Introduction
Both animals and plants associate with symbiotic bacterial communities that provide functional benefits to their hosts (Mueller and Sachs, Reference Mueller and Sachs2015). The gut microbiota comprises some of the largest and most well-studied communities of host-associated symbionts (Kamada et al., Reference Kamada, Chen, Inohara and Núñez2013). The bacterial communities that colonize the gut and skin epithelia interact directly with potential pathogens in the environment, and can influence infection by stimulation of the host-immune system, competition for space and nutrients and production of inhibitory substances such as organic acids and bacteriocins (Dillon and Dillon, Reference Dillon and Dillon2004; Round and Mazmanian, Reference Round and Mazmanian2009; Daskin et al., Reference Daskin, Bell, Schwarzkopf and Alford2014; Raymann and Moran, Reference Raymann and Moran2018). Interactions between the gut microbiota and pathogens of bees are an emerging area of research with both fundamental and applied importance (Gaggìa et al., Reference Gaggìa, Baffoni and Alberoni2018; Raymann and Moran, Reference Raymann and Moran2018). Elucidation of the antipathogenic potential of the bee microbiota may ultimately help to preserve the pollination services provided by both wild and managed bees, which improve yields of over two-thirds of common agricultural crops (Klein et al., Reference Klein, Vaissière, Cane, Steffan-Dewenter, Cunningham, Kremen and Tscharntke2007) and contribute to the >$150 billion per year in economic value supplied by animal pollination (Gallai et al., Reference Gallai, Salles, Settele and Vaissière2009).
The gut microbiota of corbiculate (‘pollen basket’) bees, including honey and bumble bees, comprises a common core of 5 bacterial clades: Snodgrassella (Betaproteobacteria), Gilliamella (Gammaproteobacteria), Bifidobacterium and Lactobacillus clades ‘Firmicutes-4’ and ‘Firmicutes-5’ (Kwong et al., Reference Kwong, Medina, Koch, Sing, Soh, Ascher, Jaffé and Moran2017b). In addition to these core symbionts, bees may be infected by a variety of bacterial, fungal, protozoal and viral pathogens (Evans and Spivak, Reference Evans and Spivak2010), many of which are shared between wild and managed bees (Graystock et al., Reference Graystock, Blane, McFrederick, Goulson and Hughes2016), can elevate mortality (Fürst et al., Reference Fürst, McMahon, Osborne, Paxton and Brown2014) and have been implicated in declines of bee populations on multiple continents (Cameron et al., Reference Cameron, Lozier, Strange, Koch, Cordes, Solter and Griswold2011; Schmid-Hempel et al., Reference Schmid-Hempel, Eckhardt, Goulson, Heinzmann, Lange, Plischuk, Escudero, Salathé, Scriven and Schmid-Hempel2014; Goulson et al., Reference Goulson, Nicholls, Botías and Rotheray2015). Both core and non-core microbiota have been found to stimulate immunity and enhance bee resistance to pathogens (Evans and Lopez, Reference Evans and Lopez2004; Engel et al., Reference Engel, Kwong, McFrederick, Anderson, Barribeau, Chandler, Cornman, Dainat, Miranda, de, Doublet, Emery, Evans, Farinelli, Flenniken, Granberg, Grasis, Gauthier, Hayer, Koch, Kocher, Martinson, Moran, Munoz-Torres, Newton, Paxton, Powell, Sadd, Schmid-Hempel, Schmid-Hempel, Song, Schwarz, vanEngelsdorp and Dainat2016; Kwong et al., Reference Kwong, Mancenido and Moran2017a; Raymann and Moran, Reference Raymann and Moran2018). For example, depletion or perturbation of the gut microbiota increased the severity of bacterial, fungal and protozoal infections in honey bees (Schwarz et al., Reference Schwarz, Moran and Evans2016; Kwong et al., Reference Kwong, Mancenido and Moran2017a; Raymann and Moran, Reference Raymann and Moran2018), whereas supplementation with core and hive-associated bacteria improved survival of infected larvae and adults (Forsgren et al., Reference Forsgren, Olofsson, Váasquez and Fries2010; Vásquez et al., Reference Vásquez, Forsgren, Fries, Paxton, Flaberg, Szekely and Olofsson2012; Kwong et al., Reference Kwong, Mancenido and Moran2017a). Several studies have shown direct inhibitory effects of gut and hive-associated symbionts against common bee pathogens (Evans and Armstrong, Reference Evans and Armstrong2006; Sabaté et al., Reference Sabaté, Carrillo and Carina Audisio2009; Praet et al., Reference Praet, Parmentier, Schmid-Hempel, Meeus, Smagghe and Vandamme2018), which suggests a parsimonious explanation for the effects of symbionts on infection.
The gut microbiota of bumble bees (Bombus spp.) has been repeatedly associated with resistance to infection with the trypanosomatid gut parasite Crithidia bombi (Koch and Schmid-Hempel, Reference Koch and Schmid-Hempel2012; Koch et al., Reference Koch, Cisarovsky and Schmid-Hempel2012; Cariveau et al., Reference Cariveau, Elijah Powell, Koch, Winfree and Moran2014; Mockler et al., Reference Mockler, Kwong, Moran and Koch2018). This parasite has a variety of negative effects on bees. Symptoms include reduced rates of foraging, pollen collection and learning for worker bees (Shykoff and Schmid-Hempel, Reference Shykoff and Schmid-Hempel1991; Gegear et al., Reference Gegear, Otterstatter and Thomson2005, Reference Gegear, Otterstatter and Thomson2006); and reduced winter survival and spring nest-founding success for queen bees (Brown et al., Reference Brown, Schmid-Hempel and Schmid-Hempel2003). Infection with C. bombi can also exacerbate susceptibility to co-occurring stressors such as starvation, pesticides (Fauser-Misslin et al., Reference Fauser-Misslin, Sadd, Neumann and Sandrock2014) and nectar alkaloids (Palmer-Young et al., Reference Palmer-Young, Hogeboom, Kaye, Donnelly, Andicoechea, Connon, Weston, Skyrm, Irwin and Adler2017b). Trypanosomatid infections appear to be common in corbiculate bees, afflicting over half of individuals in some honey and bumble bee populations (Schmid-Hempel and Ebert, Reference Schmid-Hempel and Ebert2003; Cornman et al., Reference Cornman, Tarpy, Chen, Jeffreys, Lopez, Pettis, vanEngelsdorp and Evans2012), and have been correlated with honey bee colony collapses (Cornman et al., Reference Cornman, Tarpy, Chen, Jeffreys, Lopez, Pettis, vanEngelsdorp and Evans2012; Ravoet et al., Reference Ravoet, Maharramov, Meeus, De Smet, Wenseleers, Smagghe and de Graaf2013) and native bumble bee declines (Schmid-Hempel et al., Reference Schmid-Hempel, Eckhardt, Goulson, Heinzmann, Lange, Plischuk, Escudero, Salathé, Scriven and Schmid-Hempel2014). Both the presence and composition of the bumble bee microbiota may improve resistance to C. bombi. For example, germ-free rearing conditions and treatment with antibiotics both resulted in higher infection intensity in Bombus terrestris (Koch and Schmid-Hempel, Reference Koch and Schmid-Hempel2011, Reference Koch and Schmid-Hempel2012). In contrast, absolute and relative abundance of select core gut bacteria were correlated with resistance to infection in both field surveys and fecal transplant experiments (Koch and Schmid-Hempel, Reference Koch and Schmid-Hempel2011, Reference Koch and Schmid-Hempel2012; Cariveau et al., Reference Cariveau, Elijah Powell, Koch, Winfree and Moran2014; Mockler et al., Reference Mockler, Kwong, Moran and Koch2018). Just 3 taxa – Snodgrassella, Gilliamella and Lactobacillus Firm-5 – generally account for over 80% of the total gut bacteria in bumble bees (Koch and Schmid-Hempel, Reference Koch and Schmid-Hempel2012; Billiet et al., Reference Billiet, Meeus, Van Nieuwerburgh, Deforce, Wäckers and Smagghe2017; Mockler et al., Reference Mockler, Kwong, Moran and Koch2018). In both B. terrestris and B. impatiens, inoculation with microbiota rich in Lactobacillus Firm-5 resulted in resistance to C. bombi infection (Koch and Schmid-Hempel, Reference Koch and Schmid-Hempel2012; Mockler et al., Reference Mockler, Kwong, Moran and Koch2018). However, no study has examined the mechanisms by which these microbes influence parasite growth.
Lactobacillus Firm-5 is a group of lactic acid-producing bacteria (Praet et al., Reference Praet, Meeus, Cnockaert, Houf, Smagghe and Vandamme2015). One way that they might increase host resistance to parasites is via modification of gut chemistry. Lactic acid fermentation results in production of organic acids that lower pH and inhibit growth of organisms that cause spoilage and infection (Adams and Hall, Reference Adams and Hall1988; Lindgren and Dobrogosz, Reference Lindgren and Dobrogosz1990; Glass et al., Reference Glass, Loeffelholz, Ford and Doyle1992; Russell and Diez-Gonzalez, Reference Russell and Diez-Gonzalez1998). Indeed, lactic acid bacteria have a long history of use in food preservation in both human and insect societies (Salminen and von Wright, Reference Salminen and von Wright2004; Anderson et al., Reference Anderson, Carroll, Sheehan, Mott, Maes and Corby-Harris2014). In the host intestine, lactic acid bacteria can inhibit enteric pathogens, such as Salmonella and Escherichia coli (Gorbach, Reference Gorbach1990). This inhibition may reflect stimulation of the host-immune system (Presser et al., Reference Presser, Ratkowsky and Ross1997; Cox et al., Reference Cox, Pyne, Saunders and Fricker2010), including that of insects (Evans and Lopez, Reference Evans and Lopez2004; Evans and Armstrong, Reference Evans and Armstrong2006). However, Lactobacillus-mediated inhibition of pathogens is most simply explained by the direct antimicrobial activity of Lactobacillus metabolites. These metabolites, which include lactic acid and bacteriocins (Lindgren and Dobrogosz, Reference Lindgren and Dobrogosz1990), may reduce the suitability of the gut environment for pathogens.
In the bee gut, Lactobacillus Firm-5 has been shown to have a disproportionately large effect on gut metabolomics. In honey bees, mono-inoculation with Firm-5 accounted for over 80% of the changes seen in bees inoculated with a full complement of gut microbes (Kešnerová et al., Reference Kešnerová, Mars, Ellegaard, Troilo, Sauer and Engel2017). Firm-5 isolates also showed in vitro inhibitory activity against the pathogens Paenibacillus larvae and Melissococcus plutonius (Praet et al., Reference Praet, Parmentier, Schmid-Hempel, Meeus, Smagghe and Vandamme2018). The high relative abundance of the Firm-5 clade in bumble bees [often >30% total bacteria (Koch and Schmid-Hempel, Reference Koch and Schmid-Hempel2012; Billiet et al., Reference Billiet, Meeus, Van Nieuwerburgh, Deforce, Wäckers and Smagghe2017)], combined with its consistent association with resistance to trypanosomatid infection, suggests that Lactobacillus Firm-5 plays a major role in bumble bee resistance to trypanosomatid parasites.
We hypothesized that Lactobacillus Firm-5 enhances resistance to trypanosomatid parasites primarily by modifying the pH of the enteric environment. To test this hypothesis, we measured the inhibitory effects of spent medium from Lactobacillus bombicola, a member of the Firmicutes-5 clade that is ubiquitous in the gut microbiota of corbiculate bees, including Bombus spp. (Billiet et al., Reference Billiet, Meeus, Van Nieuwerburgh, Deforce, Wäckers and Smagghe2017; Kwong et al., Reference Kwong, Medina, Koch, Sing, Soh, Ascher, Jaffé and Moran2017b; Praet et al., Reference Praet, Parmentier, Schmid-Hempel, Meeus, Smagghe and Vandamme2018), on in vitro growth of several strains of C. bombi. We predicted that spent medium from L. bombicola would inhibit C. bombi growth, that the acidity of spent medium would be both necessary and sufficient to account for parasite inhibition, and that C. bombi strains would vary in sensitivity to spent medium.
Materials and methods
Overview of experiments
Three experiments were conducted to evaluate effects of spent medium from L. bombicola cultures on growth of C. bombi. Spent medium was generated by growth of L. bombicola in MRS broth for 3 days, followed by sterile filtration to remove live cells. For C. bombi growth assays, the MRS-based spent medium (or MRS broth control) was diluted 1:1 in fresh, Crithidia-specific ‘FPFB’ medium (Salathé et al., Reference Salathé, Tognazzo, Schmid-Hempel and Schmid-Hempel2012). (1) The neutralization experiment tested whether spent medium would inhibit growth, and whether acidity of the spent medium was necessary or sufficient for inhibition. (2) The acidification experiment tested for variation in pH-dependent growth inhibition due to various sources of acidity. (3) The strain variation experiment tested for variation in sensitivity to spent medium among different parasite strains.
Cell cultures
Lactobacillus bombicola strain 70-3, isolated from B. lapidarius collected near Ghent Belgium [isolate ‘28288T’ (Praet et al., Reference Praet, Meeus, Cnockaert, Houf, Smagghe and Vandamme2015)], was obtained from the DSMZ and grown in 2 mL screw-cap tubes in MRS broth (Research Products International, Mt. Prospect, IL) with 0.05% cysteine at 27 °C. Crithidia bombi cell cultures were isolated from bumble bee intestines by flow cytometry based single cell sorting as described previously (Salathé et al., Reference Salathé, Tognazzo, Schmid-Hempel and Schmid-Hempel2012). Cultures originated from wild infected bumble bees. Strains VT1 (Vermont, USA, 2013, courtesy Rebecca Irwin) and IL13.2 (Illinois, USA, 2013, courtesy Ben Sadd) originated from B. impatiens workers. Strains S08.1 (Switzerland, 2008, courtesy Ben Sadd) originated from B. terrestris. These same cell lines have been used to assess effects of phytochemicals on parasite growth (Palmer-Young et al., Reference Palmer-Young, Sadd, Irwin and Adler2017a). Briefly, cells from fecal samples were sorted into 96-well plates containing ‘FPFB’ culture medium with 10% heat-inactivated fetal bovine serum and incubated at 27 °C. Cultures with successful growth and absence of visible contamination were transferred to vented, 25 cm2 tissue culture flasks, grown to high density and cryopreserved at −80 °C until several weeks before the experiments began (Salathé et al., Reference Salathé, Tognazzo, Schmid-Hempel and Schmid-Hempel2012). Culture identity was confirmed as C. bombi based on glyceraldehyde 3-phosphate dehydrogenase and cytochrome b gene sequences. Cultures were inspected weekly to verify absence of contamination.
The neutralization and acidification experiments were performed with a line of strain ‘VT1’ that had been in continuous culture for 2 months at the start of the experiments presented here, with transfers to fresh medium every 3–4 days. This line is referred to as ‘VT1*’ in the multi-strain experiment, to differentiate it from the more recently thawed line of the same strain. All other strains in the multi-strain experiment were thawed 20 days prior to the assay and transferred to fresh medium every 3–7 days, depending on growth.
Generation of spent medium
To generate spent medium, L. bombicola aliquots were transferred to 8 mL fresh medium and grown in screw-cap 14 mL conical tubes at 27 °C for 3 days. The resulting spent medium (net OD630 nm = 0.500–0.700) was sterile-filtered through a 0.22 µm membrane and stored at −20 °C until use in experiments (not more than 2 weeks). Fresh MRS medium, incubated under identical conditions, was used as a control.
Neutralization experiment
To evaluate the inhibitory effects of spent medium, neutralized spent medium and acidified fresh medium, growth of C. bombi was compared across 4 MRS-based treatments: spent medium (‘Spent’, initial pH 4.8), spent medium neutralized to pH 6.2 with 1 m NaOH (‘Neutralized spent’), fresh MRS medium (‘Fresh’, pH 6.2), and fresh MRS medium acidified to pH 4.8 with 1 m HCl (‘Acidified fresh’). The C. bombi culture was diluted to an OD of 0.020 in fresh FPFB medium (pH 5.88). The resulting cell suspension (100 µL) was added to wells of a 96-well plate containing an equal volume of the MRS-based treatment medium, resulting in a final net OD630 nm of 0.010. Growth was measured twice daily by optical density (630 nm) over the ensuing 48 h on an EL-800 plate reader spectrophotometer (BioTek, Winooski, VT). Net optical density at each time point was computed by subtracting OD of wells containing the corresponding MRS-based treatment medium and FPFB medium without C. bombi; this controlled for any differences in optical density that occurred independent of C. bombi growth. The experiment included 18 replicate wells per treatment.
Acidification experiment
To compare variation in growth inhibition across different sources of acidity, growth of C. bombi was compared in dilutions of spent medium (initial pH 4.65), and in fresh medium acidified with d-lactic acid (pH 4.82), l-lactic acid (pH 4.77) or HCl (pH 4.73). Each base medium was diluted with fresh MRS medium to 0, 20, 40, 60, 80 or 100% of initial concentration. The final pH of each treatment was measured with a pH meter (‘Orion Star’, Thermo, Waltham, MA) after combination with an equal volume of FPFB medium. Growth of C. bombi (initial OD 0.010) was evaluated with a 96-well plate assay as in the neutralization experiment above. The experiment included 12 replicate wells per concentration of each acidification treatment. To verify the relative potency of spent vs acidified medium against different parasite strains, the experiment was repeated with 2 C. bombi strains (IL13.2 and VT1*) tested in parallel against 2 of the 4 acidification treatments (spent medium and HCl-acidified medium); results are shown in Supplementary Fig. 2.
Strain variation experiment
To compare susceptibility to spent medium across different C. bombi strains and degrees of acclimation to the culture environment, inhibitory concentration of spent medium was compared across 4 cell lines: the ‘VT1*’ line of strain VT1 that had been used for the above experiments, and by this time had been in continuous culture for 3 months; and strains VT1, IL13.2 and S08.1 that had been thawed 20 days prior to the experiment. Six dilutions of spent medium (0–100%) were prepared in fresh MRS medium; final pH of each treatment was measured after combination with an equal volume of FPFB medium. Growth of each line of C. bombi (initial OD 0.010) was evaluated with a 96-well plate assay as in the neutralization experiment above. The experiment included 12 replicate wells per cell line and spent medium concentration.
Statistical analysis
Statistical analysis was performed in R v3.4.3 for Windows (R Core Team, 2014). For the acidification and strain variation experiments, dose–response curves to relate growth rate to pH were estimated with the drm function in the R package drc (Ritz et al., Reference Ritz, Baty, Streibig and Gerhard2015). Under the conditions of the experiment (initial OD = 0.010, incubation temperature 32 °C), C. bombi growth was found to be exponential over the first 24 h of incubation (Supplementary Fig. 1). Therefore, the rate of growth (log2(OD/OD0)/t) over the first incubation interval (0–21 h) was used as the response variable for the dose–response models. A log-logistic function, with the lower asymptote fixed at 0, was fitted to the growth measurements for each acidification treatment (acidification experiment) or cell line (strain variation experiment), using final pH of the treatment medium at the start of the experiment (i.e. after combination of the MRS-based treatment with an equal volume FPFB medium) as the predictor variable. The 95% confidence intervals (CIs) for EC50 pH (i.e. the pH that inhibited growth by 50% relative to the unacidified control treatment), and 95% CIs for ratios of EC50 pH among acidification treatments and strains, were estimated using the delta method [drc function EDcomp (Ritz et al., Reference Ritz, Baty, Streibig and Gerhard2015)]. Acidification treatments and strains were considered significantly different when the 95% CI for the ratio of their EC50 pH values did not include 1.
Results
Neutralization experiment: acidity dependent inhibition of C. bombi by spent medium
We found that L. bombicola spent medium completely inhibited growth of C. bombi cell cultures (Fig. 1). However, neutralized spent medium had no inhibitory effect, indicating that acidity of the spent medium was necessary for inhibition (Fig. 1). Moreover, acidification of fresh (Lactobacillus-specific) MRS medium to pH 4.8 with l-lactic acid led to a level of growth inhibition that was comparable to that caused by pH 4.8 spent medium (Fig. 1). This demonstrated that spent medium from L. bombicola inhibited C. bombi growth, and that changes in pH were necessary and qualitatively sufficient to account for this inhibition.

Fig. 1. Spent medium from Lactobacillus bombicola inhibited growth of Crithidia bombi (strain VT1); acidity of the spent medium was necessary and sufficient for inhibition. Spent medium (‘Spent’, red solid line) completely inhibited parasite growth. However, spent medium neutralized to pH 6.2 with NaOH (‘Neutralized spent’, green dotted line) resulted in no inhibition relative to the fresh medium control (‘Fresh’, light green dashed line), demonstrating that acidity was necessary for inhibition. Fresh medium acidified to pH 4.8 with lactic acid (‘Acidified fresh’, blue dashed line) showed that acidity was sufficient for complete growth inhibition. Final pH of both spent medium and acidified fresh medium was 5.0 after combination with equal volume of fresh Crithidia medium (pH 5.9). Y-axis represents approximate number of parasite cell divisions, as measured by optical density (630 nm). Points and error bars show means and 95% CIs (n = 36 wells per treatment).
Acidification experiment: pH-dependent inhibition of C. bombi with different sources of acidity
Having established pH-dependent growth inhibition, we conducted a follow-up experiment to quantify C. bombi growth rates across a range of pH values, and to compare the relative inhibitory effects of L. bombicola spent medium with that of 3 other sources of acidity: HCl, l-lactic acid [the form produced by animal cells (Ewaschuk et al., Reference Ewaschuk, Zello, Naylor and Brocks2002)] and d-lactic acid [the form produced by L. bombicola (Praet et al., Reference Praet, Meeus, Cnockaert, Houf, Smagghe and Vandamme2015)]. We used log-logistic models to estimate and compare the EC50 pH (i.e. the pH that inhibited growth by 50% relative to the unacidified control treatment) for each source of acidity. All 4 sources of acidity resulted in qualitatively similar inhibition of C. bombi (Fig. 2A), with considerable inhibition achieved within the pH range previously measured in honey bee guts [vertical lines in Fig. 2A, from (Zheng et al., Reference Zheng, Powell, Steele, Dietrich and Moran2017); no data are available on pH of bumble bee guts]. However, the EC pH varied somewhat across sources of acidity, with a hierarchy of pH-dependent inhibitory potency in the order HCl < l-lactic acid < d-lactic acid < spent medium (Fig. 2B, see Supplementary Table S1 for EC50 values and Supplementary Data S1 for model parameters, CIs on EC50 ratios and raw data). An additional experiment with a second C. bombi strain (‘IL13.2’) confirmed the greater potency of spent medium relative to HCl-acidified medium across multiple parasite genotypes (Supplementary Fig. 2).
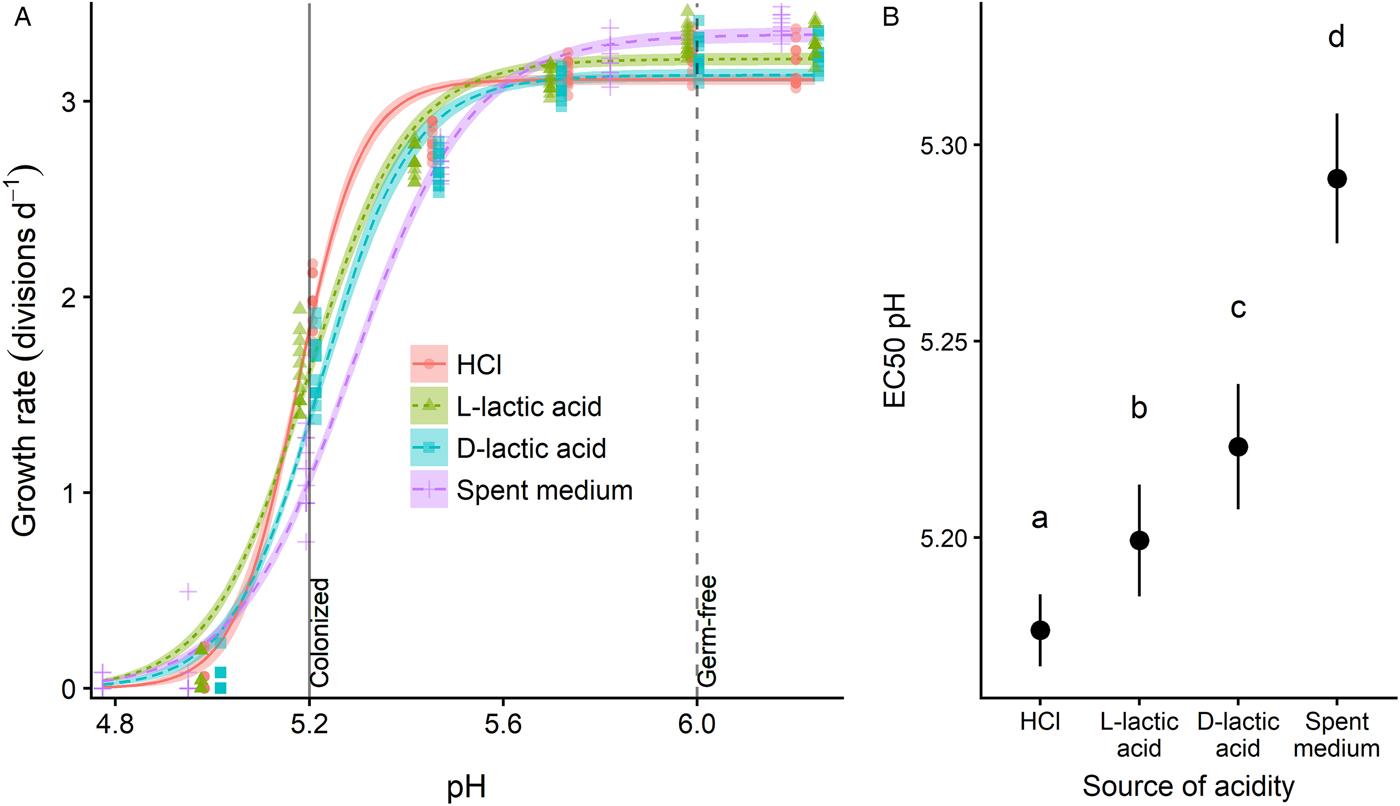
Fig. 2. Different sources of acidity varied in pH-dependent inhibitory potency against C. bombi (strain VT1). (A) Dose–response curves relating pH to growth rate. X-axis shows final pH of treatment medium after combination of L. bombicola spent medium or acidified MRS medium with an equal volume of Crithidia-specific FPFB medium. Y-axis represents growth rate over first 21 h of incubation, measured as number of doublings per day by optical density (OD: 630 nm). Lines and shaded bands represent model predictions and standard errors for each source of acidity. Points show raw data for each replicate well (n = 12). Vertical lines correspond to pH values measured in ileum and rectum of microbe-colonized and germ-free honey bees (Zheng et al., Reference Zheng, Powell, Steele, Dietrich and Moran2017), and are shown as an estimate of gut pH range in bumble bees. (B) 50% inhibitory concentrations (EC50, i.e. the concentration that inhibits growth by 50%) for different sources of acidity. Point estimates and 95% CIs are derived from model fits shown in panel (A). Higher EC50 pH estimates correspond to higher inhibitory potency for a given level of acidity. Different lower-case letters indicate statistically significant (P < 0.05) differences in pairwise comparisons of EC50 pH by source of acidity.
Strain variation experiment: sensitivity to spent medium differs across C. bombi strains and rate of growth in culture (Fig. 3)
We tested the dose-dependent effects of spent medium across 3 C. bombi strains, including 2 lines of strain VT1 – the line that had been used for the above experiments and kept in continuous culture over the preceding 3 months (‘VT1*’), and a second line that had been thawed 20 days prior to the experiment (‘VT1’). Values for EC50 pH showed statistically and biologically meaningful variation across cell lines (Fig. 3). The line most thoroughly acclimated to the culturing conditions, VT1*, exhibited both the fastest growth (Fig. 3A) and the lowest sensitivity to spent medium, as indicated by its low EC50 pH value (Fig. 3B); strain IL13.2 exhibited the second-fastest growth and the second-lowest sensitivity to spent medium. Strains VT1 and S08.1 had similar growth rates in the absence of spent medium (Fig. 3A), but had significantly different EC50 pH values (Fig. 3B; see Supplementary Table S1 for EC50 values and Supplementary Data S1 for model parameters, CIs on EC50 ratios and raw data). All C. bombi strains suffered growth inhibition within the pH range (5.2–6.0) documented in the honey bee hindgut (Zheng et al., Reference Zheng, Powell, Steele, Dietrich and Moran2017) (Fig. 3A).
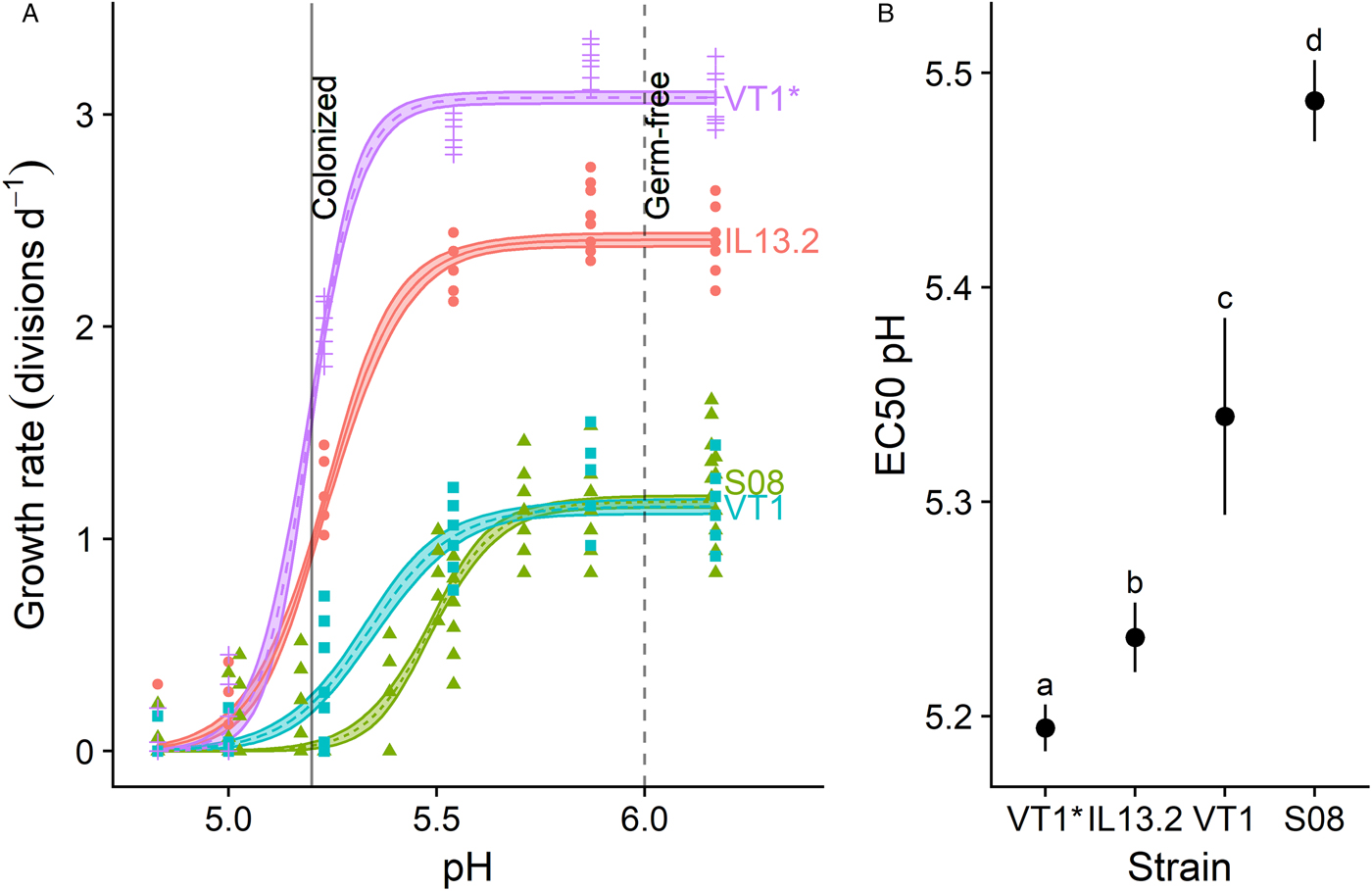
Fig. 3. Crithidia bombi pH sensitivity varied according to strain identity and rate of growth in culture. (A) Dose–response curves relating pH to growth rate for different C. bombi strains. X-axis shows final pH of treatment medium after combination of L. bombicola spent MRS medium with an equal volume of Crithidia-specific FPFB medium. Y-axis represents growth rate over first 20 h of incubation, measured as number of doublings per day by optical density (OD: 630 nm). Lines and shaded bands represent model predictions and standard errors for each strain. Points show raw data for each replicate well (n = 12). Vertical lines correspond to pH values measured in ileum and rectum of microbe-colonized and germ-free honey bees (Zheng et al., Reference Zheng, Powell, Steele, Dietrich and Moran2017), and are shown as an estimate of gut pH range in bumble bees. Strains ‘VT1*’ and ‘VT1’ are the same strain, but ‘VT1*’ had been grown in continuous culture for 3 months prior to the experiment, whereas ‘VT1’ and all other strains had been thawed from cryopreserved stock 3 weeks prior. (B) 50% inhibitory concentrations (EC50) for each strain. Point estimates and 95% CIs are derived from model fits shown in panel (A). Higher EC50 pH estimates correspond to higher sensitivity to acidity. Different lower-case letters indicate statistically significant (P < 0.05) differences in pairwise comparisons of EC50 pH by source of acidity. For all strains, inhibition occurred within the pH range measured in honey bee guts (data from Zheng et al., Reference Zheng, Powell, Steele, Dietrich and Moran2017).
Discussion
Our results show that production of acids by the core bumble bee hindgut symbiont, L. bombicola, is both necessary and sufficient to inhibit growth of the widespread trypanosomatid parasite C. bombi. The growth-inhibitory effects of L. bombicola-acidified spent medium were qualitatively similar across C. bombi isolates and occurred within a pH range that is physiologically realistic for the bee hindgut where C. bombi establishes. Because lactobacilli and other acid-producing bacteria are dominant members of the bumble bee gut microbiota, our results suggest that the inhibitory effects of bumble bee gut microbiota on C. bombi infection intensity can be largely attributed to microbial acidification of the gut. Our findings provide a mechanistic basis to understand how microbiota may affect trypanosomatid infection in corbiculate bees that share a core microbiome (Kwong et al., Reference Kwong, Medina, Koch, Sing, Soh, Ascher, Jaffé and Moran2017b) and can be infected with identical and related parasites, including trypanosomatids (McMahon et al., Reference McMahon, Fürst, Caspar, Theodorou, Brown and Paxton2015; Schwarz et al., Reference Schwarz, Bauchan, Murphy, Ravoet, de Graaf and Evans2015; Tripodi et al., Reference Tripodi, Szalanski and Strange2018).
Inhibitory activity of L. bombicola spent medium is driven by production of acids
The effects of L. bombicola spent medium on C. bombi growth could be explained by the acidity of the spent medium. Environmental pH is a recognized driver of interactions in microbial communities, where species vary in how they alter the pH of their surroundings and in the pH range at which they can grow (Morton et al., Reference Morton, Sanders, Quinn, McDonald, Gonzalez, Vázquez-Baeza, Navas-Molina, Song, Metcalf, Hyde, Lladser, Dorrestein and Knight2017; Ratzke and Gore, Reference Ratzke and Gore2018). In honey bees, microbial colonization with core symbionts resulted in acidification of the hindgut lumen (Zheng et al., Reference Zheng, Powell, Steele, Dietrich and Moran2017). In our cell culture experiments, a reduction in pH that corresponds to the difference between guts of germ-free (pH ~ 6.0) and normal, hive-reared honey bees [pH ~ 5.2 (Zheng et al., Reference Zheng, Powell, Steele, Dietrich and Moran2017)] profoundly inhibited growth of C. bombi. Our findings indicate that symbiont-mediated gut acidification could act as an important filter that prevents trypanosomatid establishment and defends bees against infection.
Spent medium was more strongly inhibitory than expected based on pH alone
Whereas all 4 tested acids inhibited C. bombi in a dose-dependent fashion, the EC50 pH varied across sources of acidity (Fig. 2). Both enantiomers of lactic acid were more inhibitory than HCl for a given pH. This finding is consistent with prior work on bacteria, which showed growth inhibition at relatively high pH when lactic acid, rather than HCl, was used as the acidulant (Adams and Hall, Reference Adams and Hall1988; Glass et al., Reference Glass, Loeffelholz, Ford and Doyle1992). For example, the inhibitory pH of E. coli was 0.1 pH units higher with lactic acid, rather than HCl, as the acidulant (Glass et al., Reference Glass, Loeffelholz, Ford and Doyle1992). This increased activity reflects the fact that lactic acid and other weak acids are undissociated at low pH, which allows them to penetrate membranes of target cells with relative ease. Once inside the relatively alkaline cytoplasm of the cell, the acid dissociates, and disrupts the proton motive force necessary for energy production and homoeostasis (Russell and Diez-Gonzalez, Reference Russell and Diez-Gonzalez1998).
We also found very slightly but significantly higher potency of d-lactic acid relative to l-lactic acid (Fig. 2). l-Lactic acid is the enantiomer more often produced by trypanosomatids, including by Leishmania species that are closely related to C. bombi (Bringaud et al., Reference Bringaud, Rivière and Coustou2006), and l-lactic acid dehydrogenase has been found in the genome of honey bee-infective trypanosomatids (Runckel et al., Reference Runckel, DeRisi and Flenniken2014). It is possible that C. bombi can use oxidative phosphorylation (Bringaud et al., Reference Bringaud, Rivière and Coustou2006) to metabolize this enantiomer, and thereby reduce its toxicity, or that C. bombi have greater resistance to the enantiomer that they produce through their own metabolism. For example, L. bulgaricus produced d-lactic acid, and were less inhibited by this self-generated enantiomer than by l-lactic acid (Benthin and Villadsen, Reference Benthin and Villadsen1995).
Prior measurements of the pH range and sources of acidity in honey bee gut both indicate that pH-mediated inhibition of trypanosomatids is achievable for corbiculate bees. The pH of a symbiont-colonized honey bee hindgut [~5.2 (Zheng et al., Reference Zheng, Powell, Steele, Dietrich and Moran2017)] was similar to the EC50 pH for HCl (5.18) and lower than the EC50 pH due to spent medium (5.29). In vitro, L. bombicola produced exclusively d-lactic acid (Praet et al., Reference Praet, Meeus, Cnockaert, Houf, Smagghe and Vandamme2015), which was the most inhibitory (i.e. highest EC50 pH) of the pure acids tested here (Fig. 3). Moreover, the specific acids that are found in the gut environment are generally more inhibitory for a given pH than those tested here. In the honey bee hindgut, the most abundant acids were acetic acid and succinic acid (Zheng et al., Reference Zheng, Powell, Steele, Dietrich and Moran2017). Both acetic acid (pK a = 4.95) and succinic acid (pK a = 4.2 for first deprotonation and 5.6 for second deprotonation) have relatively high pK as relative to lactic acid (pK a = 3.86) and HCl (pK a = −7). These high pK a values mean that at low pH, the acids are found in their more potent, undissociated form. As a result, these high-pK a acids generally have antimicrobial effects that are even stronger than those of lactic acid for a given pH (Adams and Hall, Reference Adams and Hall1988). Indeed, lactic and acetic acids can have synergistic effects against growth of E. coli (Adams and Hall, Reference Adams and Hall1988), with lactic acid producing a low pH that increases the fraction of undissociated acetic acid (Adams and Hall, Reference Adams and Hall1988).
Spent medium was slightly more inhibitory than all pure acids for a given pH (Fig. 3). Because neutralization completely removed the inhibitory activity of spent medium, it appears that some aspect of the spent medium – whether the existence of some metabolite or the relative lack of nutrients – is only inhibitory at low pH. In other words, something about the spent medium is potentiated by an acidic environment. For example, low pH could facilitate solubility or penetration of non-lactic acid components, such as bacteriocins; this type of synergy was seen in other studies of Lactobacillus spp. (Fayol-Messaoudi et al., Reference Fayol-Messaoudi, Berger, Coconnier-Polter, Moal and Servin2005; De Keersmaecker et al., Reference De Keersmaecker, Verhoeven, Desair, Marchal, Vanderleyden and Nagy2006). Within the bee gut, synergistic effects could also occur between organic acids and toxins produced by other members of the microbiota (Praet et al., Reference Praet, Meeus, Cnockaert, Houf, Smagghe and Vandamme2015; Steele et al., Reference Steele, Kwong, Whiteley and Moran2017).
Strains varied in sensitivity to spent medium
We found that sensitivity to spent medium varied by C. bombi strain and rate of growth in culture. Strain VT1 that had been in continuous culture for 3 months and had the fastest growth rate was the least sensitive to spent medium, followed by the next-fastest strain IL13.2, the recently thawed line of strain VT1, and strain S08.1. The comparison between the recently thawed VT1 and S08.1 strains – which had similar maximal growth rates, but different levels of sensitivity to spent medium – indicates that pH sensitivity can have a genotypic basis and is not purely driven by the overall growth rate in culture. Strains of C. bombi have been shown to be both genetically and phenotypically diverse, and to vary in growth rate (Imhoof and Schmid-Hempel, Reference Imhoof and Schmid-Hempel1998), infectivity (Barribeau et al., Reference Barribeau, Sadd, du Plessis and Schmid-Hempel2014), and responses to host diet composition (Sadd, Reference Sadd2011), phytochemicals (Palmer-Young et al., Reference Palmer-Young, Sadd, Stevenson, Irwin and Adler2016) and microbiota (Koch and Schmid-Hempel, Reference Koch and Schmid-Hempel2012). Our study documents variation in pH sensitivity within an ecologically relevant range of pH that is likely representative of the environment in the bumble bee gut.
Our results showed some growth inhibition of all strains – and complete inhibition of some strains – within the pH range measured in the gut of honey bees (Zheng et al., Reference Zheng, Powell, Steele, Dietrich and Moran2017). The pH found in a germ-free gut (5.8–6.0) was favourable for growth, consistent with the high C. bombi infection intensities found in germ-free and antibiotic-treated bees (Koch and Schmid-Hempel, Reference Koch and Schmid-Hempel2011), whereas pH of a symbiont-colonized gut (<5.2) would be expected to inhibit growth of all strains. Thus, pH sensitivity may constrain ability of strains to colonize certain host genotypes (Barribeau et al., Reference Barribeau, Sadd, du Plessis and Schmid-Hempel2014) or enterotypes (Koch and Schmid-Hempel, Reference Koch and Schmid-Hempel2012; Li et al., Reference Li, Powell, Guo, Evans, Wu, Williams, Lin, Moran and Zhang2015) characterized by low gut pH. For example, inoculation with a microbiota high in Lactobacillus Firm-5 resulted in lower overall infection intensity and favoured infection with a single parasite strain that was less successful in bees inoculated with microbiota low in Firm-5 (Koch and Schmid-Hempel, Reference Koch and Schmid-Hempel2012). We hypothesize that low-pH conditions favour strains that are more tolerant, whereas high-pH conditions favour strains that are strong competitors. Further experiments are needed to investigate the extent to which parasite populations are selected for pH tolerance, and possible trade-offs between growth rate, infectivity or tolerance to environmental stressors and insect immune factors. Environment-dependent selection for these other traits could maintain variation in pH tolerance within parasite populations.
Gut microbiota-driven changes in pH may explain patterns of trypanosomatid infection in bees
The pH-dependent inhibition demonstrated here is consistent with past surveys and experiments that showed negative correlations between the abundance of acid-producing gut symbionts and C. bombi infection intensity, and with associations between microbiota composition and relative infectivity of different parasite strains. The Bombus gut microbiota is dominated by 3 taxa – Lactobacillus Firm-5, Gilliamella and Snodgrassella – that made up over 80% of total gut bacteria (Koch and Schmid-Hempel, Reference Koch and Schmid-Hempel2012; Billiet et al., Reference Billiet, Meeus, Van Nieuwerburgh, Deforce, Wäckers and Smagghe2017). In fecal transplant experiments of B. terrestris and B. impatiens, relative abundances of Lactobacillus Firm-5 and Gilliamella were negatively correlated with C. bombi infection intensity (Koch and Schmid-Hempel, Reference Koch and Schmid-Hempel2011; Reference Koch and Schmid-Hempel2012; Koch et al., Reference Koch, Cisarovsky and Schmid-Hempel2012; Mockler et al., Reference Mockler, Kwong, Moran and Koch2018). Both Lactobacillus and Gilliamella ferment sugars to produce acids (Engel et al., Reference Engel, Martinson and Moran2012; Kešnerová et al., Reference Kešnerová, Mars, Ellegaard, Troilo, Sauer and Engel2017).
In contrast, Snodgrassella abundance was not correlated with resistance to C. bombi in either B. terrestris or B. impatiens (Koch and Schmid-Hempel, Reference Koch and Schmid-Hempel2012; Mockler et al., Reference Mockler, Kwong, Moran and Koch2018), and pre-inoculation with Snodgrassella alvi reduced resistance to trypanosomatid infection in Apis mellifera (Schwarz et al., Reference Schwarz, Moran and Evans2016). Whereas Lactobacillus and Gilliamella spp. produce acids from sugars, Snodgrassella consumes organic acids (Kwong and Moran, Reference Kwong and Moran2013; Kešnerová et al., Reference Kešnerová, Mars, Ellegaard, Troilo, Sauer and Engel2017). This metabolic activity could elevate gut pH to levels that are more hospitable to trypanosomatids. On the other hand, Snodgrassella abundance was negatively correlated with infection prevalence in a field survey (Cariveau et al., Reference Cariveau, Elijah Powell, Koch, Winfree and Moran2014). This correlation might reflect the association of Snodgrassella with acid-producing Gilliamella (Kešnerová et al., Reference Kešnerová, Mars, Ellegaard, Troilo, Sauer and Engel2017), rather than inhibitory effects of Snodgrassella per se. Snodgrassella and Gilliamella form a biofilm that lines the ileum (Engel et al., Reference Engel, Martinson and Moran2012) and could competitively inhibit trypanosomatid attachment to the gut wall (Gorbunov, Reference Gorbunov1996; Schwarz et al., Reference Schwarz, Bauchan, Murphy, Ravoet, de Graaf and Evans2015). Further study would be needed to determine the relative contributions of gut acidification vs biofilm formation to microbiota-induced inhibition of parasites in bee guts. It would be intriguing to investigate how Snodgrassella abundance alters gut pH, and whether the effects of this symbiont on gut pH are outweighed by its direct competition with trypanosomatids for space along the gut wall, or by its contribution to anoxic gut environments (Zheng et al., Reference Zheng, Powell, Steele, Dietrich and Moran2017) that can be toxic to insect gut trypanosomatids (Bringaud et al., Reference Bringaud, Rivière and Coustou2006). Because Apis and Bombus share a core gut microbiota (Martinson et al., Reference Martinson, Danforth, Minckley, Rueppell, Tingek and Moran2011; Kwong and Moran, Reference Kwong and Moran2016), we hypothesize that trypanosomatid parasites of honey bees (Schwarz et al., Reference Schwarz, Bauchan, Murphy, Ravoet, de Graaf and Evans2015) and bumble bees interact with similar communities of gut symbionts, and that the same pH-altering symbiont taxa could govern parasite establishment in both host genera.
Do gut microbiota shape resistance to opportunistic infection via alteration of gut pH?
In the context of prior experiments, our results suggest a pH-mediated role of the bee microbiota in defence against opportunistic infection. Multiple studies have correlated lack of core gut bacteria, or abundance of non-core bacteria, with bee infection. In North America and Europe, high diversity of non-core bacterial species correlated with higher prevalence of the pathogens Crithidia and Nosema (Koch et al., Reference Koch, Cisarovsky and Schmid-Hempel2012; Cariveau et al., Reference Cariveau, Elijah Powell, Koch, Winfree and Moran2014). Similarly, in a survey of Bombus spp. in China, bees could be grouped into 2 microbial enterotypes (Li et al., Reference Li, Powell, Guo, Evans, Wu, Williams, Lin, Moran and Zhang2015). One enterotype was dominated by core symbionts Snodgrassella and Gilliamella, while the other enterotype comprised non-core Hafnia, Enterobacteriaceae and Serratia. The latter 2 are thought to be opportunistic pathogens (Kwong et al., Reference Kwong, Mancenido and Moran2017a; Raymann and Moran, Reference Raymann and Moran2018). Finally, higher susceptibility to pathogens was found in germ-free honey and bumble bees (Koch and Schmid-Hempel, Reference Koch and Schmid-Hempel2011; Kwong et al., Reference Kwong, Mancenido and Moran2017a; Mockler et al., Reference Mockler, Kwong, Moran and Koch2018; Raymann and Moran, Reference Raymann and Moran2018).
Acidification of the bee gut lumen by symbionts (Zheng et al., Reference Zheng, Powell, Steele, Dietrich and Moran2017) could explain how a strong core microbiota resists invasion by pathogens. This hypothesis is consistent with the dominant role of pH in determination of bacterial communities in soil (Morton et al., Reference Morton, Sanders, Quinn, McDonald, Gonzalez, Vázquez-Baeza, Navas-Molina, Song, Metcalf, Hyde, Lladser, Dorrestein and Knight2017; Ratzke and Gore, Reference Ratzke and Gore2018), and correlations between low gastric acidity and opportunistic infection in human subjects (Stark and Nylund, Reference Stark and Nylund2016). However, other explanations for the effects of core gut microbiota on parasites – such as physical competition for space and resources, enhancement of the immune response and improvement of host nutritional status (Raymann and Moran, Reference Raymann and Moran2018) – also deserve consideration. It is possible that different microbial assemblages are optimal for different functions. For example, a microbiome that produces abundant acids might be optimal for direct defence against parasites, whereas other community compositions might be provide more benefits to immune system regulation, or to nutritional sufficiency that improves resistance or tolerance to infection (Scrimshaw et al., Reference Scrimshaw, Taylor and Gordon1959; Brown et al., Reference Brown, Schmid-Hempel and Schmid-Hempel2003). Further research is needed to investigate variation in gut pH within and across species, and possible causative relationships between microbiome composition and bee health under different environmental circumstances.
Conclusion
Our results build on prior associations between microbiome and infection intensity to provide mechanistic insights into how the bee gut microbiota are likely to influence trypanosomatid infection in bumble bees and possibly other corbiculate bee species. Our findings of pH-dependent parasite inhibition in vitro suggest that gut pH could be a critical determinant of trypanosomatid growth in vivo, a hypothesis that could be tested in both manipulative and observational studies that relate gut pH to trypanosomatid establishment. The role of gut pH in resistance to other enteric pathogens, and the selective forces acting on symbionts and parasites to create and tolerate different levels of acidity, warrants further study as part of continued investigations into the functional significance of the bee microbiome.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182018001555
Acknowledgements
The authors thank Ben Sadd for providing strains IL13.2 and S08.1, Rebecca Irwin for providing the bees from which strain VT1 was established, Guang Xu and Ben Sadd for sharing DNA sequences and two anonymous reviewers for comments that improved the paper.
Financial support
This project was funded by a National Science Foundation Postdoctoral Research Fellowship to EPY (NSF-DBI-1708945); USDA NIFA Hatch funds (CA-R-ENT-5109-H), NIH (5R01GM122060-02) and NSF MSB-ECA (1638728) to QSM and an NSF-CAREER grant (IOS 1651888) to TRR. The funders had no role in study design, data collection and interpretation or the decision to submit the work for publication.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical standards
Not applicable.
Data availability
All data are supplied in Supplementary Information, Data S1.






