Introduction
Functional somatic syndromes (FSS) such as chronic fatigue syndrome, irritable bowel syndrome and fibromyalgia are characterised by bodily complaints for which medical examination does not provide sufficient explanatory pathology (Henningsen et al., Reference Henningsen, Zipfel and Herzog2007). An estimated 2–4% of patients in primary and secondary care suffer from severe FSS (Fink et al., Reference Fink, Toft, Hansen, Ørnbøl and Olesen2007; Fink and Schröder, Reference Fink and Schröder2010; Jones et al., Reference Jones, Atzeni, Beasley, Fluss, Sarzi-Puttini and Macfarlane2015). In the most severe cases, patients fulfil criteria for several FSS, i.e. multiple FSS. Healthcare professionals experience difficulties diagnosing and treating these patients since most treatments target single FSS diagnoses. A new diagnosis of Bodily Distress Syndrome (BDS) which seems to cover most of the relevant FSS has been proposed (Fink and Schröder, Reference Fink and Schröder2010). Multi-organ BDS entails functional somatic symptoms from at least three of four organ systems, and clinical studies have found that patients fulfilling criteria for multi-organ BDS on average fulfil diagnostic criteria for three or more single FSS (Schröder et al., Reference Schröder, Rehfeld, Ornbøl, Sharpe, Licht and Fink2012; Agger et al., Reference Agger, Schroder, Gormsen, Jensen, Jensen and Fink2017). Recent studies have shown an unfavourable prognosis of untreated multi-organ BDS in terms of high healthcare expenditure and a high risk of new disability pension awards (Budtz-Lilly et al., Reference Budtz-Lilly, Vestergaard, Fink, Carlsen and Rosendal2015; Rask et al., Reference Rask, Ornbol, Rosendal and Fink2017; Schröder et al., Reference Schröder, Ørnbøl, Jensen, Sharpe and Fink2017). As use of the BDS diagnosis is still uncommon, we use the term multiple FSS in the remainder of this paper.
Graded exercise and psychological treatment based on cognitive and behavioural therapies show moderate long-term effects on functional level, physical health, quality of life and symptom level in patients with FSS in larger clinical trials and meta analyses (Henningsen et al., Reference Henningsen, Zipfel and Herzog2007; Busch et al., Reference Busch, Schachter, Overend, Peloso and Barber2008; Glombiewski et al., Reference Glombiewski, Sawyer, Gutermann, Koenig, Rief and Hofmann2010; White et al., Reference White, Goldsmith, Johnson, Potts, Walwyn, DeCesare, Baber, Burgess, Clark, Cox, Bavinton, Angus, Murphy, Murphy, O'Dowd, Wilks, McCrone, Chalder and Sharpe2011). We have recently demonstrated that patients with multiple FSS can feasibly be treated together in a group-based CBT programme (STreSS-1) (Schröder et al., Reference Schröder, Rehfeld, Ornbøl, Sharpe, Licht and Fink2012; Schröder et al., Reference Schröder, Sharpe and Fink2015); however, not all patients benefit from the treatment.
Acceptance and Commitment Therapy (ACT), (Hayes et al., Reference Hayes, Strosahl and Wilson1999) may be suitable as a ‘transdiagnostic’ approach to address generic illness mechanisms at work in patients with various symptom profiles/multiple FSS (Levin et al., Reference Levin, Hildebrandt, Lillis and Hayes2012). ACT aims at increasing patients’ quality of life irrespective of potential symptom relief. This is, among others, facilitated by investigating short- and long-term consequences of avoidance and control strategies, acceptance of inner experience through willingness to become connected to the present moment using mindfulness exercises, identifying life values, and challenging patients to commit to behaviour change in the therapeutic process (Dahl and Lundgren, Reference Dahl and Lundgren2006). ACT has been found effective in some trials for the treatment of chronic pain conditions and various FSS (Trompetter et al., Reference Trompetter, Bohlmeijer, Veehof and Schreurs2015; Hughes et al., Reference Hughes, Clark, Colclough, Dale and McMillan2016; Veehof et al., Reference Veehof, Trompetter, Bohlmeijer and Schreurs2016), and meta analyses comparing ACT and other evidence-based treatments have found the treatments equally effective (Kohl et al., Reference Kohl, Rief and Glombiewski2012; Öst, Reference Öst2014; Veehof et al., Reference Veehof, Trompetter, Bohlmeijer and Schreurs2016). Yet, ACT has not been tested in patients with multiple FSS. Furthermore, little is known about how much treatment is needed to obtain an effect.
The aim of this study was to investigate the effect of ACT for patients with multiple FSS delivered as an extended therapy (nine-session group therapy) and a brief therapy (1-day workshop and one consultation). Both therapies were compared with enhanced care (EC). We hypothesised that extended ACT would be superior to EC in improving the primary outcome (patient-rated overall health status) at 14 months after baseline (end-point) and in improving the secondary outcomes (physical, mental and social health) from baseline to endpoint with a sustained effect at 20 months. We additionally hypothesised that brief ACT would be superior to EC in improving primary and secondary outcomes but with lower effects than the Extended ACT.
Methods
Study design
This randomised three-armed trial took place in a university general hospital setting at The Research Clinic for Functional Disorders, Aarhus University Hospital, Denmark. The study is registered with ClinicalTrials.gov, number NCT01518647. The current trial, entitled Specialised Treatment for Severe Bodily Distress Syndrome-4 (STreSS-4), is part of a group of studies (Schröder et al., Reference Schröder, Rehfeld, Ornbøl, Sharpe, Licht and Fink2012; Fjorback et al., Reference Fjorback, Arendt, Ørnbøl, Walach, Rehfeld, Schröder and Fink2013; Agger et al., Reference Agger, Schroder, Gormsen, Jensen, Jensen and Fink2017) with the overall aim to provide evidence-based treatment options for patients with multiple FSS.
Participants
Consecutively referred patients (aged 20–50 years) with multiple, long-lasting somatic symptoms were screened for eligibility. The age range was set to enhance diagnostic certainty and a criterion for referral to the clinic. Patients with multi-organ BDS of at least 2 years’ duration leading to moderate or severe impairment in daily living were eligible. The diagnosis multi-organ BDS was established by a medical doctor after a thorough physical and psychological assessment including the SCAN diagnostic interview (Schedules for Clinical Assessment in Neuropsychiatry) (Wing et al., Reference Wing, Babor, Brugha, Burke, Cooper, Giel, Jablenski, Regier and Sartorius1990), a close review of medical history by medical records, physical examination and blood test. Patients with a lifetime diagnosis of psychosis, mania or depression with psychotic symptoms (ICD-10: F20–29, F30–31, F32.3, F33.3), current abuse of alcohol or drugs and pregnancy at the time of inclusion were excluded. Patients with concurrent medical illness or psychiatric disorders were only excluded if the concurrent illness was the primary reason for health complaints or impairment for the patient.
At assessment, patients were thoroughly informed about the diagnosis multi-organ BDS and the current knowledge regarding helpful management strategies of the disorder taking as a point of departure a bio-psycho-social model of BDS (Creed et al., Reference Creed, Henningsen and Fink2011). Patients were encouraged to modify their basic lifestyle with the intent to improve physical activity, sleep quality, diet and social network and to increase awareness of potential stress factors. If they wished to participate in the trial, they provided informed consent and were randomised at the time of inclusion to receive either (1) EC, (2) Brief ACT or (3) Extended ACT.
Interventions
See online Supplementary Appendix A for the depiction of the intervention.
Enhanced care
Patients allocated to EC received one manualised follow-up consultation with the physician conducting the clinical assessment 1–2 weeks after randomisation. Duration of the consultation was 1–1½ h and aimed at enhancing the patient's understanding of the BDS diagnosis, optimising further treatment initiatives in the healthcare system, increasing awareness of stress factors and motivation for lifestyle changes. The general practitioner received written suggestions for further treatment after this manualised follow-up consultation for the EC group.
Brief ACT
Patients allocated to Brief ACT received the same manualised follow-up consultation 1–2 weeks after randomisation as patients allocated to EC but without a treatment plan to the GP at this time. Additionally, they participated in a 1-day workshop from 10 am to 4 pm. The workshop consisted of information about multi-organ BDS and introduction to ACT concepts such as acceptance, mindfulness, and life values. The workshop alternated between psycho-education, experiential exercises and group discussions. Up to 15 patients participated in each workshop. One to 2 weeks after the workshop, patients attended a follow-up consultation with one of the therapists from the workshop evaluating and individualising the content of the workshop. The workshop was carried out by two to three therapists trained in ACT and management of BDS. After the last follow-up consultation, the general practitioner received a summary of treatment progress and written suggestions for further treatment.
Extended ACT
Patients allocated to Extended ACT received the same manualised follow-up consultation 1–2 weeks after randomisation as patients allocated to EC but without a treatment plan to the GP at this time. The following group therapy consisted of 9 weekly 3-h sessions over a period of 3 months led by two therapists; a psychiatrist and a psychologist, who both were trained in ACT and management of BDS. We chose to use two therapists to ensure continual peer-supervision and to allow for more time for each patient. Especially, with two therapists we were able to split the group when planning and evaluating homework assignments. Each session had an overall theme representing the core elements of the hexaflex model (Hayes et al., Reference Hayes, Luoma, Bond, Masuda and Lillis2006): acceptance, contact to the present moment, cognitive diffusion, self as context and life values. Commitment was integrated in the other themes and did not represent an independent theme in the material. Experiential exercises and group discussions were used to examine the workability of control and avoidance strategies such as thought suppression or distraction to eliminate symptoms. Mindfulness exercises were introduced to increase awareness and tolerance of especially physical symptoms but also negative thoughts and distressing emotions. Commitment processes were emphasised in each session in that patients committed to short- and long-term goals consistent with chosen values using steps of objectives. An overview of content in each session can be found in the online Supplementary (Appendix B). At end-of-treatment, the general practitioner received a summary of treatment progress and written suggestions for further treatment.
Randomisation
The randomisation code was computer-generated by a statistician, who also provided sequentially numbered sealed opaque envelopes containing allocated intervention for each of the 180 patients. The envelopes were serially administered according to date of inclusion. The envelope was opened by the physician in presence of the patient, and the patient was hence informed of the allocated intervention immediately after inclusion.
Outcomes
Patients completed questionnaires at baseline (before randomisation) and 6, 14 and 20 months after baseline. All questionnaire data were obtained as self-report using a web-based programme.
The primary outcome was self-rated global health improvement after 14 months using the clinical improvement scale [clinical global improvement (CGI)], a five-point Likert scale. Patients rated their general health as much worse, worse, unchanged, better or much better in response to the following question: ‘How do you consider your health status now compared with when you first came to the clinic?’. For statistical analysis, the scale was collapsed into three categories (worse, same or better). This simple scale correlates with other specific outcomes in this population including physical functioning (PF) and symptom scores and is important to patients. Further it is recommended by consensus groups in pain research and the field of FSS (Dworkin et al., Reference Dworkin, Turk, Wyrwich, Beaton, Cleeland, Farrar, Haythornthwaite, Jensen, Kerns, Ader, Brandenburg, Burke, Cella, Chandler, Cowan, Dimitrova, Dionne, Hertz, Jadad, Katz, Kehlet, Kramer, Manning, McCormick, McDermott, McQuay, Patel, Porter, Quessy, Rappaport, Rauschkolb, Revicki, Rothman, Schmader, Stacey, Stauffer, von Stein, White, Witter and Zavisic2008; Moore et al., Reference Moore, Straube and Aldington2013; Rief et al., Reference Rief, Burton, Frostholm, Henningsen, Kleinstäuber, Kop, Löwe, Martin, Malt, Rosmalen, Schröder, Shedden-Mora, Toussaint and van der Feltz-Cornelis2017). Moreover, we wanted to compare this primary outcome with another parallel-running trial at the clinic on the effect of imipramine on self-rated global health improvement (Agger et al., Reference Agger, Schroder, Gormsen, Jensen, Jensen and Fink2017).
Secondary outcomes were changes in physical, mental and social health assessed with the physical and mental component summary, the individual subscales of the Short Form-36 Health Survey (SF-36, version 1) except for the scales Role Emotional and Role Physical, and an aggregate score of the SF-36 subscales ‘physical functioning’, ‘bodily pain’ and ‘vitality’, which measure physical health domains usually affected in multiple FSS (Schröder et al., Reference Schröder, Rehfeld, Ornbøl, Sharpe, Licht and Fink2012; Rief et al., Reference Rief, Burton, Frostholm, Henningsen, Kleinstäuber, Kop, Löwe, Martin, Malt, Rosmalen, Schröder, Shedden-Mora, Toussaint and van der Feltz-Cornelis2017). Data were scored and interpreted according to the Danish manual. Similarly, changes over time (from baseline to 20 months) in symptoms of depression, anxiety and somatic symptoms were assessed by subscales of the 92-item version of the Hopkins Symptom Checklist (SCL-92) and the BDS checklist to measure symptom score, the Whiteley-7 to measure illness worry and The WHODAS 2.0 to measure disability. All secondary outcomes were assessed as changes over time from baseline to endpoint at 14 month and additionally from baseline to follow-up at 20 months. Treatment response was defined on the domains physical health (as a four-point improvement on the SF-36 aggregate score) and symptom burden (as a 0.35 points decrease in the SCL somatic symptom score), respectively, and calculated as changes from baseline to 14 months. These changes equal half a standard deviation and may be considered clinically significant improvements.
Sample size
The power calculation was based on the CGI using the three categories (worse, same or better) and a proportional odds model. With a level of significance of 0.05 and a realistic sample size of 60 patients in each of the three groups, we performed simulations of data comparing Extended ACT with EC. A total of 17 different scenarios of the treatment effects was created from a large positive (OR = 5.4, power = 99.3) to small effect (OR = 1.3, power = 9.7). A model reflecting reasonable expectations of treatment effects (OR = 3.0) and also providing adequate power (power = 87%) gave basis for proceeding with the study. We expected 15% loss to follow-up in each group.
Statistical analysis
The Intention-to-Treat sample (ITT) consisted of all included patients and the per protocol sample (PP) of patients providing full data and receiving sufficient treatment according to protocol. Sufficient treatment was defined as follows: for Extended ACT a minimum of seven sessions, for Brief ACT patients attending both the 1-day intervention and the following consultation, and for EC patients who attended the follow-up consultation. All analyses were conducted using Stata SE v. 13.1 for Windows.
For both primary and secondary outcome measures, we first compared Extended ACT to EC and then Brief ACT to EC. In addition, it was planned to compare Extended ACT with Brief ACT on all outcome measures only if results from both above mentioned analyses would be significant.
All analyses were performed both for the ITT and the PP samples.
The analysis of the CGI score was based on the above-mentioned combined outcome groupings using one unadjusted proportional odds model. The primary analysis was based on all patients in the ITT sample who provided primary outcome data. Worst case scenarios were calculated for missing values if the main result was statistically significant giving patients receiving the treatment of interest an outcome of ‘worse’, the control treatment (EC) an outcome of ‘better’ and the intervention of no interest in the analysis an outcome of ‘unchanged’.
For each of the secondary outcomes, we fitted a linear mixed model with a random intercept and a cluster effect for treatment group. Each model included group (Extended ACT v. Brief ACT v. EC), time and their interaction as explanatory variables. Using this model, we tested whether the groups differed with regard to changes over time (i.e. test of interaction between group and time) and then calculated within-group changes from baseline to primary endpoint at 14 months. The analyses were repeated while adjusting for age, gender, the assessing physician, psychiatric comorbidity and work status.
Finally, in each of the three groups, we estimated the proportion of patients who improved in perceived physical health (the aggregate score of the SF-36) and in symptom burden (the SCL physical symptom score) × from baseline to endpoint at 14 months.
Results
Subjects
Figure 1 shows the trial profile. Patients were screened for eligibility between 25 January 2012 and 19 May 2014. We included and randomised 180 patients: 59 were allocated to Extended ACT, 61 to Brief ACT and 60 to EC. Baseline characteristics of all included patients are displayed in Table 1.

Fig. 1. Trial profile. ITT, Intention to treat; PP, per protocol.
Table 1. Patient characteristics

a Current, other: personality disorder, OCD. ADHD, PTSD.
b Life-time psychiatric comorbidity: anxiety disorder, depression disorder, eating disorders, personality disorder, OCD. ADHD, PTSD.
c Other medication: antiepileptics, low-dose TCA, other pain medications, anxiolytics, antipsychotics, psychostimulants.
d Data are missing for one patient in the ACT-g group, data are missing for one patient in the ACT-w group and two in the ACT-g group. Major depression of any severity or in treatment for major depression.
Attendance and data collection
Adherence to allocated intervention and data collection is shown in Fig. 1. The primary reasons for not attending allocated interventions were practical issues or regret of participation. Patients provided baseline data on average 0.8 months (s.d. 0.5 months) before inclusion. Mean time from inclusion to intermediate data was 6.4 months (s.d. 1.0), to primary endpoint 14.5 months (s.d. 1.3) and to follow-up 20.5 months (s.d. 0.9). Data collection was completed at 9 February 2016. Reasons for not providing data were primarily loss of interest to participate or loss of contact. In total, 154 patients provided primary outcome data, and 145 provided full data. The ITT analysis included all available data. The PP population consisted of 139 patients.
Primary outcome
The raw data for the primary outcome are displayed in Table 2. Extended ACT was superior to EC in providing improvement after 14 months with an OR of 2.9 (95% CI 1.4–6.2; p = 0.006) when including all patients who provided data (n = 154). The PP analyses (n = 139) yielded an OR of 3.1 (95% CI 1.4–7.0; p = 0.005). When applying a worst-case scenario for the 26 patients with missing outcome, the OR dropped to 1.0 (95% CI 0.5–2.0; p = 0.983). We found no difference between Brief ACT and EC.
Table 2. Primary outcome CGI-5 raw score

Data are in n (%) in response to the question: ‘How do you consider your health status now compared with when you first came to the clinic?’.
Secondary outcomes
We found no differences in changes over time on the 18 secondary outcomes between groups when comparing Extended ACT or Brief ACT to EC in neither the ITT sample nor the PP sample except for PF which increased significantly for Extended ACT compared with EC in both analyses. The results of the secondary outcomes for the PP sample are shown in Fig. 2 and in online Supplementary Appendix C and D. The results of the adjusted analyses comparing Extended ACT to EC did not change the interpretation of our results.
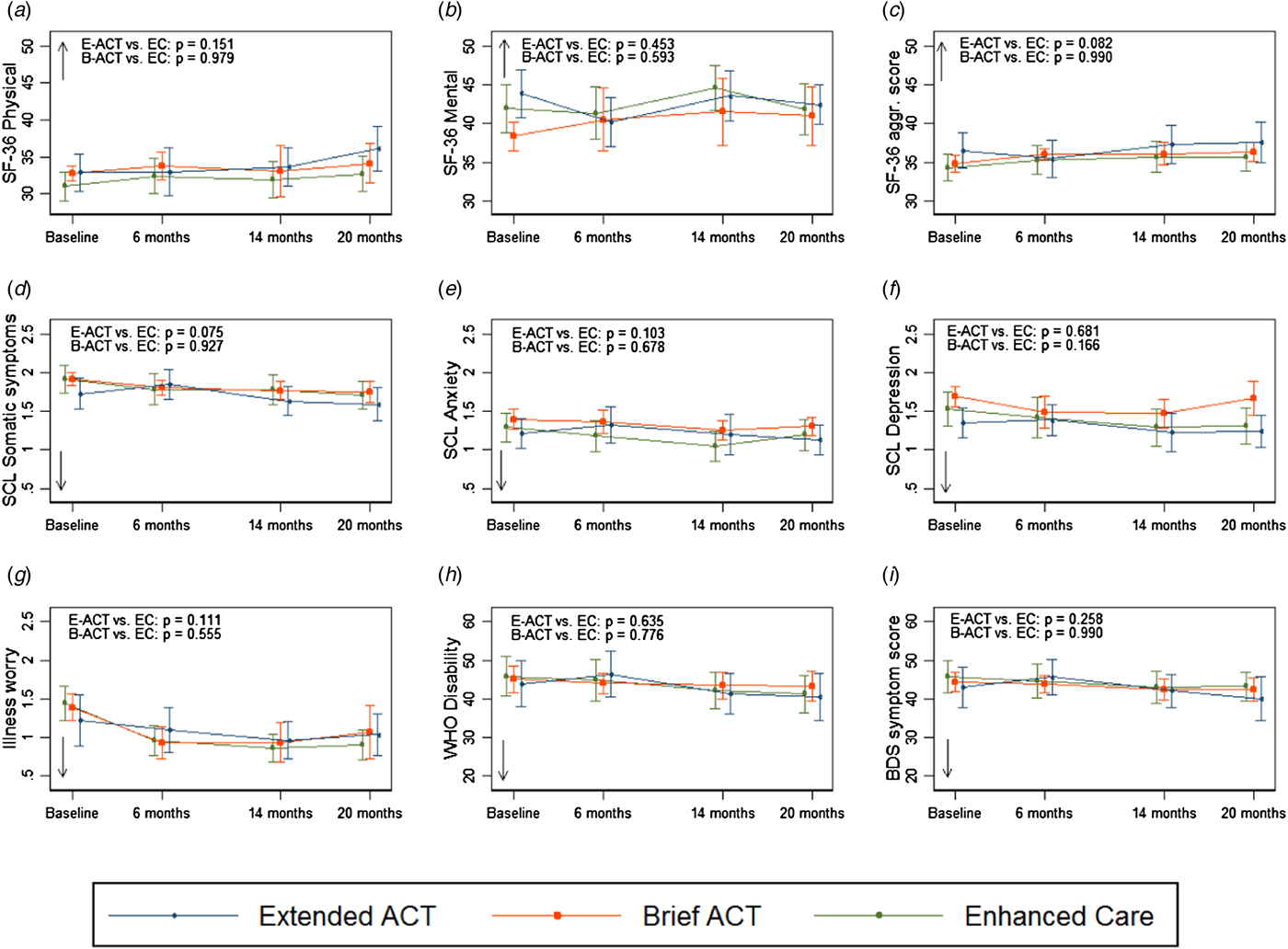
Fig. 2. Effect of ACT in extended and brief version on secondary outcomes. The three curves of each graph give the mean values and 95% confidence intervals for the Extended ACT (E-ACT), Brief ACT (B-ACT), and Enhanced Care (EC) groups, respectively, at baseline and during follow-up. The p values are for the group × time interaction for E-ACT v. EC and B-ACT v. EC, respectively (i.e. test of no difference in change over time from baseline to 20 months according to an unadjusted linear mixed model in the PP population). Arrows indicate the direction of improvement. a–c Health-related quality of life measured with SF-36 (physical and mental component scores and aggregate score of physical functioning, bodily pain, and vitality), higher scores indicating better health. D-G Somatic symptoms, anxiety, depression, and illness worry measured with subscales of the Symptom Check List-92, Revised version, and the 7-item Whiteley-Checklist. Lower scores indicate less symptom burden. H Disability measured with WHO-Disability Adjustment Scale version 2.0, lower score indicating less disability. I Somatic symptom score measured with Bodily distress syndrome checklist, lower scores indicating less symptom burden.
The within-group differences from baseline to 14 months showed a statistically significant modest reduction of illness worry measured by Whiteley-7 for all three groups. Perceived physical health (the aggregate score of SF-36) improved after EC and the Brief ACT, and the SCL score for depression and anxiety improved for patients receiving EC, but the observed effects were small (online Supplementary Appendix D).
Figure 3 displays the changes in perceived physical health (the aggregate score of the SF-36) and symptom burden (the SCL physical symptom score) in the three groups. There were no statistically significant differences between groups in the proportion of patients who improved at least 4 points in physical health or by at least 0.35 points in somatic symptoms.

Fig. 3. Improvement in physical health (a) and symptom burden (b) from baseline to 14 months. The figure displays the proportions of patients in each group with corresponding changes from baseline to trial endpoint at 14 months in physical health (measured with SF-36 aggregate score) and symptom burden (measured with SCL-92 somatic symptoms score). Plots present the observed data with each dot representing the observed change score for an individual patient who provided data at 14 months. Vertical lines with arrows indicate levels of improvement: treatment response, i.e. change scores ⩾0.5 s.d. unit; marked improvement, i.e. change scores ⩾1.0 s.d. unit.
Post-hoc analyses provided similar results and did not change the interpretation of the results (data not shown).
Discussion
Principal findings
The present study is, to our knowledge, the first larger randomised controlled trial examining the effect of two different intensities of ACT for patients with multiple FSS. The study compared the effect of nine 3-h sessions of group-based ACT therapy (Extended ACT) with EC and a 1-day ACT workshop and follow-up consultation (Brief ACT) with EC in 180 patients with multiple FSS. We found no differences between the low-intensity, Brief ACT, and EC on any outcome, whereas the high-intensity group therapy, Extended ACT, showed a statistically significant improvement compared to EC for the primary outcome reflecting patients’ self-rated overall health improvement. However, secondary outcomes did not show clinically relevant differences. Together, the results suggest limited or no clinical effect of ACT as compared with EC.
Existing evidence on the effectiveness on ACT for patients with multiple FSS
The literature suggests that out of a range of health conditions such as anxiety, depression, and pain, the strongest evidence established is for the effectiveness of ACT on chronic pain including FSS defined by diagnostic criteria for fibromyalgia, whiplash-associated disorders, and chronic widespread pain (Öst, Reference Öst2014). However, effect sizes are small to moderate depending on the measure and the quality of the design, and ACT has not been found to outperform CBT (Hughes et al., Reference Hughes, Clark, Colclough, Dale and McMillan2016; Veehof et al., Reference Veehof, Trompetter, Bohlmeijer and Schreurs2016). Furthermore, the majority of controlled studies on chronic pain and ACT are suffering from a low number of participants, which might bias results in existing meta-analyses. Worthy of note, only two controlled trials using therapist-guided ACT face-to-face treatment have included more than 30 participants in each study arm, (Wetherell et al., Reference Wetherell, Afari, Rutledge, Sorrell, Stoddard, Petkus and Atkinson2011; Luciano et al., Reference Luciano, Guallar, Aguado, Lopez-Del-Hoyo, Olivan, Magallon, Alda, Serrano-Blanco, Gili and Garcia-Campayo2014) .
Among those, Luciano et al. (Reference Luciano, Guallar, Aguado, Lopez-Del-Hoyo, Olivan, Magallon, Alda, Serrano-Blanco, Gili and Garcia-Campayo2014) found group-based ACT therapy delivered as eight 2.5-h sessions for patients with fibromyalgia to be more effective compared to recommended pharmacological treatment and wait-list control in reducing, amongst others, functional impairment, pain catastrophising, anxiety, depression, and subjective pain both at end-of-treatment and at 6-month follow-up with large effect sizes at follow-up for functional impairment and pain acceptance (d = 1.0–2.4). The only other study including more than 30 participants was a study comparing ACT with CBT delivered as eight weekly 1.5-h sessions in a primary care setting for patients with a range of chronic pain conditions (Wetherell et al., Reference Wetherell, Afari, Rutledge, Sorrell, Stoddard, Petkus and Atkinson2011). They found significant effects in both groups on pain interference, depression, and pain-related anxiety, but no effect on physical or mental quality of life or pain intensity. There was no difference in improvement between the two treatments; however, patients receiving ACT were more satisfied with the treatment compared to patients receiving CBT.
All in all, existing evidence for ACT in the treatment of severe chronic pain or single FSS is not abundant, and the largest effect sizes for ACT are found for specific processes such as increased pain acceptance and pain interference, measures not included in the present study (Wetherell et al., Reference Wetherell, Afari, Rutledge, Sorrell, Stoddard, Petkus and Atkinson2011; Luciano et al., Reference Luciano, Guallar, Aguado, Lopez-Del-Hoyo, Olivan, Magallon, Alda, Serrano-Blanco, Gili and Garcia-Campayo2014). However, patients’ subjective experience of treatment effect is important alongside psychometric measures such as pain and pain-related disability. We observed for the primary outcome that fewer patients receiving E-ACT were represented in the category of feeling worse compared to EC and B-ACT. Thus, the more extensive interventions may actually have prevented deterioration for some of the patients. Further, we observed that a higher proportion of patients receiving E-ACT reported feeling better when compared to EC and B-ACT. One may hypothesise that the improvement in our primary outcome could reflect an improved ability to accept symptoms and act with awareness in accordance with life values. If so, the patients may have experienced this as a huge improvement in their overall quality of life. On the other hand the improvement observed on primary outcome may just as well reflect patient's need to express gratitude for having received this intense treatment rather than real improvement.
The target group
It is important to bear in mind the illness severity of the patients included in our study when evaluating the effectiveness of ACT for multiple FSS. In general, patients with multiple FSS represent the most severely affected and possibly more treatment-resistant FSS patients. In this trial in particular, the patients may have been even more severely affected than in other trials of multiple FSS; reported rates of disability pensions at baseline were lower in previous trials conducted at outpatient clinics (Schröder et al., Reference Schröder, Rehfeld, Ornbøl, Sharpe, Licht and Fink2012; Fjorback et al., Reference Fjorback, Arendt, Ørnbøl, Walach, Rehfeld, Schröder and Fink2013; Agger et al., Reference Agger, Schroder, Gormsen, Jensen, Jensen and Fink2017). We observed that an equal amount of patients across all three groups (approximately 41%) reported no change on the primary outcome. This might indicate that a proportion of the patients, irrespective of treatment intensity, did not respond to this kind of treatment. One may hypothesise that the social problems, which many of these patients experience, are not sufficiently addressed in the interventions tested in this trial. Perhaps, a more comprehensive and supportive interdisciplinary effort are needed for these patients.
ACT v. CBT for patients with multiple FSS
Generally, studies using CBT for patients with FSS have produced the most convincing results, including the first trial from our clinic, STreSS-1 (Schröder et al., Reference Schröder, Rehfeld, Ornbøl, Sharpe, Licht and Fink2012). Comparing the treatment in STreSS-1 with the treatment in this present study reveals some important differences. In STreSS-1, the therapy was highly structured using few recurrent models such as the cognitive diamond and exposure strategies stringently applied to challenge unhelpful thoughts and behaviours. We have previously found that change in symptom catastrophising and illness perceptions partly mediated the treatment effect (Christensen et al., Reference Christensen, Frostholm, Ørnbøl and Schröder2015; Frolund Pedersen et al., Reference Frolund Pedersen, Frostholm, Sondergaard Jensen, Ornbol and Schroder2016) and the literature suggests that these elements may play an important role in the perpetuation of FSS and related conditions (Henningsen et al., Reference Henningsen, Zipfel and Herzog2007). However, symptom catastrophising and illness perceptions may not have been targeted directly and in a structured manner in the present study. A structured intervention that focus directly on changing these beliefs systems may add to more immediate change in coping behaviour and result in increased experience of control, which may be important in keeping the patient motivated and engaged in the therapy. Furthermore, no simple recurrent explanatory model was used in the ACT therapy, and exposure exercises were not applied stringently. Due to the severity of this patient group, who often report attention and memory problems, one might favourably use a few, recurrent explanatory models together with exposure targeting core symptoms. Such changes could quite easily be incorporated into an ACT framework. Finally, we also note that for some, the group-based format with little flexibility and more reliance on group-processes may have served as a barrier to engage actively in therapy. Although some patients may benefit from meeting other patients in terms of normalisation and recognition of their condition, less time is reserved for each patient to address their individual challenges. Thus, some may benefit more from an individual treatment format, and more research aimed at exploring what works for whom is needed.
Limitations and strengths of the study
This study has several limitations. First, regarding the treatment, sessions were not video-monitored, and adherence to the treatment manual was not monitored. We were therefore not able to systematically assess the quality of the therapy delivered. Though the therapist and the psychiatrist who delivered the majority of the treatment were skilled and had several years of experience with the patient group and psychotherapy, and both were trained in ACT, some intervention elements may not have been delivered as planned. However, the therapy was mainly delivered by the same two therapists who also participated in manual preparation which may have enhanced manual adherence, albeit this also reduced the objectivity and standardisation of the therapy method. During the approximate same time period as this study was undertaken, another RCT on group-based ACT for severe health anxiety was conducted at the clinic with therapists who had received the same ACT training as those conducting the ACT therapy in this present study. The therapists from both studies participated in regular supervision together, and manual preparation for this present study was inspired by the manual for health anxiety. The study on ACT for health anxiety also suffers from of the same weaknesses regarding treatment integrity and lack of monitoring of core elements delivered. However, we did find large effects of this intervention on both primary outcome and most secondary measures (Eilenberg et al., Reference Eilenberg, Fink, Jensen, Rief and Frostholm2016). Second, regarding the quality of our data, we had several drop-outs of the treatment as well as missing data, which entails diminished power. Third, outcomes were based on patients’ self-reports, and the study did not include a blinded clinical assessment at endpoint or other more objective outcome measures. More measurement points would have given a more detailed picture of possible differences in changes between groups. Especially, measurements after the clinical assessment and follow-up consultation could have revealed a possible specific effect of the psycho-education delivered which was the same for all three groups (Creed et al., Reference Creed, Henningsen and Fink2011). Furthermore, specific process measures could have illuminated possible changes in dimensions such as psychological flexibility and pain acceptance.
This study also has several strengths. First, this is to our knowledge the largest randomised controlled study on ACT for patients with multiple FSS including 180 patients in two different conditions and one control group, providing sufficient power to detect even smaller treatment benefits. Second, patients were thoroughly clinically assessed by means of a standardised structured diagnostic interview, and the study had well-defined transparent diagnostic criteria for inclusion. Third, we had a long follow-up period eliminating doubt about whether lack of treatment response was caused by a delayed treatment effect. Fourth, data collection and data analyses were performed by researchers who were not involved in the actual treatment process securing some objectivity towards the data.
Conclusion
We did not find support for the efficacy of neither brief nor extended ACT when compared with EC in improving core outcomes such as physical health, mental health, and symptom burden; yet patients receiving extended ACT reported experienced improvement more often than those receiving EC only. More trials are needed before conclusions can be drawn as regards the effectiveness of ACT for patient with multiple FSS.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718001666.
Acknowledgements
We want to thank Malene Skjøth for proofreading, the patients for their participation, and the therapist involved in the treatment.
Financial support
This work was supported by the Danish foundation Trygfonden (number 7-11-1062). The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Conflict of interest
None.
Ethical standards
The study was approved by The Danish Data Protection Agency, number 2007-58-0010 and The Ethics Committee of Central Denmark Region number 20110265 and was done in accordance with the provisions of the Declaration of Helsinki and current regulatory requirements.







