Introduction
In northern Europe, deciduous forests with temperate broad-leaved trees, such as small-leaved lime (Tilia cordata Mill.), Norway maple (Acer platanoides L.), common ash (Fraxinus excelsior L.) and wych elm (Ulmus glabra Huds.) are distributed mainly in the hemiboreal subzone of the boreal forest zone and are restricted to areas with soils of high fertility (Valk & Eilart Reference Valk and Eilart1974; Diekmann Reference Diekmann1996). Anthropogenic pressure on broad-leaved forests on fertile soils has been long-term; stands have been cut for valuable timber and forest land has been used for agriculture (Laasimer Reference Laasimer1965; Hansson Reference Hansson1997). Now, as most original forests have been replaced by young or middle-aged secondary forests, old-growth deciduous forests are particularly scarce in northern Europe (Nilsson Reference Nilsson and Hansson1992; Esseen et al. Reference Esseen, Ehnström, Ericson and Sjöberg1997; Lõhmus et al. 2004).
Fragmentation and degradation of old-growth deciduous forests have exerted negative effects on persistence of many organisms, including lichens growing on temperate broad-leaved trees (Thor Reference Thor1998; Berg et al. Reference Berg, Gärdenfors, Hallingbäck and Nordén2002). Although epiphytic lichens depend primarily upon the host tree species and its properties (Barkman Reference Barkman1958; Brodo Reference Brodo, Ahmadjian and Hale1973), stand age and spatial-temporal continuity are considered key factors for the development of highly complex lichen assemblages in forests (Boudreault et al. Reference Boudreault, Gauthier and Bergeron2000; Price & Hochachka Reference Price and Hochachka2001; Snäll et al. Reference Snäll, Ribeiro and Rydin2003; Ellis & Coppins Reference Ellis and Coppins2007a). This can be explained by the fact that many epiphytic forest-dwelling lichens have a long generation time as well as poor dispersal and colonization ability (Sillett et al. Reference Sillett, McCune, Peck, Rambo and Ruchty2000; Scheidegger & Goward Reference Scheidegger, Goward, Nimis, Scheidegger and Wolseley2002).
Lichen assemblages on broadleaved trees, which are dominant in forests of temperate regions (e.g. oak, ash, lime), have been intensively studied in central Europe (e.g. Barkman Reference Barkman1958; Cieśliński et al. Reference Cieśliński, Czyżewska, Klama and Żarnowiec1996) but more rarely at the boundary of their dispersal range in northern Europe (Löbel et al. Reference Löbel, Snäil and Rydin2006; Jüriado et al. Reference Jüriado, Liira, Paal and Suija2008; Mežaka et al. 2008). The composition of lichen communities could depend upon the location of the host tree in relation to its natural distribution range, as the number of epiphyte species tends to decrease on trees growing near the limits of their natural distribution area (Barkman Reference Barkman1958; Jüriado et al. Reference Ingerpuu, Vellak, Liira and Pärtel2003). Moreover, to cover the full spectrum of habitat conditions suitable for different threatened species, a study of sites with similar substrata, but with different soil moisture and nutrient conditions, is needed (Berg et al. Reference Berg, Gärdenfors, Hallingbäck and Nordén2002). In the context of long-term anthropogenic impact on the boreal landscape it is also important to identify remaining habitats with potentially high biodiversity values (Gustafsson et al. Reference Gustafsson, de Jong and Norén1999).
The so-called klint forest of Estonia is an unusual habitat for temperate broad-leaved tree species in northern Europe. It occurs as a narrow stripe on the talus slopes and at the base of the limestone escarpment along the northern coastline of Estonia (North-Estonian Klint) (Paal Reference Paal1998). In places, due to inaccessibility, the forests are thought to be among the very few primary forest stands surviving in Estonia (Kalda Reference Kalda and Rebane1962). The historical continuity of the forests, mild climatic conditions in proximity of the sea, and shade provided by the cliffs, render the forests on the talus slope of the North-Estonian Klint unique among habitats in the boreal forest zone (Paal Reference Paal1998). According to the criteria of the EU Habitat Directive (EC 1992), these forests belong to the north-easternmost variety of type 9180 ‘Tilio-Acerion forests of slopes, screes and ravines’, and have in the context of biodiversity protection the rank of high priority for all Europe. These forests are characterized by high richness of vascular plant species and bryophytes, among them many red-listed species (Paal Reference Paal2001; Ingerpuu et al. Reference Ingerpuu, Vellak, Liira and Pärtel2003). In this study, we quantify the richness and composition of epiphytic lichen species on four temperate broad-leaved tree species (Acer platanoides, Fraxinus excelsior, Tilia cordata and Ulmus glabra) in the Estonian klint forests, and examine multiple environmental drivers influencing epiphytic lichen communities.
Materials and Methods
Study area
The North-Estonian Klint forms part of the larger Baltic Klint, creating a conspicuous geographical boundary in northen Europe (Raukas & Teedumäe Reference Raukas and Teedumäe1997). The line of the klint follows the northern and north-western boundaries of the Ordovician limestone outcrop (Raukas & Teedumäe Reference Raukas and Teedumäe1997); the upper part of the klint exposes calcareous rocks and the lower layers consist of clays, silts, sandstones and argillites (Miidel Reference Miidel, Raukas and Teedumäe1997). In Estonia, the klint is distributed across more than 200 km (Miidel Reference Miidel, Raukas and Teedumäe1997). The height of the escarpment increases from west to east, varying between 24 and 67 m a.s.1.; its slope varies from almost vertical (90°) to gently sloping (<20°), with one to five terraces. In many places the klint falls almost directly to the sea, but more often its base and the sea are separated by a coastal plain of variable width (Suuroja Reference Suuroja2005). The dominant tree species in the forests on the talus slope of the escarpment are Fraxinus excelsior, Tilia cordata, Ulmus glabra and Acer platanoides and the characteristic species of the field layer are Lunaria rediviva L. and Mercurialis perennis L. (Paal Reference Paal1997; Paal et al. Reference Paal2001).
A large expanse of klint forests is located in north-eastern Estonia, the area most severely affected by the simultaneous presence of alkaline cement dust and oil shale fly ash, as well as by acid gaseous pollutants (mainly SO2 but also volatile organic compounds, ammonia and hydrogen sulphide) (Mandre Reference Mandre1995; Liblik Reference Liblik and Punning2007). The pollutants originate from several electricity power plants fired by oil shale and from chemical industry plants in north-eastern Estonia (Fig. 1) and also from Russia (Haapala et al. Reference Haapala, Goltsova, Seppälä, Huttunen, Kouki, Lamppu and Popovichev1996). The simultaneous presence of alkaline emissions and acid gaseous pollutants has the potential to neutralize the effects of acidification (Haapala et al. Reference Haapala, Goltsova, Seppälä, Huttunen, Kouki, Lamppu and Popovichev1996; Liblik & Pensa Reference Liblik and Pensa2001). The concentrations of pollution in north-east Estonia were extremely high up until 1990, since then emissions have been progressively decreasing (Liblik Reference Liblik and Punning2007).

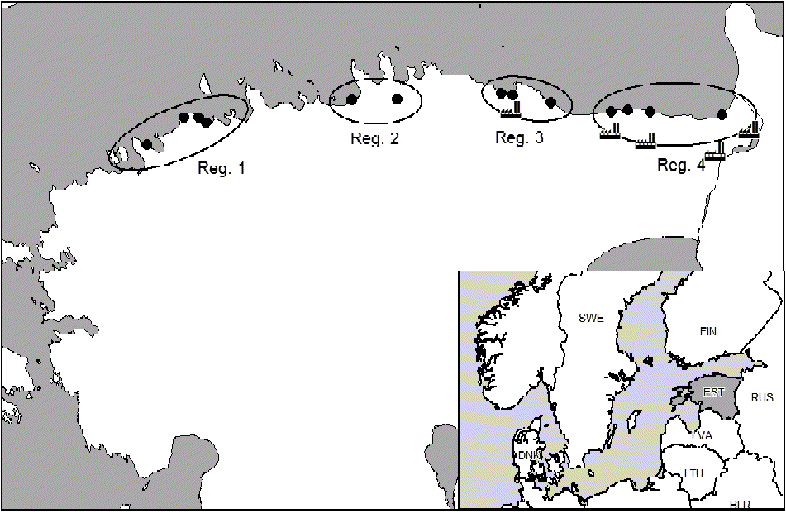
Fig. 1. Location of the study sites (•) in four study regions (Reg.) along the North-Estonian Klint. ![]() = Pollution sources (cement plant in study region 3 and electricity power plants in the vicinity of study region 4).
= Pollution sources (cement plant in study region 3 and electricity power plants in the vicinity of study region 4).
Data collection
Field data were collected in 2002 as part of a project describing the typology and soils of the Estonian klint forests (Paal et al. Reference Paal2001; Paal Reference Paal2001, Reference Paal, Puura, Pihu and Amon2007). On the basis of the composition of tree species recorded in stands, 13 localities along the talus slope of the escarpment from west to east were selected for this study. For data analysis, the stands studied were grouped into four study regions according to their geographic location in Estonia (Fig. 1; Table 1). As the studied forests occur in a line on the talus slopes and at the base of the limestone escarpment along the northern coastline, the size of each locality was determined by the length of an unbroken forest fragment (km) measured using a digital ruler on the Internet-serviced map of Estonia (Web map server of Estonian Land Board 2007). The same map was also used to measure the distance of the stand from the sea. The approximate height of the escarpment in each locality was provided by Suuroja (Reference Suuroja2005) (Table 1).
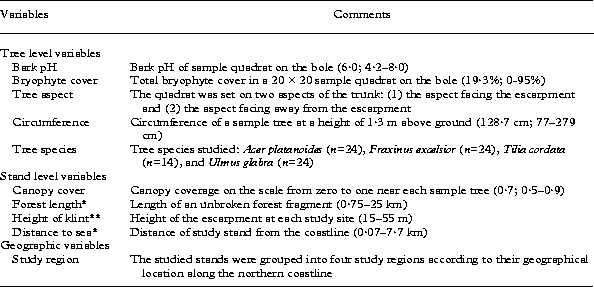
Table 1. Explanatory variables employed in the data analyses. The average values and minimum-maximum range of the variables are presented in the brackets

* The value of the parameter was obtained from the digital map of Estonia (Web map server of Estonian Land Board 2007).
** The variable was provided by Suuroja (Reference Suuroja2005).
In western Estonia (study regions 1 and 2), two sample trees of four temperate broad-leaved species, Acer platanoides, Fraxinus excelsior, Tilia cordata and Ulmus glabra, were studied in each locality. In eastern Estonia, Tilia cordata was generally absent, and in one locality only lichens on Acer platanoides were studied. Altogether 86 tree trunks were analysed (Table 1). Owing to the poor accessibility of the trees on the steep slope of the escarpment, sample trees (>20 cm diam.) were chosen subjectively within the study localities established by Paal et al. (Reference Paal2001). The circumference of each sample tree was measured at breast height (1·3 m above ground level) and light conditions (% canopy cover) were estimated visually near each sample tree on the scale from zero to one (Table 1).
The epiphytic lichen communities on the tree trunks were sampled using a 20 × 20 cm quadrat, at a height of 1·3 m above ground level. As the trees on the talus slope of the escarpment were in many cases leaning, the quadrat was placed on two aspects of the trunk (one facing, the second facing away from the escarpment). To estimate the % cover of lichen species and the total cover of bryophytes, the sample quadrat was divided into 100 subquadrats. For measurement of bark pH, samples of the bark were removed with a knife from each 20 × 20 cm sample quadrat on both sides of the tree trunk. Bark samples were air dried and stored in paper bags until laboratory analysis. The bark (1 g) was incubated in distilled water (1·5 ml g−1) for 24 hours and the pH of the solution was then measured with a standard pH meter (E6121) (Table 1).
The specimens which were not identified in the field were studied in the laboratory, using a stereomicroscope, a light microscope, ‘spot tests’, UV light and standardized thin-layer chromatography (TLC). Owing to their difficult taxonomy, species of Arthopyrenia were treated at the generic level. Reference materialsare deposited in the lichen herbarium at the Natural History Museum of the University of Tartu (TU). The nomenclature of the lichens follows Randlane et al. (Reference Randlane, Saag and Suija2007). Data on species frequency in Estonia are derived from Randlane and Saag (Reference Randlane and Saag1999) and updated according to the Database of Estonian Lichens (2008). The list of protected lichen species is presented according to legal documents (Keskkonnaministri määrus nr 51, 2004; Vabariigi Valitsuse määrus nr 195, 2004) and red-listed lichen species are presented following Randlane et al. (Reference Randlane, Jüriado, Suija, Lóhmus and Leppik2008).
Data analysis
Before the data analysis, some explanatory variables were transformed to improve the assumptions of homoscedacity and to render units of the variables comparable. The cover values of bryophytes were square-root transformed and the variables ‘circumference’, ‘forest length’ and ‘distance to sea’ were log-transformed. The cover values of lichen species were also square-root transformed, to lessen the disproportionate effects of the dominant species (McCune & Grace Reference McCune and Grace2002).
The main gradient structure of the species data was examined using Detrended Correspondence Analysis (DCA) (Hill & Gauch Reference Hill and Gauch1980) implemented in the program package PC-Ord version 4.25 (McCune & Mefford Reference McCune and Mefford1999). The axes were rescaled using 26 segments. To reduce noise, the species appearing only in 1 or 2 sample quadrats were removed from the data set prior to ordination. The proportion of variance represented by ordination axes was estimated by the after-the-fact method using relative Euclidean distance (McCune & Mefford Reference McCune and Mefford1999). Spearman rank order correlations (implemented in the program package Statistica 7.1; Statsoft Inc. 2005) were estimated among the environmental variables and the ordination axes.
Canonical Correspondence Analysis (CCA) (ter Braak Reference ter Braak1986) implemented in the program package CANOCO ver. 4.5 (ter Braak & Šmilauer Reference ter Braak and Šmilauer2002) was used to evaluate the descriptive power of environmental variables on species composition. The variables were grouped into three subsets: tree level, stand level and geographic variables (Table 1). To evaluate the explanatory power of each set of the environmental variables, singly and in combination, the variation partitioning approach (Borcard et al. Reference Borcard, Legendre and Drapeau1992) in partial correspondence analysis (pCCA) (ter Braak Reference ter Braak1986), was used. The forward selection procedure with randomization tests (Monte-Carlo permutation test, 1000 unrestricted permutations) was employed to select the most important environmental variables influencing species composition, retaining the variables with an independent significant contribution at the P<0·05 level. The statistically significant environmental variables had the value of inflation factors less than 10·0, i.e. below the suggested limit value of 20 (ter Braak & Šmilauer Reference ter Braak and Šmilauer2002).
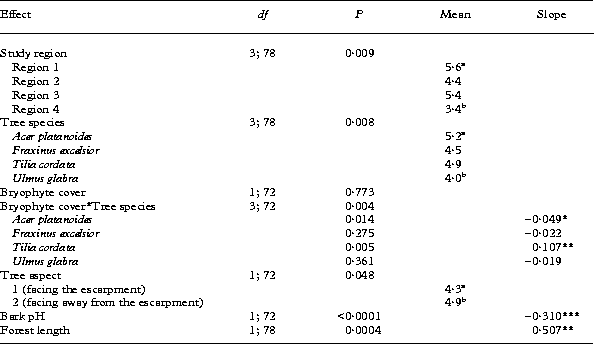
The response of species richness to the studied environmental variables was tested using a general linear mixed model (GLMM) (Littell et al. Reference Littell, Milliken, Stroup and Wolfinger1996) with the stepwise selection procedure implemented in the program package SAS ver. 8.2 (proc MIXED) (SAS Institute Inc. 1989). The response variable ‘number of lichen species’ and the predictor variable ‘circumference’ were log-transformed; and the variable ‘bryophyte cover’ was square-root transformed to obtain homoscedacity of model residual variation and normality of residuals. The locality of each stand studied was considered the fixed factor and the factor ‘tree aspect’ was treated as the result of repeated observations per sample tree. We tested interactions of the factor ‘tree species’ with the continuous factors ‘bryophyte cover’, ‘circumference’ and ‘bark pH’. Backward elimination was used to select the final model for species richness. At each step the variable with the highest P value was removed, and all variables at P<0·05 were retained in the final model. Akaike's information criterion (AIC) (Akaike Reference Akaike, Petrov and Csáki1973) and the significance of the factors were used to identify optimal parameterization of the models (Shao Reference Shao1997). For multiple comparisons between tree species and different aspects of the tree trunk, the Tukey-Kramer adjustment was used. The GLMM analysis was also applied to evaluate the influence of ‘bryophyte cover’, ‘circumference’ and ‘study region’ on bark pH using the same model settings as in the models described above.
Results
Bark pH of temperate broad-leaved trees
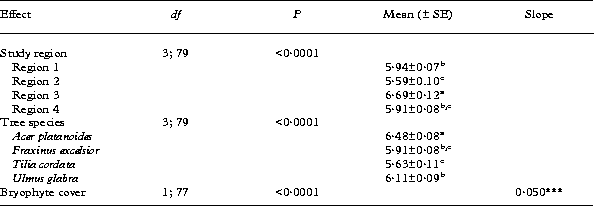
The mean values of bark pH were significantly different among the study regions (Table 2). The bark pH of trees in region 3 differed from that of trees in all other study areas in having subneutral mean values (6·69 ±0·12). The mean bark pH of region 2 was the lowest (5·59±0·10) being marginally significantly different from the corresponding value for region 1 (5·94±0·07, Table 2).
Table 2. The results of general linear mixed model analysis (GLMM) for bark pH on temperate broad-leaved trees

Explanations of the explanatory variables are given in Table 1. Within-group mean values (mean) with standard errors (± SE) are presented for categorical variables and slope estimates (slope) are presented for continuous variables. Letter labels denote homogeneity groups according to the results of the Tukey-Kramer multiple comparison test. The significance test for the slope estimate:
*** P<0·001.
Bark pH varied significantly between the temperate broad-leaved tree species studied. The bark of Acer platanoides was less acid (mean pH = 6·48 ±0·08) than the bark of the other broad-leaved tree species (mean pH values for Fraxinus excelsior, Tilia cordata and Ulmus glabra being 5·91±0·08, 5·63±0·11 and 6·11±0·09, respectively) (Table 2). The value of the bark pH of Tilia cordata differed significantly from that of Ulmus glabra, the bark of T. cordata being more acid. In addition, there was an overall positive relationship observed between cover of bryophytes and bark pH (Table 2).
Composition of lichen community
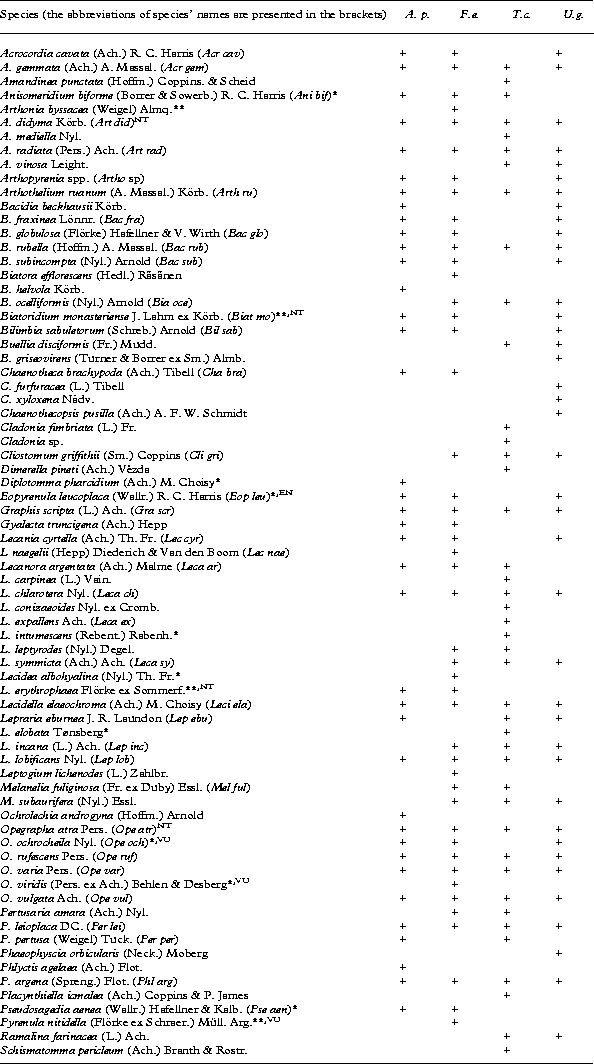
A total of 74 lichen species was found on the trunks of the four broad-leaved tree species. The highest number was recorded on Fraxinus excelsior with 46 species, followed by Tilia cordata with 42 species, Ulmus glabra with 39 species, and Acer platanoides with 38 species (Appendix 1). Most of the recorded lichens (92%) were crustose, while only six foliose or fruticose species (Cladonia fimbriata, Leptogium lichenoides, Melanelia fuliginosa, M. subaurifera, Phaeophyscia orbicularis and Ramalina farinacea) were recorded. The most frequent lichen species on the temperate broad-leaved trees were Graphis scripta, Opegrapha rufescens and Lecidella elaeochroma, occurring in more than 40% of the sample quadrats. Out of the total of 74 species, 20% of the taxa (15 species) are either rare in Estonia (having less than ten localities), or are red-listed or protected by law (e.g. Arthonia byssacea, A. didyma, Biatoridium monasteriense, Lecidea erythrophaea, Opegrapha ochrocheila and Pyrenula nitidella) (Appendix 1).
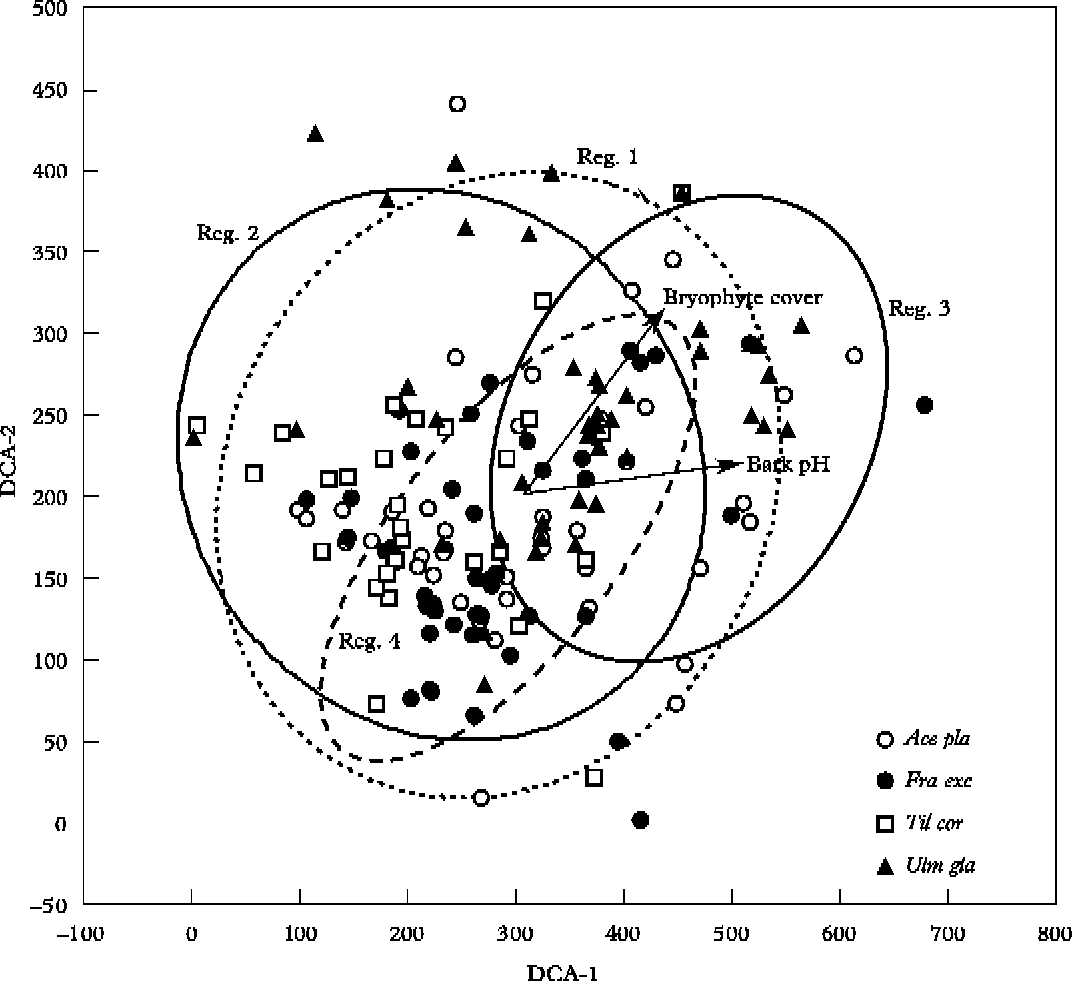
In the DCA ordination, the first two axes described 36% of variance in the composition of the lichen communities, (25% for axis 1 and 11% for axis 2). Since axis 3 did not show a significant contribution to the results of ordination (3·1%), the results are presented only for the first two axes. The sample quadrats from regions 1 and 2 are scattered all over the DCA ordination plot; the other two study regions are more clustered. The quadrats from region 3 are mostly located on the right side of the ordination plot and the quadrats from region 4 form a compact cluster in the centre of the ordination plot (Fig. 2). The sample quadrats from Tilia cordata are located almost exclusively on the left side of the ordination plot and the quadrats of Ulmus glabra are represented in a large part on the right side of the ordination plot (Fig. 2). The variable ‘bark pH’ had the strongest correlation with axis 1 (r=0·62, P<0·0001), and the variable ‘bryophyte cover’ had an almost equally strong correlation with axis 1 (r=0·50, P<0·0001) and axis 2 (r=0·56, P<0·0001) (Fig. 2). The variable ‘height of klint’ was also weakly correlated with axis 1 (r=0·27, P = 0·005).
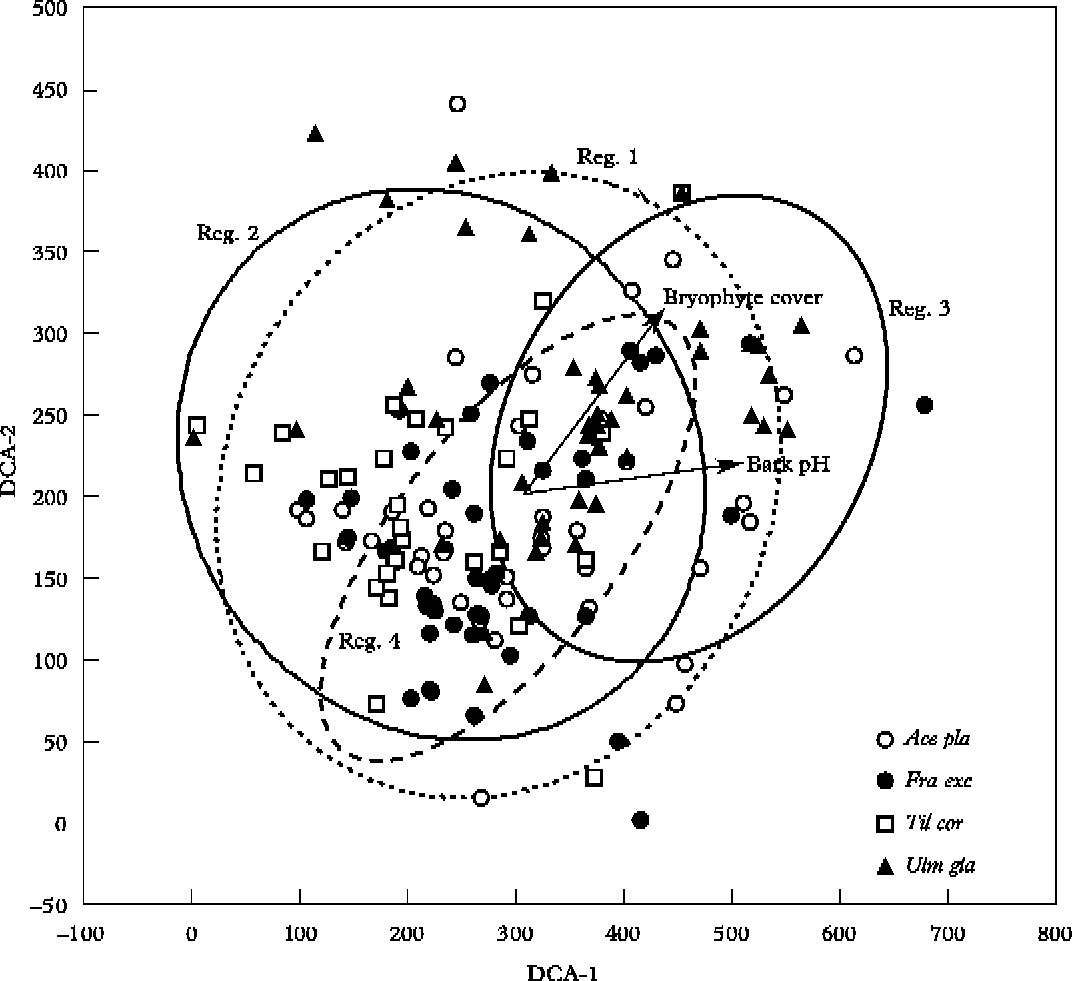
Fig. 2. DCA ordination diagram of the sample quadrats with significantly correlated explanatory variables along axis 1 and axis 2. Notations: Ace pla – Acer platanoides (○), Fra exc – Fraxinus excelsior (•), Til cor – Tilia cordata (□), Ulm gla – Ulmus glabra (▲), Reg. – study region. The ellipses denote the 95%-range of sample quadrats belonging to respective study region.
The CCA ordination of the lichen species and the environmental variables revealed more specifically the influence of the measured environmental factors on the composition of lichen species. In the CCA forward selection procedure most of the geographic, stand and tree level variables considered revealed a significant contribution to the results of ordination, except ‘canopy cover’, ‘tree aspect’ and the tree species Acer platanoides and Tilia cordata (P≥0·05). The final CCA model with all significant predictors explained 21·4% of total variance in the species data (total inertia was 9·331).
The CCA biplot of the species and explanatory variables demonstrates a change in species composition of lichen communities along the first ordination axis, explained here by a gradient in bark pH and cover of bryophytes (Fig. 3). Lichen species found predominantly on the most alkaline and mossy tree bark showed the highest positive correlation with axis 1 (e.g. Bilimbia sabuletorum, Biatoridium monasteriense and Eopyrenula leucoplaca) while lichens associated with the moderately acid bark of broad-leaved trees showed the highest negative correlation with axis 1 (e.g. Cliostomum griffithii, Biatora ocelliformis, Lecanora argentata and Opegrapha vulgata) (Fig. 3). Owing to the high value of bark pH and the extensive cover of bryophytes on the tree trunks, region 3 shows the greatest difference from the other study areas.

Fig. 3. The lichen species and the explanatory variables on the biplot of CCA of axis 1 and axis 2. The eigenvalue of the CCA axis 1 was 0·55 and of the CCA axis 2 was 0·34. The tree species and the study regions (dummy variables) are represented by their centroids (Fra exc – Fraxinus excelsior, Ulm gla – Ulmus glabra, Reg – study region). Explanations of the explanatory variables are given in Table 1, for abbreviations of the lichen species see Appendix 1.
Along the second axis of the CCA plot, the change in the composition of the lichen species on the tree trunks is determined by the trunk circumference (Fig. 3). Ulmus glabra trees had generally the largest circumference and also had a high cover of bryophytes on the trunk; the lichens Bacidia rubella, B. subincompta and Lepraria lobificans were the dominant species. Opegrapha ochrochela and Lepraria eburnea also preferentially occurred on larger trees, while Lecania naegelii, L. cyrtella and Lecidella elaeochroma were predominantly found on smaller trees.
The third axis of CCA is explained by the environmental conditions of klint forests and separates the sites of region 4 from the other study sites (Fig. 4). Stands in region 4 comprised the longest fragment of the klint forest, located close to the coastline and on the talus slope of the highest part of the escarpment. Lichens characteristic of stands in region 4 are those located on the upper left side of the ordination plot (e.g. Opegrapha atra, O. ochrochela and Chaenotheca brachypoda) (Fig. 4).

Fig. 4. The lichen species and the explanatory variables on the biplot of CCA of axis 1 and axis 3. The eigenvalue of the CCA axis 1 was 0·55 and of the CCA axis 3 was 0·30. Notations as in Fig. 3, explanations of the variables are given in Table 1 and for abbreviations of the lichen species see Appendix 1.
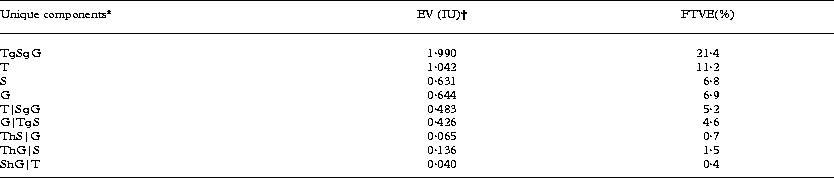
The partial CCA model indicated that the largest amount of variance in the composition of lichen communities on the tree bole was explained by the set of tree level variables [11·2% of TVE (total variation explained)], followed by the set of geographic variables (6·9% of TVE) and the set of stand level variables (6·8% of TVE) (Table 3). Each subset of variables explained an independent component of variation, while the fraction of shared variation was marginal (Table 3). Tree level variables alone (‘bark pH’, ‘bryophyte cover’, ‘circumference’, ‘Ulmus glabra’ and ‘Fraxinus excelsior’) explained 8·5% of TVE when the other two environmental data sets (geographic and stand level variables) were used as the covariables. The unique variation component of the stand level variables (‘forest length’, ‘height of klint’ and ‘distance to sea’) accounted for 5·2% of TVE (tree level and geographic variables were used as the covariables) and that of the geographic variables (Regions 1, 2, 3 and 4) accounted for 4·6% of TVE (tree and stand level variables were used as the covariables) (Table 3).
Table 3. Partitioning of variation in epiphytic lichen communities using three sets of explanatory variables in variation partitioning analysis (pCCA)

* T = tree level environmental variables (‘bark pH’, ‘bryophyte cover’, ‘circumference’, ‘Ulmus glabra?’ and ‘Fraxinus excelsior’); S = stand level variables (‘forest length’, ‘height of klint’ and ‘distance to sea’); G = geographical variables (Regions 1, 2, 3 and 4) (see Table 1). Symbol ‘|’ means covarying out variable sets, ‘∪’ refers to union of variable sets, ‘∩’ refers to intersection of variable sets.
† EV (the sum of all canonical eigenvalues) is given in inertia units (IU; total inertia 9.331 TU) as well as the fraction of the total variation explained (FTVE; %).
Richness of lichen species
The stepwise building of the general linear model suggested that several environmental variables have a significant influence on the species richness of epiphytic lichen communities (Table 4). In regions 1 and 4 the mean number of lichen species differed significantly, being higher and lower, respectively, than those of regions 3 and 4. The significant effect of tree species indicates that on average, there was one more lichen species in the sample quadrats from Acer platanoides compared with those from Ulmus glabra (Table 4). There were also significant differences in species richness related to the aspect of the tree trunk relative to the escarpment. The number of lichen species was lower on the sample quadrats facing the escarpment compared to the quadrats facing away from the escarpment (Table 4).
Table 4. The results of general linear mixed model analysis (GLMM) for number of lichen species (log-transformed) on temperate broad-leaved trees

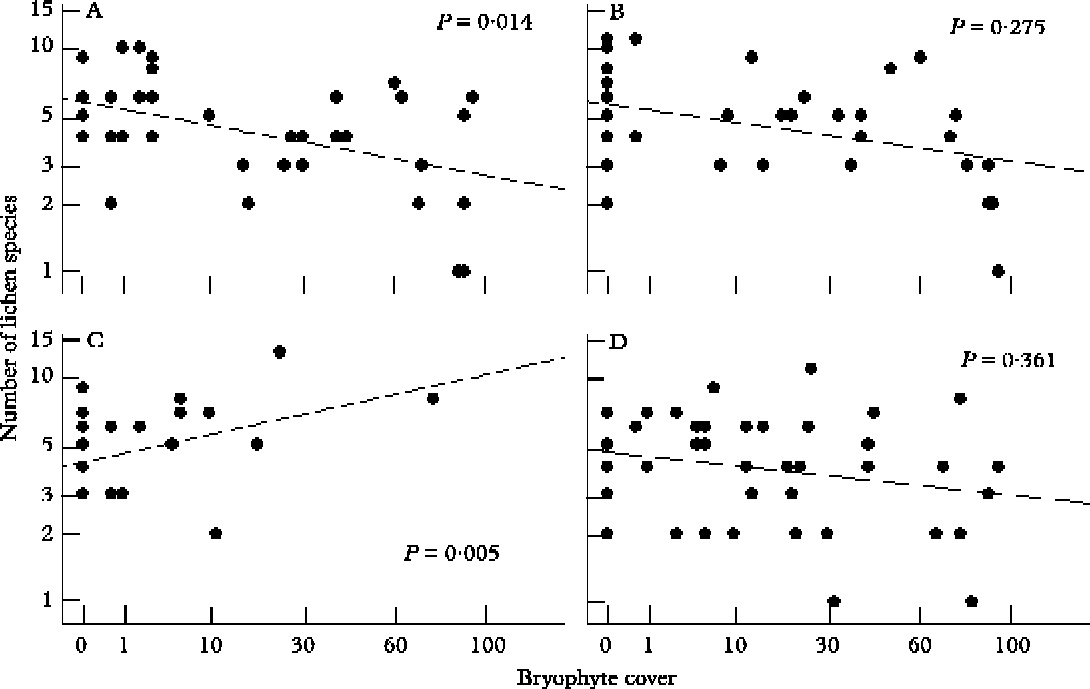
Species richness of lichens had an overall negative correlation with bark pH (Table 4). The effect of bryophytes on species richness of lichen communities was dependent upon tree species. On Tilia cordata, lichen species richness increased with increasing cover of bryophytes, while the number of lichen species decreased with increasing moss cover on Acer platanoides (Table 4, Fig. 5). The length of the fragment of klint forest was a significant positive predictor of species richness of epiphytic lichen communities (Table 4).

Fig. 5. Relationship between number of lichen species and cover of bryophytes according to general linear mixed model analysis (GLMM) for different broad-leaved tree species (see Table 4). A, Acer platanoides; B, Fraxinus excelsior, C, Tilia cordata, D, Ulmus glabra. The scale of ‘Number of lichen species’ is log-transformed and the scale of ‘Bryophyte cover’ is square-root transformed
Discussion
Species richness and composition of lichens on temperate broad-leaved trees on the talus slope of the limestone escarpment of the North-East Estonian Klint is substantially influenced both by tree level variables, unique habitat conditions of klint forest (length of the forest fragment, proximity of the stand to the sea and height of the escarpment) as well as geographical location of the stand. However, the tree level variables explained the largest fraction of variation in richness or composition of lichen species, as has also been reported in other studies where lichen diversity was evaluated at the tree level within a limited study area (Aude & Poulsen Reference Aude and Poulsen2000; Löbel et al. Reference Löbel, Snäil and Rydin2006; Belinchón et al. Reference Belinchón, Martínéz, Escudero, Aragón and Valladares2007).
As in a study of lichen communities on temperate broad-leaved trees in floodplain forests (Jüriado et al. Reference Jüriado, Liira, Paal and Suija2008), changes in lichen assemblages on broad-leaved trees in klint forest are mainly caused by the co-variation of the bryophyte cover with bark pH and trunk circumference (Figs 3 & 4). As the mean bark pH of the studied trees was usually subneutral, a decrease of species richness with increasing bark pH, as well as the negative effect of bryophyte cover on species richness of lichen communities, were expected (Jüriado et al. Reference Jüriado, Liira, Paal and Suija2008). In this study, we also detected a reverse effect of moss cover on lichen species richness on Tilia cordata. Tilia had a comparatively low cover of bryophytes on the tree bole compared with the other tree species studied (Fig. 5); increasing bryophyte cover appears to promote lichen species richness on this tree species. This relationship could be attributed to a situation in which both lichens and bryophytes have enough space on the tree bole and optimal conditions for growth.
In the case of mature or older broad-leaved trees, the effect of trunk size on lichen species richness on the tree bole has proved to be negative (Jüriado et al. Reference Jüriado, Liira, Paal and Suija2008), or is of minor importance when compared to other factors (Löbel et al. Reference Löbel, Snäil and Rydin2006; Johansson et al. Reference Johansson, Rydin and Thor2007). However, changes in the composition of lichen specie communities with increasing bole size have been documented in many other studies (Burgaz et al. Reference Burgaz, Fuertes and Escudero1994; Moe & Botnen Reference Moe and Botnen1997; Belinchón et al. Reference Belinchón, Martínéz, Escudero, Aragón and Valladares2007). Alterations in the physical and chemical properties of tree bark with tree ageing may induce a transition from a crustose community, dominated by sexually reproducing species, to an assembly dominated by asexually reproducing species (Ellis & Coppins Reference Ellis and Coppins2007b).
Owing to the steep slope of the escarpment, the trunks of the trees growing on it are leaning which can explain the difference in the species richness of lichens between the different aspects of the trunk. On the side of the trunk facing the escarpment, bryophytes are probably favoured by the larger amount of stem flow as well as by increased shade (Gustafsson & Eriksson Reference Gustafsson and Eriksson1995; Moe & Botnen Reference Moe and Botnen2000). The side of the trunk facing away from the escarpment is apparently drier and better illuminated, which has led to the dominance of lichen species.
The extraordinary environmental conditions of klint forests have a unique effect on the composition and richness of lichen communities. Stands on the longest stretch of the klint forest, close to the sea, and on the highest slope of the escarpment support lichens that are not found, or are rare, in other regions (e.g. Opegrapha atra, O. ochrochela and Pyrenula nitidella). For dispersal of epiphytic lichens (Löbel et al. Reference Löbel, Snäil and Rydin2006; Ellis & Coppins Reference Ellis and Coppins2007a), the connectivity of narrow forest fragments along the talus slope of the escarpment may be important whereas in more extensive landscapes such stands are usually distinctly isolated from other forest areas.
In general, habitat area has proved to be positively correlated with number of species (Gotelli & Graves Reference Gotelli and Graves1996; Berglund & Jonsson Reference Berglund and Jonsson2001; Jüriado et al. Reference Jüriado, Suija and Liira2006). We found that the fragment length of klint forest is a significant positive predictor of species richness of lichen communities on the temperate broad-leaved trees (Table 4). However, previous studies on the diversity of vascular plants and bryophytes in Estonian klint forests have not found a significant relationship between length of the forest fragment and species richness (Ingerpuu et al. Reference Ingerpuu, Vellak, Liira and Pärtel2003). The difference between the results of our study and those reported by Ingerpuu et al. (Reference Ingerpuu, Vellak, Liira and Pärtel2003) might be due to the difference in the treatment of respective variables in data analysis. Ingerpuu et al. (Reference Ingerpuu, Vellak, Liira and Pärtel2003) divided forest fragments into three size classes, while in the present study the absolute dimensions of forest fragments were used.
We found a significant effect of stand locality on the composition and richness of lichen communities on the tree bole, which is most likely explained by a gradient in local air pollution. Owing to the extraordinarily high level of alkaline pollutants originating from a local cement plant during the past decades (Mandre Reference Mandre1995), the epiphytic lichen communities have changed near study region 3 (Martin & Nilson Reference Martin and Nilson1992; Nilson Reference Nilson and Mandre1995). In this area of klint forests, bark is alkaline and under the shelter of the closed tree canopy, the tree trunks are covered with an extensive mat of bryophytes accompanied by bryophilous lichens (e.g. Bilimbia sabuletorum), or lichen species favouring high bark pH (e.g. Biatoridium monasteriense and Eopyrenula leucoplaca) (Figs 3 & 4).
In the easternmost part of the klint forest (study region 4) increasing acidification could explain the lower species richness of lichens compared with the klint forests in the western part of the country (Table 4). In recent decades, the proportion of SO2 in the ratio of alkaline dust to acid components in the north-eastern part of Estonia (Fig. 1) has increased, which might indicate that SO2 emissions have reached a level affecting lichen and bryophyte species (Liblik et al. Reference Liblik, Pensa and Kundel2000; Liblik & Pensa Reference Liblik and Pensa2001).
Paal et al. (Reference Paal2001) and Ingerpuu et al. (Reference Ingerpuu, Vellak, Liira and Pärtel2003) have emphasized the high conservation value of Estonian klint forests for vascular plants and bryophytes and the need for the strict protection of these habitats throughout their range in Estonia. We found that klint forests support lichen species restricted to only a few localities in Estonia (e.g. Anisomeridium biforme, Arthopyrenia spp., Lecidea albohyalina) or not found in other forest types here (Eopyrenula leucoplaca) (Database of Estonian Lichens 2008). In addition, klint forests account for a substantial proportion of the national localities of red-listed or protected lichens (Appendix 1). In spite of the presumably detrimental effect of air pollution on epiphytic lichens in some places, we consider that klint forests form a valuable habitat from the lichenological perspective.
The authors are grateful to A. Jüriado, K. and Kr. Jürgens, and R. Vaske for assistance in fieldwork. Special thanks belong to L. Saag who carried out TLC analyses and identified some specimens. We also thank A. Suija and two anonymous referees for their helpful comments on the manuscript. E. Jaigma is acknowledged for revising the English text. Financial support was received from the Estonian Ministry of Education and Research (targeted financing Nos. SF0182639s04, SF0180153s08 and SF0180098sO8). This research was also supported by the European Union through the European Regional Development Fund and by the Archimedes Foundation (grant RLOOMTIPP).
Appendix 1 List of the recorded species and their occurrence on four broad-leaved tree species in boreo-nemoral forest on the talus slope of the North-Estonian limestone escarpment
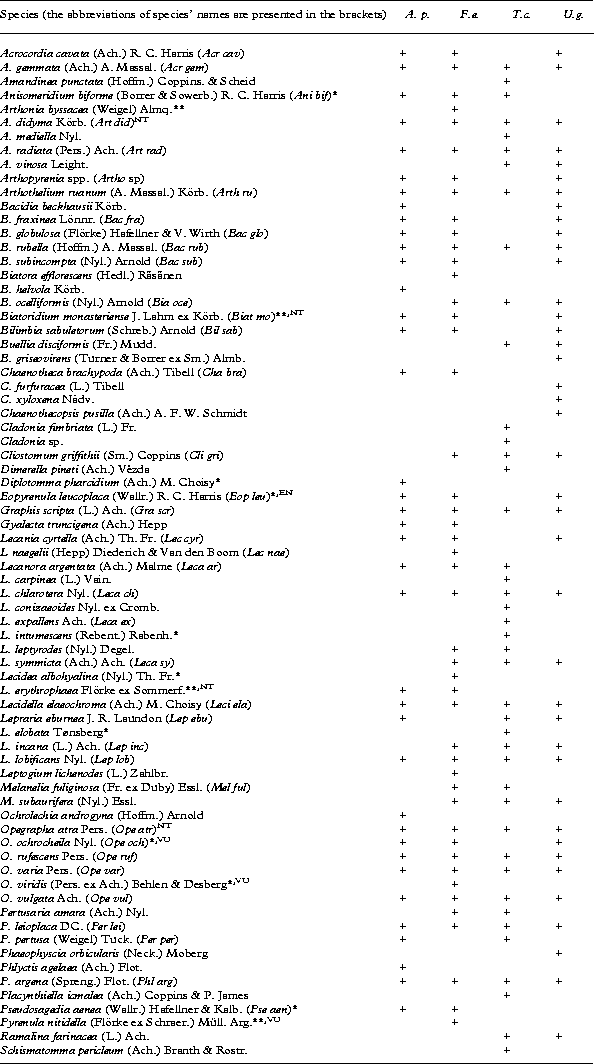
A. p. – Acer platanoides, F. e. – Fraxinus excelsior, T. c. – Tilia cordata and U. g. – Ulmus glabra. Rare species in Estonia are marked with an asterisk (*) and species under protection are marked with two asterisks (**). Red-listed lichen species are assigned the abbrevation of red-list category: EN – endagered, NT – near threatened, VU – vulnerable.