics of the human filarial parasite Wuchereria bancrofti. For successful transmission of the parasite, the vector must survive longer than the 12 days it takes the parasite to develop to the stage of infective larva. Not all infected mosquitoes survive that long. Apart from meteorological factors and age, parasite load and parasite stage distribution influence the survivorship of an infected mosquito. In a previous experimental transmission study we observed that the relationship between W. bancrofti microfilarial (Mf) load in the human host and uptake of Mf by C. quinquefasciatus and parasite development was non-linear and saturating, which is suggestive of density-dependent regulation of parasite in the vector (Subramanian et al. 1998). Further, the yield of L3 larvae (ratio of L3 to Mf ingested) decreased with increasing Mf-uptake by C. quinquefasciatus (‘limitation’). The main mechanisms proposed to drive limitation are increased mortality of the larvae (loss of parasites) and parasite-induced vector mortality particularly at high parasite densities (Brengues & Bain, 1972; Pichon, Prodhon & Riviere, 1980a; Subramanian et al. 1994; Das et al. 1995; Dye & Williams, 1995). Both experimental studies (Crans, 1973; Maeda & Kurihara, 1980; Saporu, 1993; Failloux et al. 1995; Subramanian et al. 1998) and field studies (Samarawickrema & Laurence, 1978; Subramanian et al. 1994) found excess-mortality among heavily infected mosquitoes. In field studies, it is difficult to distinguish the effect of vector-age from the effect of parasite burden on the survival of mosquitoes because the wild population is a mixture of members with different ages. Most of the experimental studies were designed to quantify the relationship between infection in the human and uptake by the vector, and not to assess the risk of parasite load on the survival of infected/uninfected vector population. Therefore, an experimental study was carried out in which laboratory reared C. quinquefasciatus mosquitoes were allowed to engorge blood from human volunteers with known W. bancrofti Mf density. The results of the analysis of the data on mosquito survival, parasite density in the volunteers, and parasite counts in dead mosquitoes are reported in this paper. The analysis focuses on the relationship of the survival of the mosquitoes to microfilarial load in human volunteers and larval counts in the dead mosquitoes.
Male and female human volunteers were randomly selected from a list of Mf-carriers from a blood sample survey conducted in and around Pondicherry. Informed consent was obtained from all volunteers. Newly emerged female C. quinquefasciatus mosquitoes from the laboratory-reared colony were used. Female mosquitoes were starved for 48 h before engorgement. Prior to the feeding experiment three 20 μl blood smears were taken from each of the Mf-carriers by the finger prick method. One arm up to the elbow of the volunteer was exposed for 30 min between 20.00 h and 21.00 h to the mosquitoes kept in a cage to engorge. Immediately after the feeding, 3 more blood smears were collected from the volunteers. All volunteers were given a full course of diethylcarbamazine following the experiment.
Fully fed female mosquitoes were caught, counted and released into another cage. Mosquitoes were maintained on raisin and water till their death at a controlled room temperature of 28–30 °C and relative humidity of 80–85%. Each day, dead mosquitoes were removed and dissected to assess the infection load. The number of parasites in relation to the stage was recorded separately for abdomen, thorax and head. To assess the natural survival, another batch of female mosquitoes was fed on amicrofilaraemic persons (determined on the basis of 3 blood smears) and was also followed up until death.
The mosquitoes were classified into 4 groups according to human Mf-density: 0, 0·3–7·0, 7·0–21·0 and 21·0–440 per 20 μl of peripheral blood (Table 1). The classification of mosquitoes fed on Mf-volunteers was made in such a way that the percentile distributions of the specimens in each Mf-density category were approximately equal: 28·6, 33·8 and 37·6% respectively fed on low, medium and high Mf-density categories. The geometric mean was used to express the parasite load in infected mosquitoes fed on different Mf-density categories. The 95% asymmetric confidence intervals for the means were used to compare the difference in mean larval loads within or between Mf-density categories. The chi-square heterogeneity test was used to compare the difference in proportion infected between time-periods. The generalized Wilcoxon test (Breslow) was used to compare the survival distributions of different Mf-density categories. Since the observed survivorship curves for mosquitoes fed on low (0·3–7·0) and medium Mf-density (7·0–21·0) categories appear to be overlapping with each other (Fig. 1) these two categories were pooled for fitting survival models and hence all the mosquitoes were classified under 3 Mf-density categories: 0, 0·33–21·0 and >21·0–440 per 20 μl of peripheral blood.

Fig. 1. Observed survival function of Culex quinquefasciatus mosquitoes fed on human volunteers in 4 different Mf density categories: No Mf (open circles), 0·3–7·0 Mf (closed circles), 7·0–21·0 Mf (open squares), and 21·0–440 Mf (closed squares) per 20 μl blood.

Using the observed survival times, the survivor function S(t), can be estimated. Since there is no censoring, the Kaplan–Meier estimate of S(t) will be the same as that of the empirical survivor function and life-table method (Collett, 1994). The empirical survival distributions for each of the Mf-density category were used to determine a suitable baseline hazard function. Visual inspection suggested that the hazards in the different Mf-categories are not proportional (when the log-cumulative hazard – i.e. log(−log S(t)) – is plotted against log t the lines are far from being parallel) (Collett, 1994). Therefore a (non-proportional) Weibull hazard model was fitted to the data. For a mosquito that fed on a person in Mf-category j (j∈0,1,2) and that had parasite load x at death, it is assumed that the hazard hj(t;x) of dying at time t follows a Weibull probability distribution with cumulative hazard:

with shape parameter γj(>0) and a scale parameter λj(x) (>0) which depends on the parasite load x in a mosquito. The log-cumulative hazards will be parallel if the shape parameters γj are equal between human Mf-categories. The scale parameter is assumed to depend on the human Mf-category and to increase exponentially with increasing parasite load x:
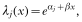
with αj, logarithm of the ratio of the hazard (relative risk, RR) for a mosquito feeding on a human in Mf-category j, to that of a mosquito feeding on a human in Mf-category 0; β, change in the logarithm of the hazard ratio for a unit increase in the number of parasites it harbours.
For the special case of γj=1, the hazard takes a constant value λj and the mosquito survival has an exponential distribution. For other values of γj, the hazard of dying increases or decreases monotonically with time.
The parameters αj, β and γj are estimated from the observed parasite loads xij and times of death tij by maximizing the following likelihood function for the 3 parasite load categories (j∈0,1,2) and the nj mosquitoes (i=1,…,nj) in each of these load categories:

with fij(t) being the Weibull probability density function of the survival distribution Sij(t) which is given by:
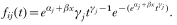
Maximizing the likelihood function was achieved using the GLIM software package and a modification of its associated library macro Weibull (Crawley, 1993).
A total of 69 human volunteers participated in this experiment, including 10 amicrofilaraemic persons and 59 microfilaraemic carriers. Out of the 59 microfilaraemic individuals, 15 were categorized as having low Mf-density (ranging from 0·3 to 7·0 per 20 μl of peripheral blood), 17 as having medium Mf-density (7·0–21·0) and the other 27 individuals having high Mf-density (higher than 21·0; the highest average Mf count in 6 smears of 20 μl was 440). A total of 2187 mosquitoes were full-fed on these volunteers: 440 on amicrofilaraemics and the remaining were fed on low, medium and high microfilaraemic individuals.
Table 1 shows the summary of dissection results of the mosquitoes that died on different days post-engorgement (p.e.). The proportion infected among mosquitoes fed on humans in the high Mf-density category was significantly higher (72%) than among those fed on humans in the low (17%) or medium (30%) Mf-density categories (P<0·005).
In the high Mf-density category, the percentage of mosquitoes with parasitic infection was the same among those that died within 12 days p.e. compared to those that died after 12 days p.e. (72%, P>0·05). A similar comparison among the mosquitoes fed on volunteers with low Mf-density also did not show a significant difference between those which died within 12 days or in the latter period (19% vs. 16%) although the difference (24% vs. 34%) was significant for those fed on persons with medium Mf-density. The percentage infected was found to be significantly higher among mosquitoes that died within 12 days compared to the 12-day survivors (45% vs. 39%, P<0·005).
Table 1 also shows the geometric mean parasite load in positive mosquitoes (parasite density) and its 95% asymmetric confidence interval. The overall parasite density was significantly higher among those fed on humans with high Mf-density category (8·9) compared to low (1·6) or medium (1·7) (95%CI do not-overlap). The parasite density was much higher in mosquitoes that died on days 0–2 than in mosquitoes that survived into the later periods, especially in mosquitoes that fed on humans with high Mf-load, (non-overlapping CI). Mosquitoes, which died within 12 days, had an average 10 larvae per infected mosquito, compared to 2·2 for mosquitoes dying after 12 days. This decline in parasite load with duration post-engorgement can be caused by selective mortality of highly infected mosquitoes but may also be related to loss of parasites during development.
Of the mosquitoes fed on humans with high Mf-density, only 37% survived 12 days compared to 55%, 60% and 71% of those fed in the low, medium and amicrofilaraemic persons respectively (Table 1). The difference in survival between those fed in microfilaraemic (50%) and amicrofilaraemic persons (71%) is highly significant (P<0·0001). Fig. 1 gives the observed survival functions for the mosquitoes, by level of Mf-density of the human volunteers. The sharp crossing of the survival curves strongly suggests non-proportional hazards, between the four groups: the survival sharply declines after 30 days for the uninfected mosquitoes. This was confirmed in a formal analysis, (see Materials and Methods section) which did not reject proportionality of the mortality hazards for mosquitoes that fed on humans in the low, medium and high Mf density categories (P>0·05), but with a non-proportional hazard when compared to mosquitoes that engorged blood from Mf negative persons (P<0·05). Further, the survivorship curves overlap for the 2 intermediate Mf-categories (low and medium), suggesting that their survival distributions are not different. Therefore, in the following analyses, the survival distributions for these Mf-categories were combined and hence there are only 3 Mf-density categories: 0, 0·3–21·0 and 21–440 Mf per 20 μl of peripheral blood.
We first considered models in which the shape-parameter γ has the same value for the 3 Mf-classes. When we assume exponentiality (i.e. γj=1 in eqn. (4)), we get a very poor fit to the data of Fig. 2 (open circles). The maximum likelihood estimate of a common shape-parameter γ equals 1·32, indicating a moderately increasing hazardous effect of mosquito age. When allowing for different shape-parameters for each Mf-class, the fit to the data improves significantly (Table 2, difference in deviance 90·0 for 2 D.F. P<0·0001). See Fig. 2 for comparison of simulated and observed survival. The estimated values of the shape parameter γ are almost equal for the 2 non-zero Mf categories, close to exponential (≈1·2), suggesting that the mortality hazard is nearly constant with mosquito age. The shape parameter for mosquitoes that fed on amicrofilaraemic individuals is significantly higher (γ=1·92), indicating that the mortality hazard strongly increases with mosquito age. In the mosquitoes that fed on microfilaraemic individuals the modest effect of mosquito age occurred combined with a pronounced effect of parasite load on mortality already during the early stage of parasite development.

Fig. 2. Observed (symbols) and expected (lines) survival of mosquitoes that fed on humans in three different Mf density categories: (A) No Mf, (B) 0·3–21·0 Mf, and (C) 21·0–440 Mf per 20 μl blood. Expected survival is given for the model in which the shape parameter γ differs between these 3 Mf density classes, and is calculated as the average of the expected survival of the mosquitoes, which in turn depends on the human Mf-density category and the parasite load of the mosquito.

The cumulative hazard of dying of a mosquito is estimated to increase by a factor of eβ(≈1·01) for every unit increase in parasite load. Hence, for mosquitoes that fed on low and high Mf-density persons respectively and that harbour 100 parasites, the model predicts that 74% and 87% will die within 12 days p.e., compared to 25% for mosquitoes fed on persons without microfilariae.
This paper reports the analysis of survival times under laboratory conditions of a cohort of C. quinquefasciatus mosquitoes fed on W. bancrofti uninfected and infected human volunteers. We obtained quantitative results on the effect of parasite density on the survival and hence on the transmission potential of the infected vectors.
Further analysis of the results in Table 1 show that a considerable fraction (17%, 127 out of 736) of mosquitoes with any stage of the parasite died in less (i.e. γj=1 in eqn. (4)) than 2 days post-engorgement. This fraction was higher (21%, 98 out of 472) among those exposed to humans with high Mf-load. Mosquitoes dying in days 0–2 have a much higher parasite load than those dying in later days. This decrease in parasite load during the early phase of parasite development is in agreement with our field study in which we have found evidence for density-dependent regulation between Mf and L1 stage (Subramanian et al., unpublished observations).
For mosquitoes that fed on Mf-positive persons, both the percentage infected and the geometric mean parasite density were much higher among mosquitoes that died before 13 days than those surviving beyond 13 days (45% vs. 39% infected, with on average 10 vs. 2·2 larvae per infected mosquito). This suggests a parasite loss of more than 80% during the extrinsic incubation period as a consequence of 13% excess mortality among infected vectors. This result is close to our earlier semi-experimental study in which we found that 64% of mosquitoes that died before 12 days were infected vs. 42% of mosquitoes dying later, with 73·1 and 10·0 larvae per positive mosquito (arithmetic means), a reduction of 91% (Subramanian et al. 1998) as well as other field transmission studies (Samarawickrema & Laurence, 1978: 10·3 to 2·6 larvae per positive mosquito, 75% reduction). The relatively higher mosquito mortality in the semi-experimental (Subramanian et al. 1998) study can be attributed to the added effect of age, which was controlled in the present lab experiment by using mosquitoes of the same age. These results support the conclusions derived from both experimental and field studies that the ‘limitation’ phenomenon for W. bancrofti (parasite) and C. quinquefasciatus (vector) complex might be due to increased mortality of heavily infected mosquitoes (Pichon, 1974; Rajagopalan, Kazmi & Mani, 1977; Samarawickrema & Laurence, 1978; Subramanian et al. 1994, 1998; Das et al. 1995; Dye & Williams, 1995; Subramanian et al., unpublished observations).
Rapid formation of crystals and peritrophic membrane has been proposed as a barrier for the migration of Mf from the gut into the haemocele or through the epithelial cells in the vector. Loss of parasites immediately after feeding could be related to the presence and shape of the cibarial and pharyngeal armatures in the vectors. McGreevy et al. (1978) have reported that Anopheles farauti and Anopheles gambiae, which have well-developed cibarial armatures, killed 36–96% of the ingested Mf. Bryan & Southgate (1988) observed 57–60%, 33–51%, 39–56% and 25% loss of W. bancrofti Mf ingested by An. gambiae, An. arabiensis, An. melas and An. funestus respectively. In C. quinquefasciatus, having a poorly developed cibarial armature, the loss of Mf was reported to be only 6% (McGreevy et al. 1978). However, large proportions of the larvae die in the mid-gut or during their development in the thorax. Jordan & Goatly (1962) reported considerable loss (55–99%) in ingested Mf during their development to infective larvae and showed many Mf in the dejecta of mosquito 2–3 days post-engorgement. Although the present study was not aimed at examining the mechanisms of parasite elimination, we did not observe melanized or degenerated larvae in mosquitoes that died during our observation.
Filarial parasites are known to influence the survival of vectors. Field studies indicate that W. bancrofti infection not only reduces the survival (Das, 1976; Jordan & Goatly, 1962; Samarawickrema, 1967; Nathan, 1981) and the fecundity of C. quinquefasciatus but also increases the duration of gonotrophic cycle (Rajagopalan, Kazmi & Mani, 1977). Subramanian et al. (1994) reported that the survival of wild C. quinquefasciatus infected with W. bancrofti declined by 25–33% during development from Mf to L3. Crans (1973) observed a 2-fold increase in mortality rate of C. quinquefasciatus fed on persons harbouring W. bancrofti compared to mosquitoes fed on normal individuals. Parasite-related reduction in survival has also been reported for Simulium vectors infected with Onchocerca volvulus (Basanez et al. 1996), Aedes polynesiensis, Anopheles funestus and C. quinquefasciatus infected with W. bancrofti (Pichon, Prodhon & Riviere, 1980b). The present study also provides supporting evidence of parasite-induced mortality and quantifies the effect of parasite load on vector survival. The effect of parasitic infection alone was examined using mosquitoes of the same age. Mortality due to age during parasite development was corrected from the cohort of mosquitoes fed on amicrofilaraemic individuals, to reflect the excess mortality in vector mosquitoes due to parasite infection alone.
The percentage of mosquitoes dying before 13 days (during the extrinsic period) was significantly higher among those fed on microfilaraemic persons (50%) than on amicrofilaraemic (29%), accounting for an excess mortality of 21%. The excess mortality is likely to be a consequence of the level of infection in mosquitoes, which in turn is directly related to the human Mf-density. The overall mortality risk for mosquitoes fed on Mf-carriers was estimated (eαj) to be 11–15 times higher than for those fed on amicrofilaraemic persons. Further, the mortality risk was found to be increased by the level of parasite in a mosquito. For example, among those exposed to microfilaraemic persons, if 100 is the average number of parasites supported per positive mosquito up to 10 days of p.e., the mortality is estimated to be about 70–80% compared to 30–45% for those fed on Mf-volunteers but without any parasite at the time of death. The absence of parasites, in the latter group of mosquitoes, could be due to either density-dependent mortality of the parasites or failure of these mosquitoes to ingest Mf, which increases with decrease in Mf-density in the peripheral blood (Subramanian et al. 1998). As a consequence of parasite-induced reductions in survival, the life-expectancy of infected mosquitoes was reduced significantly with increasing number of parasites acquired, leaving less chance to transmit infection. Saporu (1993) by fitting both the Cox's proportional hazard model, and the Weibull accelerated failure time model to survival times of the onchocerciasis vector, Simulium damnosum, showed that a higher Mf-uptake reduced the survival of vector by a factor of 1·3 times compared to normal fly.
In estimating the survival function in mosquitoes we have used the human Mf-density as a factor and the parasite load in dead mosquitoes as a covariate. Instead, an estimate of the Mf-uptake (obtained by dissecting a sample of mosquitoes) could have been used as a co-variate or a factor as had been done for the onchocerciasis vector S. damnosum (Saporu, 1993). We consider that this would lead to problems in accuracy of the estimation of survival function because the validity of an estimate of the Mf-uptake depends on the number of mosquitoes dissected. The factors, which could influence Mf-uptake, include spatial and temporal distribution of Mf in human blood, duration of feeding, amount of blood imbibed by the mosquitoes and their efficiency to ingest Mf. Further, a fraction of the mosquitoes have to be sacrificed at the initial stage itself to assess the Mf-uptake by mosquitoes.
An important assumption in the survival model is that the parasite load as observed in dead mosquitoes influences the mortality hazard during the total life-span of the mosquito. Thus, loss of parasites is not taken into account. Such loss would mean that the hazard is relatively high in the first days, and later on lower.
A close look at the observed survival functions in Fig. 1 shows that the Mf-carrier fed mosquitoes have over the whole range an approximately exponential survival distribution, while the mosquitoes fed on uninfected persons have an accelerating mortality hazard after day 24, eventually crossing the survival curves of the Mf-carrier fed mosquitoes. This is a puzzling phenomenon. On the one hand it could be that long-term survival in Mf-carrier fed mosquitoes is enhanced by the parasite symbiont (Wolbachia), facilitating in this way the possibility of finding a new host. On the other hand, the survival of the mosquitoes fed on uninfected persons suddenly deteriorates. But, it is difficult to see how environmental problems could have caused such excess mortality as all the batches of mosquitoes were reared and maintained under the same laboratory conditions. Experimental infection studies with parasite symbiont Wolbachia may be useful in understanding the survival phenomenon of infected vectors.
The conclusion that the survival of C. quinquefasciatus infected with W. bancrofti declines with increasing parasite load has important implications for the control/elimination of lymphatic filariasis through mass drug administration (MDA) programmes. MDA with either single drug (DEC/ivermectin) or 2-drug (DEC/ivermectin+albendazole) regimens and DEC fortified salt distribution have been recommended for the control/elimination of filariasis (Ottesen & Ramachandran, 1995). These strategies are aimed at interruption of transmission by liquidating parasite load in the community. Following each round of mass treatment the parasite (Mf) density in the human host is reduced gradually and thereby the quantum of Mf available for the vector mosquitoes and the number of infective larvae for transmission is reduced. However, as a consequence of reduction in parasite load in the community, parasite-induced vector-mortality will be reduced and hence the vector will facilitate (instead of ‘limitation’) the successful development of the larvae. Since in any endemic area only 5–10% of the Mf-carriers harbour high Mf-counts (Das et al. 1990; Dye & Williams, 1995), and this percentage will fall into low Mf-category following MDA. The present study showed that 55 and 60% of the mosquitoes fed on low and medium Mf-categories survived beyond 12 days. Further, 46 and 94% of the Mf ingested by these mosquitoes developed into L3, whereas the corresponding figures for the mosquitoes fed on high Mf-category is 37% and 7%. Comparison of the above results suggest that the low Mf-density carriers would remain a potential threat for total interruption of transmission following repeated MDA as has been reported elsewhere (Basu & Rao, 1939; Jayasekera, Kalpage & De Silva, 1991; Krishnaswami, Pattanayak & Raghavan, 1959). The threat of low Mf density carriers can be controlled either by increasing the duration and coverage of MDA or by including vector control measures as an adjunct following 5 rounds of MDA, which is expected to reduce the Mf prevalence below 1%.
The Vector Control Research Centre (VCRC) is an Institute of the Indian Council of Medical Research. We gratefully acknowledge Dr K. Balaraman, Deputy Director (Sr Grade), Dr P. Vanamail and Ms A. Srividya, Technical Officers, VCRC for critically reviewing the manuscript. We would like to express our sincere gratitude to the volunteers who participated in the study and Mr A. Arokiadas and Mr M. Murugan, VCRC for their technical assistance. S. Subramanian received support from the special programme UNDP/World Bank/WHO for Research and Training in Tropical Diseases (ID-920743 and 950247).

Fig. 1. Observed survival function of Culex quinquefasciatus mosquitoes fed on human volunteers in 4 different Mf density categories: No Mf (open circles), 0·3–7·0 Mf (closed circles), 7·0–21·0 Mf (open squares), and 21·0–440 Mf (closed squares) per 20 μl blood.

Table 1. Summary of dissection results of mosquitoes fed in humans with different Mf-density

Fig. 2. Observed (symbols) and expected (lines) survival of mosquitoes that fed on humans in three different Mf density categories: (A) No Mf, (B) 0·3–21·0 Mf, and (C) 21·0–440 Mf per 20 μl blood. Expected survival is given for the model in which the shape parameter γ differs between these 3 Mf density classes, and is calculated as the average of the expected survival of the mosquitoes, which in turn depends on the human Mf-density category and the parasite load of the mosquito.

Table 2. Parameter estimates and their standard errors (S.E.) for the Weibull survival models, with shape parameters γj are different between Mf density classes