Published online by Cambridge University Press: 09 October 2003
Studies of invertebrate–parasite interactions frequently report that infection reduces host fecundity. The extent of the reduction is likely to be determined by a wide range of host and parasite factors. We conducted a laboratory experiment to evaluate the role of parasite genetics and infection genetic diversity on the fecundity of mosquitoes carrying malaria parasites. The malaria vector Anopheles stephensi was infected with either of 2 different genotypes of the rodent malaria parasite Plasmodium chabaudi, or by a mixture of both. Mixed genotype infections reduced mosquito fecundity by 20%, significantly more than either of the 2 single genotype infections. Mixed genotype infections were associated with high gametocyte densities and anaemia in mice, both of which were correlated with reduced bloodmeal size in mosquitoes. Bloodmeal size was the most important predictor of mosquito fecundity; the presence and number of parasites had no direct effect. Parasite density influenced the propensity of mosquitoes to feed on infected mice, with a higher percentage of mosquitoes taking a meal as asexual parasite and gametocyte density increased. Thus mosquitoes may preferentially feed on hosts who will most impair their fecundity.
Parasite virulence is generally described as a reduction in host fitness accompanying infection. Virulence of malaria parasites towards their mosquito vectors is most commonly reported as a reduction in their fecundity (Hacker, 1971; Hacker & Kilama, 1974; Freier & Friedman, 1976; Hogg & Hurd, 1995 a, b, 1997; Carwardine & Hurd, 1997; Jahan & Hurd, 1997, 1998; Ahmed et al. 1999). The extent by which infection reduces vector fecundity is likely to be the product of a wide variety of mosquito and parasite factors. The contribution of parasite genetics to malaria vector fecundity reductions has never been quantified. As parasite genetics are an important predictor of the virulence of malaria parasites in their vertebrate hosts (Rowe et al. 1997; Taylor, Mackinnon & Read, 1998; Mackinnon & Read, 1999 a, b; Ariey et al. 1999; Ofosu-Okyere et al. 2001; Timms et al. 2001; Mackinnon, Gaffney & Read, 2002), and of vector longevity (Ferguson & Read, 2002), it is possible they also influence vector fecundity.
There are at least two reasons for examining the relationship between parasite genetics and mosquito fecundity. First, the net effects of parasites on vector populations cannot be estimated without considering the range of fitness costs they elicit and, in nature, many malaria-infected mosquitoes have more than one Plasmodium genotype (e.g. Babiker et al. 1994). Secondly, identification of a range of parasite genotypes with different effects on vector fecundity would permit investigation of parasite traits that imbue virulence and their potential adaptive nature (Hurd, 1998, 2001). For example, it would permit tests of whether parasite strains that elicit the greatest reduction in vector fecundity have the greatest transmission success into new hosts (adaptive for the parasite).
It is possible that a vector's fecundity is influenced not only by the particular Plasmodium genotype with which it becomes infected, but also by the number of genotypes it receives. There are several reasons why infection genetic diversity should increase virulence. First, it may be more difficult to mount an immune response against a genetically diverse infection, and/or competition between genetically distinct parasites may trigger the release of toxins that are harmful to the hosts as well as the parasite competitor (discussed by Ofosu-Okyere et al. 2001 and Read & Taylor, 2001). Also, evolutionary theory predicts that parasites should facultatively increase their replication rate, causing a correlated increase in virulence, when their host is infected with another parasite strain (Sasaki & Iwasa, 1991; Frank, 1992, 1996; van Baalen & Sabelis, 1995). Such a strategy would ensure the parasite obtained some nutrients from their host before they are depleted by their competitor. A pattern of increased virulence in mixed genotype infections has been found in some but not all studies (as reviewed by Read & Taylor (2001)). In studies of rodent malaria, mixed infections generate greater weight loss and anaemia in mice (Taylor et al. 1998), and are more deadly to mosquitoes under some environmental conditions (Ferguson & Read 2002). Whether genetically diverse infections also impose greater costs on vector reproduction is unknown.
Parasites that reduce host fecundity may select for infection avoidance. However, many studies of infectious diseases other than malaria have shown that insect vectors preferentially bite infected hosts (Mahon & Gibbs, 1982; Turell, Bailey & Rossi, 1984; Coleman & Edman, 1988; Coleman, Edman & Semprevivo, 1988; Baylis & Mbwabi, 1995). The evidence that this occurs in malaria is mixed. Some studies have reported increased feeding on infected hosts (Day, Ebert & Edman, 1983; Rossignol et al. 1985), others have reported avoidance of infected hosts (Freier & Friedman, 1976) and others that host infection status has no influence on feeding (Burkot et al. 1989). The reason for this variation is uncertain, and may be due to the co-evolutionary history of the host, vector and parasite species involved (whether selection could have acted), environmental and genetic variation, or because different Plasmodium species incur different fitness costs so that the strength of selection for infection avoidance varies.
Here we carry out a simultaneous investigation of the effects of parasite genetic diversity on vector fecundity and blood-feeding tendency. We conducted a laboratory study using the Plasmodium chabaudi–laboratory mouse model to test whether (1) mosquito fecundity is generally reduced by infection, (2) the magnitude of fecundity reduction varies with parasite genotype, (3) infections consisting of 2 parasite genotypes are more virulent than those with 1 and (4) mosquito blood feeding tendency is influenced by host infection status.
Anopheles stephensi were maintained in standard insectary conditions of 27±1 °C, 70% humidity and a 12[ratio ]12 light[ratio ]dark cycle. Larvae were reared in plastic trays at a density of 500/1·5 l of distilled water (Ferguson & Read, 2002). On days 10–13 after egg hatching, groups of 250 pupae were randomly selected from the rearing trays and placed in 1 of 24 emergence cages (16×16×16 cm), giving rise to 160–240 adults that were fed ad libitum on a 10% glucose solution supplemented with 0·05% para-aminobenzoic acid.
Two clones of P. chabaudi known as CR and ER were used (from the World Health Organization's Registry of Standard Malaria Parasites, University of Edinburgh; Beale, Carter & Walliker, 1978). Groups of 6 mice (C57BL/6J) were infected with either 106 CR parasites, 106 ER, 106 of a 1[ratio ]1 mix of CR and ER, or were left uninfected (controls). From the 5th day of infection onwards, thin blood smears were taken on a daily basis to assess parasitaemia (proportion of red cells infected with asexual parasites). Mosquito feeds took place 14 days after mouse infection, when all infected mice had sufficiently high gametocytaemia (proportion of red blood cells infected with gametocytes >0·1%). A few hours before the feed, anaemia was recorded by counting the number of red blood cells in a 2 μl sample of mouse blood. Asexual parasite and gametocyte densities were calculated as the number of red blood cells multiplied by the parasitaemia and gametocytaemia of the blood respectively.
To increase appetite, mosquitoes were deprived of glucose for 24 h before feeding on the mice. One anaesthetized mouse was placed on each cage (n=24) for 20 min. Immediately after the feed, 4–6 fully engorged mosquitoes were removed from each cage and individually placed into 30 ml vol. plastic tubes (9×2·5 cm) covered with mesh (total number of mosquitoes per treatment: CR=24, ER=23, CR/ER=25, Uninfected=20). Cotton pads soaked in a 10% glucose solution with 0·05% PABA were placed on top of each tube and replaced daily.
Bloodmeal size was estimated indirectly as the amount of haematin excreted over a 3-day period by the mosquitoes in tubes (as in Briegel (1980)). This assay estimates bloodmeal size as the mass of blood protein obtained from the meal, not the volume of liquid taken in. Excretia collected in the bottom of mosquito holding tubes was dissolved in 1 ml of a 1% LiCO3 solution. The absorbance of the resulting solution was read at 387 nm, and compared to a standard curve made from porcine haematin (Sigma-Aldrich). Solutions with an absorbance of less than [les ]0·01 were classified as being from mosquitoes that had not blood fed, as this absorbance was indistinguishable from that of the LiCO3 control.
After the 3-day haematin collection period, mosquitoes were moved to new tubes filled with 2 ml of water to allow oviposition. Fecundity was measured as the number of eggs laid over the following 3 days. Mosquitoes were subsequently moved into further new tubes for 1–2 days before being killed with chloroform. The midguts of mosquitoes that fed on infected mice were dissected under a microscope in a drop of 0·01 M phosphate-buffered saline solution. A cover-slip was placed over each midgut which was observed under a compound microscope to assess infection rate (% of mosquitoes with oocysts) and oocyst intensity (no. oocysts per gut).
The entire experiment was repeated 4 months later (hereafter called block 2). The experimental procedure was identical in this second experimental block except that 35–40 mosquitoes were placed in tubes from each of 13 cages (3 cages per infection treatment: CR, ER, CR/ER, and 4 uninfected controls). In contrast to the first block, the female mosquitoes used in this analysis were selected arbitrarily from each cage after blood-feeding (selection not restricted to those that were fully engorged). Thus in this block, it was possible for mosquitoes that did not take a bloodmeal to be selected. In this block we used the haematin assay to identify the proportion of non-feeders in each sample, and tested whether host infection status influenced feeding propensity.
We used General Linear Models to assess whether P. chabaudi infection and genotype influenced (1) mosquito fecundity and (2) mosquito bloodmeal size (SAS, 1997). Variation between the mean fecundity of mosquitoes feeding on a single mouse was modelled as a function of parasite genotype, experimental block, asexual and gametocyte density, mean bloodmeal size, and the mean infection rate and abundance of parasites on mosquito midguts (oocysts). As block main effects are of little biological interest in their own right, they are reported only if they interacted significantly with other explanatory variables in the model. Individuals that did not feed (as defined above) were excluded from the analysis of fecundity and bloodmeal size. We used logistic regression to investigate the association between the proportion of mosquitoes that fed and parasite genotype, host anaemia, asexual parasite and gametocyte density. This analysis was only possible for block 2. Gametocyte densities and oocyst burdens were log transformed prior to statistical analysis. In the first block, 1 of the control mice died during blood feeding and mosquitoes that had fed on it were not included in our analysis.
Of mosquitoes that did take a bloodmeal, 14% and 32% did not lay any eggs in block 1 and block 2 respectively. Controlling for this block difference, the proportion of mosquitoes from each mouse that did not oviposit was unrelated to infection treatment (F3,31=0·69, P=0·57). All subsequent analyses exclude individuals that did not lay eggs.
There was an overall effect of Plasmodium on mosquito fecundity: the mean fecundity of mosquitoes feeding on infected mice was lower than those feeding on the controls (F1,33=5·83, P=0·03). Restricting analysis to the infected groups, parasite genotype did influence mean mosquito fecundity (Fig. 1, F2,23=3·41, P=0·05). The mean fecundity of mosquitoes that fed on mixed infections was lower than those fed on CR-infected or uninfected blood (Fig. 1, pairwise t-tests: Bonferroni adjusted P<0·025 in both cases). The fecundity of mosquitoes with single genotype infections (CR and ER) did not differ from each other, or from the controls (pairwise t-tests, Bonferroni adjusted P>0·20 in all 3 cases). Although mosquitoes in block 2 laid a greater number of eggs than in block 1 (F1,31=73·9, P<0·01), the effects of parasite genotype on mosquito fecundity did not differ between blocks (Block*genotype interaction N.S.).

Fig. 1. The mean fecundity of mosquitoes (no. eggs/mosquito ±1 S.E.) after feeding on mice infected with different genotypes of Plasmodium chabaudi. Each bar represents the grand mean of the mean mosquito fecundity per mouse.
Several infection properties varied between parasite genotypes (Fig. 2). Gametocyte density in mice with mixed infections was higher than those with either of the 2 single genotype infections (F2,23=4·77, P=0·02), although the mean infectivity and number of oocysts was not (infection rate: F2,23=0·37, P=0·69, mean oocyst burden: F2,23=0·69, P=0·51). On the days of blood feeding, asexual parasite density did not differ between parasite genotypes (F2,23=0·04, P=0·96). Pooling all parasite treatments, bloodmeals taken from infected mice were approximately 25% smaller than those taken from the uninfected controls (F1,33=16·13, P<0·01). Bloodmeal size varied between infected groups also, with only the mixed infections and the ER genotype causing a reduction in bloodmeal size relative to the controls (Bonferroni adjusted P<0·01 in both cases). Finally, the red blood cell density of mice that had experienced Plasmodium infection was approximately 20% lower than those that had not (F1,33=13·78, P<0·01), but did not differ significantly between the 3 infected groups (F2,23=0·44, P=0·65, Fig. 2).

Fig. 2. Properties of blood from uninfected mice and those infected with 3 different Plasmodium chabaudi infections (mean value ±1 S.E.). Data are grand means of values from separate feeding trials (no. mosquitoes per trial=4–5 in block 1, 35–40 in block 2).
Can the effects of parasite genotype on mosquito fecundity be explained by the dynamics of each type of infection? Restricting analysis to the infected groups, neither mean mosquito infection rate (F1,22=0·17, P=0·69), mean oocyst burden (F1,22=1·52, P=0·23), red blood cell density (F1,22=0·10, P=0·91), asexual parasite density (F1,22=0·482, P<0·50), nor gametocyte density (F1,22=0·002, P=0·96) could explain variation in mean fecundity when added to a statistical model including parasite genotype and experimental block. Furthermore, none of these variables had any association with fecundity when considered independently (P>0·1 in all cases). Mean bloodmeal size, however, did explain additional variation in fecundity when added to a statistical model that included parasite genotype (F1,22=4·15, P=0·05), and was also significant when considered on its own (F1,24=5·95, P=0·02, Fig. 3). Furthermore, the inclusion of bloodmeal size reduced the explanatory power of parasite genotype to the point where it was no longer significant (F2,22=2·59, P=0·10). This suggests that parasite genetic variation in mosquito fecundity is driven by differences in the bloodmeal size.

Fig. 3. Relationship between the mean bloodmeal size taken from infected mice and the mean fecundity of Anopheles stephensi mosquitoes. Symbols indicate the parasite genotype fed on. Block 1 data are open symbols and block 2 data are black symbols. The slopes of the regression lines do not differ between blocks, but the intercepts do.
Parasite genotype, red blood cell density, asexual parasite density, gametocyte density and their experimental block interactions were combined in a statistical model to test their relationship to mean bloodmeal size. Across the infected groups, both red blood cell density (F1,23=11·87, P=0·01, Fig. 4A) and gametocyte density (F1,23=10·51, P=0·01, Fig. 4B) were related to bloodmeal size. Bloodmeal size increased with host red blood cell density, but fell with increased gametocyte density. Both red blood cell density and gametocyte density remained significant in the absence of one another (P<0·01 in both cases), and regression analysis indicated that these two variables were not strongly correlated (F1,25=1·41, P=0·25, r2=0·02). Parasite genotype was no longer a significant predictor of bloodmeal size when gametocyte density and red blood cell density were included as explanatory variables (when both factors fit together, parasite genotype: F2,21=1·81, P=0·19, when parasite genotype fit on its own: F2,23=4·53, P=0·02).

Fig. 4. Relationship between (A) red blood cell density of mice and mean bloodmeal size and (B) mouse gametocyte density and mean bloodmeal size. Error bars are 1 S.E. Symbols indicate the parasite genotype fed on. Block 1 data are open symbols, block 2 data are black symbols. Two regression lines are shown only when experimental block influenced the relationships ((A) black line, block 1; grey line, block 2).
To test the significance of red blood cell density in the absence of parasitism, a second analysis was conducted on uninfected mosquitoes only. Although the mean red cell density of infected mice was lower than the controls, both groups encompassed a similar range of values (range of red cell densities for control mice=4·13–6·54×109 RBC/ml, range for infected mice=3·27–9·54×109 RBC/ml). Amongst uninfected mice, red cell density was unrelated to bloodmeal size (F1,7=0·61, P=0·46).
Across both blocks, 71–97% of the mosquitoes we collected for haematin analysis had taken a bloodmeal within the 20 min mouse exposure period. A greater proportion of mosquitoes fed when exposed to infected hosts (92% vs 81%, χ21=11·90, P<0·01). Restricting analysis to infected mice, the proportion of mosquitoes that fed was not influenced by the Plasmodium genotype of their host (χ22=2·49, P=0·29). On their own, both asexual parasite density (χ21=5·24, P=0·02) and gametocyte density (χ21=3·28, P=0·07) showed a positive association with the proportion of mosquitoes that fed (Fig. 5), with red blood cell density being unimportant (χ21=0·03, P=0·86). On the day of blood feeding, asexual parasite density was positively correlated with gametocyte density (F1,7=5·31, P=0·06). When asexual parasite density, gametocyte density, and red blood cell density were combined in a single model (non-significant terms eliminated), only asexual parasite density retained a statistically significant association with mosquito feed proportion (χ21=5·24, P=0·02).

Fig. 5. Relationships between the asexual parasite and gametocyte density of mice and the proportion of mosquitoes that took a bloodmeal from them in a 20 min period (data from block 2 only). The fitted lines give the best fit relationships as modelled by the logit function. For asexual parasite density, feeding proportion=e1·69+3·191(asexual density)/(1+e1·69+3·19(asexual density)), for gametocyte density, feeding proportion=e0·70+4·70logl(gametocyte density)/(1+e0·70+4·70log(gametocyte density)).
So far as we are aware, this study provides the first evidence that genetic diversity within Plasmodium infections plays a role in determining the magnitude by which mosquito fecundity is reduced. It is also the first demonstration that P. chabaudi reduces vector fecundity, strengthening the case that this is a general outcome of Plasmodium infections in mosquitoes (Hacker, 1971; Hacker & Kilama, 1974; Freier & Friedman, 1976; Hogg & Hurd, 1995 a, b, 1997; Jahan & Hurd, 1997; Ahmed et al. 1999). Mosquitoes with mixed infections laid approximately 20% less eggs than those fed uninfected blood. These infection diversity-effects were similar in both blocks. Although genetic diversity (one genotype or two) is important, the effect of genotype per se is more ambiguous. We found no strong differences between the fecundity of mosquitoes with single genotype infections (CR and ER) and those that fed on uninfected mice.
Our analysis suggests that increased pathogenicity of mixed infections arises indirectly from the interaction between gametocyte abundance, red blood cell density and bloodmeal size. Mixed infections produced higher gametocyte densities and made mice more anaemic in this experiment and others (Taylor et al. 1998). As we found a positive relationship between anaemia and haematin intake and a negative relationship between gametocyte density and haematin intake, mosquitoes feeding on mixed genotype infections tended to take smaller bloodmeals than those feeding on hosts with single-genotype infections. As bloodmeal size is a prime correlate of mosquito fecundity both in this study and in others (Reisen & Emory, 1976; Briegel, 1990 a, b), the small bloodmeals taken from hosts with mixed infections likely rendered mosquitoes less fecund.
It is not possible to pinpoint which factor, anaemia or gametocyte density, was responsible for the decrease in mosquito bloodmeal size. The fact that these two variables were not correlated with each other, and that both were significant when combined and in isolation, suggests they have independent effects. Further evidence that the association between bloodmeal size and gametocyte density is not simply facilitated by anaemia comes from the observation that amongst uninfected mice, red cell density was unrelated to bloodmeal size (over a wide range of red cell densities). This apparent co-dependence of bloodmeal size on both anaemia and gametocyte abundance contrasts with results from another rodent malaria species, P. yoelii (Hogg & Hurd, 1995 a). Unlike P. chabaudi, P. yoelii gametocytes emerge in mouse blood several days before anaemia develops. Plasmodium yoelii-associated reductions in mosquito bloodmeal size occurred when hosts were anaemic (and had high asexual densities) but not when gametocytaemia was highest (Hogg & Hurd 1995 a).
It is not surprising that we found a positive association between red blood cell density and bloodmeal size. Haematin content, our measure of bloodmeal size, is related to the number of red cells that a mosquito digests, not necessarily the volume of liquid it imbibes. Mosquitoes feeding on an anaemic host will therefore ingest a lower haematin mass than those feeding on a healthy host, even if both ingest a similar volume of blood. The association between bloodmeal size and anaemia we report is thus likely a product of variation in the quality of parasitized blood (as indexed by the amount of red cells per meal), rather than differences in the quantity of blood they consume. It is less clear how gametocyte abundance could influence bloodmeal size. One possibility is that mosquitoes choose to reduce their intake when they encounter heavily parasitized blood, and another that heavily parasitized blood is more difficult to imbibe. There has been little investigation of the first, intriguing possibility, but the second is strengthened by evidence that several properties of host blood that vary in response to infection, including anaemia (Shieh & Rossignol, 1992; Taylor & Hurd, 2001), molecular composition (Hosoi, 1959) and the presence of antibodies (Srikrishnaraj, Ramasamy & Ramasamy, 1993), are known to influence different aspects of female mosquito feeding and fecundity. Furthermore, the presence of parasites may reduce the efficiency with which mosquitoes can concentrate red cells while feeding (a mechanism that could otherwise compensate for low cell densities in uninfected host blood, discussed by Taylor & Hurd, 2001).
If the correlation between gametocyte density and bloodmeal size proves to be a general phenomenon, between-genotype variation in fecundity may occur in nature even though we did not detect it in our study. The two single genotype infections we used (CR and ER) produced similar levels of gametocytes. More extensive studies of genetic variation both within this Plasmodium species (Mackinnon & Read, 1999 a) and the human malaria parasite P. falciparum (James, Nicol & Shute, 1932; Graves, Carter & McNeill, 1984) have shown that gametocyte density can vary significantly between parasite genotypes. If we had used 2 genotypes that produced different gametocyte densities, we may have found genotype-specific effects on fecundity as well as an effect of infection diversity.
Despite having higher gametocyte densities, mixed infections did not produce higher infection rates or oocyst burdens in mosquitoes. This result contrasts with an earlier study where the increased gametocyte densities of mixtures did translate into higher oocyst prevalence (Taylor et al. 1998). Variation between these studies may be due to the fact that, in contrast to our study, the parasite dose given to mice in the mixed infection group in the previous experiment was higher than given to the single dose treatments, or that the CR and ER genotypes were not combined in a 50[ratio ]50 ratio.
We found no association between oocyst presence or number and mosquito fecundity, suggesting that reductions in egg output do not arise from direct nutrient competition between host reproductive tissue and growing parasites, a hypothesis strengthened by similar findings in other studies (Hacker & Kilama, 1974; Hogg & Hurd, 1995 b; Ahmed et al. 1999). Most likely, fecundity reductions are due to the reduced size of infected bloodmeals, and not to the action of the parasites within the mosquito. However, we cannot rule out the possibility that parasites had direct effects on mosquito resources immediately after ingestion. Mosquitoes may have expended a large amount of resources mounting immune responses to invading parasites, stopping them from developing into oocysts. Immune responses are costly in insects, and can drain resources that would have otherwise have been directed to survival and reproduction (Ferdig et al. 1993; Moret & Schmid-Hempel, 2000). Additionally, parasites may have directly reduced mosquito fecundity by interfering with the rate of egg production (Carwardine & Hurd, 1997; Jahan & Hurd, 1998).
In addition to its effects on bloodmeal size, parasite density was also associated with the propensity of mosquitoes to blood feed. Mosquitoes fed on infected hosts at a higher frequency than the controls, and across infected mice, feeding propensity rose both with asexual parasite and gametocyte density. It is difficult to envisage why mosquitoes have an increased tendency to feed on hosts of poor quality (those whose blood generates low fecundity). It is possible that parasites could be manipulating mosquito behaviour to increase their own fitness, perhaps by making infected hosts more attractive. Alternately, increased feeding on parasitaemic mice may simply be a consequence of changes in host physiology or odour that are associated with specific symptoms of illness (Penn & Potts, 1998; Braks, Anderson & Knols, 1999). For example, Plasmodium-infected mice undergo periods of hyperthermia (Day & Edman, 1984) and may emit more carbon dioxide than healthy mice, both of which could increase their attractiveness to mosquitoes (Grossman & Pappas, 1991; Takken & Knols, 1999). In any case, this finding challenges the notion that increased feeding of mosquitoes on gametocytaemic mice is simply due to the reduced defensive behaviour of infected individuals (Day & Edman, 1983). In our experiment, all mice were under anaesthetic. Thus host odour or physiology must also be responsible for preferential feeding, in addition to any effect of host behaviour.
Variation between parasite genotypes for their effect on mosquito fecundity is a necessary requirement to study whether vector fecundity decreases are adaptive for parasites, mosquitoes, or both. They could be beneficial to mosquitoes if reduced fecundity increases longevity, and thus lifetime reproductive success. As we did not monitor mosquito survival in this experiment, we could not test this hypothesis directly. However, evidence from a separate study of mosquito survival suggests that, under similar conditions, the infection generating the greatest fecundity reduction (mixture of CR and ER) is also the one that causes the greatest vector mortality (Ferguson & Read, 2002). Thus, even if fecundity reductions offset the mortality costs of infection, they do not eliminate them. Future studies are required to examine whether fecundity reductions are associated with components of parasite fitness other than vector survival such as sporozoite load or infectivity to new vertebrate hosts. If such a relationship exists it will both increase our understanding of the adaptive nature of parasite effects on host fecundity, and facilitate prediction of how fitness costs imposed by parasites should evolve.
We thank K. Grech and H. Hurd for discussion, B. Chan and A. Graham for experimental assistance and the staff of the Edinburgh University animal house for superb animal maintenance. A University of Edinburgh Faculty Studentship and an Overseas Research Studentship funded H. F., a Leverhulme grant to Stuart West funded A. R., and a BBRSC grant to A. F. R. funded the work.
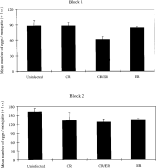
Fig. 1. The mean fecundity of mosquitoes (no. eggs/mosquito ±1 S.E.) after feeding on mice infected with different genotypes of Plasmodium chabaudi. Each bar represents the grand mean of the mean mosquito fecundity per mouse.

Fig. 2. Properties of blood from uninfected mice and those infected with 3 different Plasmodium chabaudi infections (mean value ±1 S.E.). Data are grand means of values from separate feeding trials (no. mosquitoes per trial=4–5 in block 1, 35–40 in block 2).

Fig. 3. Relationship between the mean bloodmeal size taken from infected mice and the mean fecundity of Anopheles stephensi mosquitoes. Symbols indicate the parasite genotype fed on. Block 1 data are open symbols and block 2 data are black symbols. The slopes of the regression lines do not differ between blocks, but the intercepts do.

Fig. 4. Relationship between (A) red blood cell density of mice and mean bloodmeal size and (B) mouse gametocyte density and mean bloodmeal size. Error bars are 1 S.E. Symbols indicate the parasite genotype fed on. Block 1 data are open symbols, block 2 data are black symbols. Two regression lines are shown only when experimental block influenced the relationships ((A) black line, block 1; grey line, block 2).

Fig. 5. Relationships between the asexual parasite and gametocyte density of mice and the proportion of mosquitoes that took a bloodmeal from them in a 20 min period (data from block 2 only). The fitted lines give the best fit relationships as modelled by the logit function. For asexual parasite density, feeding proportion=e1·69+3·191(asexual density)/(1+e1·69+3·19(asexual density)), for gametocyte density, feeding proportion=e0·70+4·70logl(gametocyte density)/(1+e0·70+4·70log(gametocyte density)).