Introduction
Resistance genes exist naturally in the environment owing to a range of selective pressures in nature, and Antarctica is no exception to this situation (Martinez Reference Martinez2008). However, in recent years, scientists and tourists have introduced non-indigenous microorganisms with antibiotic resistance to the Antarctic (Cowan et al. Reference Cowan, Chown, Convey, Tuffin, Hughes, Pointing and Vincent2011). This has been confirmed by several studies that have demonstrated the presence of culturable coliforms related to sewage pollution around scientific stations (Sjoling & Cowan Reference Sjoling and Cowan2000, Hughes & Thompson Reference Hughes and Thompson2004, Martins et al. Reference Martins, Aguiar, Wisnieski, Ceschim, Figueira and Montone2014). More recently, some extended-spectrum β-lactamase (ESBL)-producing strains of Escherichia coli (Migula), carrying the gene bla CTX-M, were isolated from seawater samples collected close to scientific stations in the South Shetland Islands (Hernandez et al. Reference Hernandez, Stedt, Bonnedahl, Molin, Drobni, Calisto-Ulloa, Gomez-Fuentes, Astorga-España, González-Acuña, Waldenström, Blomqvist and Olsena2012). In this environmental context, it is possible that Antarctic marine invertebrates could accumulate large numbers of resistant bacteria. In this regard, Antarctic sea urchins could be a reservoir for non-native bacteria that may be associated with abnormal mortalities or diseases.
In Antarctica, the Archaea belonging to group I Crenarchaeota and bacterial strains belonging to α-Proteobacteria, γ-Proteobacteria and Bacteroidetes have been isolated from coastal and oceanic waters (Murray & Grzymski Reference Murray and Grzymski2007). These cultivable bacteria may be cosmopolitan and endemic heterotrophic bacteria; however, information about the normal bacterial flora associated with Antarctic marine invertebrates is limited. Only a few studies of culturable heterotrophic bacteria (Tropeano et al. Reference Tropeano, Coria, Turjanski, Cicero, Bercovich, Mac Cormack and Vazquez2012) and bacteria resistant to heavy metals and antibiotics have been carried out on Antarctic marine organisms (Mangano et al. Reference Mangano, Michaud, Caruso, Brilli, Bruni, Fani and Lo Giudice2009, Reference Mangano, Michaud, Caruso and Lo Giudice2014).
The Antarctic sea urchin (Sterechinus neumayeri (Meissner)) is commonly distributed around the Antarctic continent and plays a key role in the ecosystem structure (Brey & Gutt Reference Brey and Gutt1991). Bacterial communities are a key component in Antarctic marine environments and most of them are associated with invertebrates. These microbes play important roles in host physiology. In recent years, research has mainly focused on microorganisms living inside organisms such as mammals and fishes, mainly highlighting the role of the bacterial community on the animal’s health. These communities have been analysed to understand the complex relationships between the bacteria and its host in several model organisms (Cheesman & Guillemin Reference Cheesman and Guillemin2007, Llewellyn et al. Reference Llewellyn, Boutin, Hoseinifar and Derome2014).
In the present investigation, the microbial communities present in S. neumayeri from Maxwell Bay (King George Island, South Shetland Islands) were characterized using culture-dependent techniques. The aim of the study was to assess if the cultivable bacteria were resistant to heavy metals and antibiotics. The correlation between plasmid presence and resistance was also analysed.
Materials and methods
Isolation of bacteria from sea urchins
Antarctic sea urchins (S. neumayeri) were collected by SCUBA divers at depths of 4–10 m in Maxwell Bay, Fildes Peninsula, King George Island (62°12'S, 58°57'W) during the summer of 2011. Coelomic fluids were obtained from a pool of six animals. Briefly, this fluid was collected by cutting the peristomial membrane with a scalpel and the coelomic fluids were collected in chilled tubes. The collected coelomic fluid (25 ml) was then placed in a Falcon tube and centrifuged for 10 min at 700 g (4°C). The pellet with coelomocytes was removed and the supernatant liquid was used to isolate bacteria. Heterotrophic bacteria were isolated using R2A, marine agar and Actinomycetes agar media. Inoculated agar plates were incubated at 4°C for 10 days. Bacterial colonies with unique morphologies were isolated and stained using a Gram stain and was observed under the microscope with a 100x magnification. Stock cultures were stored in 15% glycerol at -80°C.
PCR amplification of 16S rDNA and phylogenetic analysis
Genomic DNA was extracted and purified according to the methods described by Sambrook & Russell (Reference Sambrook and Russell2001). Universal primers 8-27F AGAGTTTGATCCTGGCTCAG and 1422R, GGTTACCTTGTTACGACTT were used to amplify 16S rRNA genes. The PCR amplifications were performed with an Eppendorf Mastercycler gradient PCR system. The 25 µl reaction mixture consisted of 50 ng of template DNA, 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 200 µM of each deoxynucleotide, 3 mM MgCl2, 2.5 U of Taq DNA polymerase (Invitrogen) and 0.2 µM primers. The PCR conditions were 94°C for 10 min, followed by 30 cycles of 94°C for 30 sec, 60°C for 30 sec, and 72°C for 30 sec, and a final extension at 72°C for 7 min. The PCR products were analysed using a 0.8% agarose gel in Tris-Acetate-EDTA buffer, pH 8.0 (40 mM Tris, 20 mM acetic acid and 1 mM EDTA), stained with ethidium bromide and visualized under a UV transilluminator. The partial sequences of the 16S rRNA gene of the 42 strains are available in GenBank with the following accession numbers: KP849515 to KP849561.
The phylogenetic analysis was carried out using Bosque software (Ramírez-Flandes & Ulloa Reference Ramírez-Flandes and Ulloa2008). Partial sequences obtained were used and aligned to sequences from NCBI using MUSCLE 3.6 (Edgar Reference Edgar2004). The phylogenetic tree was inferred by maximum-likelihood. This analysis was performed based on the HKY85 model (Hasegawa et al. Reference Hasegawa, Kishino and Yano1985) using phylogenetic inference based on Phyml (Guindon & Gascuel Reference Guindon and Gascuel2003). Statistical evaluation of tree topologies was performed by bootstrap analysis with 1000 resamplings.
Antibiotic and metal resistance
The activity of each antibiotic was determined using the standard method of disk diffusion on a Mueller–Hinton (MH) agar plate. The following antibacterial disks were used: ampicillin (10 µg), cephalotine (30 µg), cefotaxime (30 µg), amikacin (30 µg), gentamicin (30 µg), trimethoprim/sulphamethoxazole (1.25/23.75 µg), chloramphenicol (30 µg), nalidixic acid (30 µg) and tetracycline (30 µg). A bacterial inoculum of 5×108 CFU ml-1 was used. Plates were incubated at 15°C for 24–48 h and the inhibition zone around the disk was registered. An inhibition zone diameter of ≥12 mm indicated susceptibility to an antibacterial agent. Tests were performed twice in triplicate.
The minimal inhibitory concentration (MIC) of zinc, mercury and silver was determined against 38 bacterial strains by serial dilution on agar plates of MH or MH supplemented with 2% NaCl for halophilic bacteria. The assayed MIC ranges were: ZnSO4 32–1024 µg ml-1, HgCl2 2–16 µg ml-1 and AgNO3 2–128 µg ml-1. The breakpoints used to define resistance were 800 µg ml-1 for Zn, 16 µg ml-1 for Hg and 128 µg ml-1 for Ag. All plates were incubated at 15°C for at least 48 h and any evidence of bacterial development was considered a positive growth.
Plasmid isolation
Bacterial strains were grown in 10ml of marine broth medium. The plasmids were extracted using the Axyprep plasmid miniprep kit (Axygen) according to the instructions in the manual. Extracted DNA was analysed using a 1.0% agarose gel in Tris-Acetate-EDTA (TAE) buffer, stained with ethidium bromide and visualized under a UV transilluminator.
Statistical analyses
A two-tailed Pearson’s correlation coefficient (r) was used to assess the correlation between plasmid presence and resistance against one antibiotic, multi-drug resistance to antibiotics, resistance to metals or co-resistance (metals and antibiotics). A p-value <0.05 was considered statistically significant. The results were interpreted as a strong relationship (r>+0.5 to +1.0), weak relationship (+0.5>r) and negative relationship (r>-0.5 to -1.0). Statistical analyses were performed using the GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA). Additionally, the effect of plasmid presence on resistance to an antibiotic, multi-drug resistance, resistance to metals or co-resistance was tested using a permutational analysis of variance (PERMANOVA; Anderson Reference Anderson2001).
Results
A total of 42 isolates were purified from the coelomic fluids of S. neumayeri and identified based on 16S rRNA gene sequences. Most of the isolates were Gram-negative (n=39) and only three were Gram-positive. We isolated seven different bacterial genera and the predominant classes of bacteria were the γ-Proteobacteria (n=25) and Flavobacteria (n=14). The minority class was the Actinobacteria (n=3) represented by Agreia and Microbacterium genera. The most frequently recovered bacteria were members of the Flavobacterium (n=14) from the Bacteroidetes phylum. Many of them had the closest match to the Flavobacterium frigidarium (Fig. 1).

Fig. 1 Composition of the culturable bacteria associated with the Antarctic sea urchin Sterechinus neumayeri. The number of strains for each bacterial genera are shown in parentheses.
The phylogenetic tree constructed with sequences obtained from isolated strains and those used for phylogenetic assignment is presented in Fig. 1. The sequences obtained from a culture-dependent method are grouped into seven Families (Shewanellaceae, Pseudomonadaceae, Microbacteriaceae, Flavobacteriaceae, Moraxellaceae, Pseudoalteromonadaceae and Colwelliaceae) with a total of 11 genera. Of 42 strains, 25 were γ-Proteobacteria from four genera: Psychrobacter (n=9), Pseudomonas (n=8), Pseudoalteromonas (n=6) and Shewanella (n=2) (Fig. 2). In terms of phylogenetic diversity, Flavobacterium was the most diverse, showing nine different sequences, followed by Psychrobacter (n=6)=Pseudoalteromonas (n=6)>Pseudomonas (n=4)>Shewanella (n=1)=Microbacterium (n=1)=Agreia (n=1)=Persivirga (n=1)=Leeuwnhokella (n=1)=Colwellia (n=1) (Fig. 2; Table S1 found at http://dx.doi.org/10.1017/S0954102016000109).

Fig. 2 Phylogenetic analysis of 16S sequences isolated from the coelomic fluids of the Antarctic sea urchin Sterechinus neumayeri. The tree is based on the maximum-likelihood principle using 784 positions of the 16S amplicons alignment. A number of reference strain sequences are also included: A-Shewanellaceae, B-Pseudomonadaceae, C-Microbacteriaceae, D-Flavobacteriaceae, F-Moraxellaceae, G-Pseudoalteromonadaceae and H-Colwelliaceae.
The majority (n=21) of the 38 bacterial strains analysed were sensitive to all nine of the antibiotics tested (Table I). All of the isolates were sensitive to trimethoprim/sulphamethoxazole. Most of the strains were resistant to ampicillin (n=15), chloramphenicol (n=13) and cefalotin (n=12). A small number of the strains were resistant to cefotaxime (n=8), amikacin (n=3), gentamicin (n=3), nalidixic acid (n=2) and tetracycline (n=1) (Table I). Seventeen of the isolates were resistant to more than one of the antibiotics tested. The strains that most commonly demonstrated multi-drug resistance (i.e. resistance to at least three different antibiotics) belonged to Pseudomonas, Flavobacterium and Psychrobacter genera. Some of the Flavobacterium were resistant to five or more antibiotics. For example, strain E4R was resistant to seven of the nine antibiotics tested, while strains E6FA6 and E29R were resistant to six and five antibiotics, respectively.
Table I Resistance profiles and presence of plasmids in bacterial strains isolated from coelomic fluids of Antarctic sea urchins (Sterechinus neumayeri).

AMK: amikacin, AMP: ampicillin, CAF: chloramphenicol, CEF: cefalotin, CTX: cefotaxime, GEN: gentamicin, NAL: nalidixic acid, SXT: trimethoprim/sulphamethoxazole, TET: tetracycline.
ND: not determined, R: resistant strain, S: sensitive strain.
Hg: mercury, Zn: zinc.
+: strain with plasmid, -: strain without plasmid.
All of the 38 strains exposed to three heavy metals were sensitive to silver. None of the isolates grew in the presence of silver, even at the lowest concentration (MIC<32 µg ml-1). Twelve bacterial strains (mainly from the Flavobacterium genera) were resistant to either mercury or zinc. Flavobacterium and Psychrobacter were resistant to mercury and zinc at MIC50<16 µg m l-1 and <1024 µg ml-1, respectively. Only one Pseudomonas strain was resistant to zinc. Three strains belonging to Flavobacterium (E6FA4, E6FA6) and Psychrobacter (E6FA5) showed resistance to metals. The Flavobacterium strain E6FA6 showed co-resistance to metal and antibiotics (seven antibiotics; Table I). This strain has high identity with Flavobacterium frigidarium.
Of the 38 strains, 17 (44.7%) and 12 (31.5%) showed resistance to antibiotics and metal, respectively. Seven (18.4%) strains showed co-resistance, representing half of the bacteria that showed some kind of resistance (Fig. 3a).

Fig. 3 Resistance to antibiotics and metals in bacteria from the Antarctic sea urchin Sterechinus neumayeri. a. Comparison of resistance to antibiotics and metals in bacterial isolates. b. Distribution of most resistant bacteria at genera level.
Of the strains that were resistant to antibiotics, metals or had co-resistance, 33 were Flavobacterium sp. (Fig. 3b). Psychrobacter bacteria also showed a higher resistance to mercury and zinc. In contrast, a higher percentage of Pseudomonas spp. were resistant to antibiotics. However, equal numbers of Pseudomonas spp. and Psychrobacter spp. showed co-resistance.
The presence of extrachromosomal DNA was analysed in 38 bacterial strains. Plasmid DNA bands were detected in 13 bacterial strains (32.5%). Bacteria belonging to Flavobacterium had the highest frequency of plasmid incidence (15%), followed by Pseudomonas (7.5%), Psychrobacter (5.0%), Microbacterium (2.5%) and Shewanella (2.5%) (Table I). Some of the plasmid bearing strains had one or two plasmid bands with sizes ranging from 0.2 to >1.0 kb (Fig. S1 found at http://dx.doi.org/10.1017/S0954102016000109). A weak correlation was found between the presence of plasmids and metal resistance, as well as plasmids and co-resistance (Table II). PERMANOVA results confirmed significant differences in the response of bacterial strains associated with plasmids in relation to metal resistance (F1,37=27.632, P<0.001) and co-resistance (F1,37=47.368, P<0.001).
Table II Pearson correlation coefficient (r) between the presence of plasmids and resistance against one antibiotic, multi-drug resistance to antibiotics, metal resistance and co-resistance (metals and antibiotics).
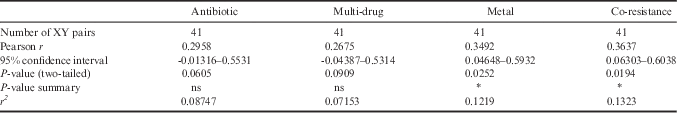
* Statistically significant, ns: non-significant.
Discussion
This is the first report of multi-drug and metal resistant cultivable bacteria in Antarctic echinoderms. Our results show the presence of multi-antibiotic and metal resistant bacterial isolates in the coelomic fluids of the Antarctic sea urchin S. neumayeri. These findings provide useful information for understanding the role of the bacterial community as a reservoir of resistance genes in pristine environments.
Sea urchins have an internal environment highly similar to seawater. Bacteria from the environment, such as Flavobacterium, Psychrobacter and Pseudoalteromonas, are also found in sea urchins. Unfortunately, the bacterial flora associated with Antarctic marine organisms have received relatively little attention. Bacteria isolated from S. neumayeri belong to three phyla, namely Proteobacteria (class γ-Proteobacteria), Bacteroidetes (class Flavobacteria) and Actinobacteria (class Actinobacteria). The predominant culturable bacterial group in S. neumayeri belong to γ-Proteobacteria, with 25 isolates affiliated to the Pseudoalteromonas, Psychrobacter, Shewanella and Pseudomonas genera. The presence of these groups of bacteria is consistent with studies of several marine sediment samples from Antarctica (Maugeri et al. Reference Maugeri, Gugliandolo and Bruni1996, Michaud et al. Reference Michaud, Di Cello, Brilli, Fani, Lo Giudice and Bruni2004, De Souza et al. Reference De Souza, Nair, Bharathi and Chandramohan2006, Tropeano et al. Reference Tropeano, Coria, Turjanski, Cicero, Bercovich, Mac Cormack and Vazquez2012). The phyla Bacteroidetes, represented by Flavobacteria, is commonly found in Antarctic biotopes (Webster et al. Reference Webster, Negri, Munro and Battershill2004, De Souza et al. Reference De Souza, Nair, Bharathi and Chandramohan2006). In addition, Pseudomonas, Pseudoalteromonas and Psychrobacter were recovered from almost all samples showing their abundance in Antarctic coastal ecosystems (Tropeano et al. Reference Tropeano, Coria, Turjanski, Cicero, Bercovich, Mac Cormack and Vazquez2012, Lo Giudice et al. Reference Lo Giudice, Casella, Bruni and Michaud2013).
Interestingly, the coelomic fluid bacterial flora of the Antarctic sea urchin is very different to that found in sea urchins from temperate and tropical waters. Bacteria from S. neumayeri are predominantly psychrophilic. In contrast, bacteria from coelomic fluids of the European edible sea urchin (Echinus esculentus L.) is dominated by Vibrio, Pseudomonas, Flavobacterium and Aeromonas bacteria, although in low numbers (Unkles Reference Unkles1977). Similarly, the coelomic fluids of the purple sea urchin (Strongylocentrotus purpuratus (Stimpson)) are dominated by Aeromonas, Flavobacterium, Pseudomonas and Vibrio (Gilles & Pearse Reference Gilles and Pearse1986), and Paracentrotus lividus (Lamarck) is dominated by the plus γ-Vibrionaceae (Becker et al. Reference Becker, Egea and Eeckhaut2008).
The results of our antibiotic resistance study are consistent with those previously reported for seawater, sponges, soil, freshwater lakes and penguin guano sampled from different parts of the Antarctic (De Souza et al. Reference De Souza, Nair, Bharathi and Chandramohan2006, Miller et al. Reference Miller, Gammon and Day2009, Mangano et al. Reference Mangano, Michaud, Caruso and Lo Giudice2014, Tam et al. Reference Tam, Wong, Yong, Blamey and Gonzalez2015). Psychrotrophic bacteria are commonly found to be resistant to conventional antibiotics such as ampicillin, chloramphenicol, kanamycin and streptomycin (De Souza et al. Reference De Souza, Nair, Bharathi and Chandramohan2006, Miller et al. Reference Miller, Gammon and Day2009, Lo Giudice et al. Reference Lo Giudice, Casella, Bruni and Michaud2013). The percentage of bacteria that are resistant to ampicillin reported here is similar to values (26–28%) reported for bacteria isolated from shallow marine sediment in Terra Nova Bay, Ross Sea (Mangano et al. Reference Mangano, Michaud, Caruso and Lo Giudice2014). In contrast, De Souza et al. (Reference De Souza, Nair, Bharathi and Chandramohan2006) found a much higher percentage of resistant bacteria among isolates from Antarctic seawater.
The resistance profiles to ampicillin and chloramphenicol among the bacteria in this study are very similar to the resistance to cephalotin, which is a first-generation cephalosporin. Miller et al. (Reference Miller, Gammon and Day2009) reported similar results in previous studies conducted with bacteria isolated from pristine environments. However, surprisingly important percentages (19%) of the bacteria were also resistant to cefotaxime, a third-generation cephalosporin. This result strongly suggests that the Gram-negative bacteria isolated from S. neumayeri probably produce AmpC or ESBL as an important mechanism to provide resistance to β-lactam broad spectrum antibiotics (Davies & Davies Reference Davies and Davies2010). Feller et al. (Reference Feller, Sonnet and Gerday1995) reported a class C β-lactamase in strains of Psychrobacter immobilis Juni and Heym collected near the Dumont d’Urville Station, and this enzyme exhibited cephalosporinase activity. In our research, the resistance to cefotaxime was characteristic for several strains isolated from S. neumayeri belonging to Psychrobacter and Flavobacterium, while resistance to cephalotin was observed among strains of Pseudomonas, Psychrobacter and Flavobacterium.
It is possible that some Antarctic marine invertebrates like sponges, mollusks or echinoderms may be able to accumulate essential and non-essential heavy metals at higher concentrations in their tissues (Bargagli et al. Reference Bargagli, Nelli, Ancora and Focardi1996, De Moreno et al. Reference De Moreno, Gerpe, Moreno and Vodopivez1997, Negri et al. Reference Negri, Burns, Boyle, Brinkman and Webster2006). Bacterial resistance to certain metals, such as zinc, can be explained by the need for optimum metabolic functions in some bacteria. Our results are in accordance with the only previous report existing on zinc tolerance in S. neumayeri (Moreno et al. Reference De Moreno, Gerpe, Moreno and Vodopivez1997), which showed high concentrations of zinc in tissues of S. neumayeri. In the case of mercury and cadmium, bacteria have probably developed resistance through constant exposure to toxic compounds in the environment or accumulation in the host tissues (Truzzi et al. Reference Truzzi, Annibaldi, Illuminati, Bassotti and Scarponi2008, Mangano et al. Reference Mangano, Michaud, Caruso and Lo Giudice2014).
The Antarctic bacteria analysed in this study were resistant to mercury and zinc but not silver. Our results on the tolerance levels are consistent with studies conducted on bacteria from seawater, marine sediments and sponges (De Souza et al. Reference De Souza, Nair, Bharathi and Chandramohan2006, Mangano et al. Reference Mangano, Michaud, Caruso and Lo Giudice2014). However, the predominant bacteria from these biotopes exhibited resistance to different types of metals. For example, Flavobacterium and Psychrobacter are predominantly resistant to mercury and zinc, while Psychrobacter and Pseudoalteromonas bacteria from the Antarctic sponge (Hemigellius pilosus (Kirkpatrick)) showed high tolerance levels to mercury (Mangano et al. Reference Mangano, Michaud, Caruso and Lo Giudice2014). However, in our study all Pseudoalteromonas strains from S. neumayeri were found to be sensitive to mercury.
The multi-drug and metal resistance among Antarctic bacteria is probably conferred by genes on plasmids (Smith et al. Reference Smith, Howington and McFeters1993, Bennett Reference Bennett2008). The percentage of plasmid occurrence in this study is 32.5%, which is high compared to reports from several Antarctic matrices of 14–23% (Kobori et al. Reference Kobori, Sullivan and Shizuya1984, Michaud et al. Reference Michaud, Di Cello, Brilli, Fani, Lo Giudice and Bruni2004, Miller et al. Reference Miller, Gammon and Day2009). These results suggest that mobile genetic elements, such as plasmids, probably promote the transfer of resistance genes from one bacterium to another in the coelomic fluids. Hence, the possibility of native Antarctic bacteria harbouring multi-drug resistance traits via horizontal gene transfer should be addressed in future studies.
Natural ecosystems do not harbour many human pathogens, and Antarctica is considered to be a non-polluted continent. However, in recent years, antibiotic-resistant bacteria have been isolated in the Antarctic (Hernandez et al. Reference Hernandez, Stedt, Bonnedahl, Molin, Drobni, Calisto-Ulloa, Gomez-Fuentes, Astorga-España, González-Acuña, Waldenström, Blomqvist and Olsena2012). The increasing number of scientific and tourist activities may introduce non-native microorganisms, such as viruses, bacteria or fungi (Hughes et al. Reference Hughes, Convey, Maslen and Smith2010, Cowan et al. Reference Cowan, Chown, Convey, Tuffin, Hughes, Pointing and Vincent2011). Furthermore, the release of sewage and spreading of faecal matter in water bodies near Antarctic stations may propagate antibiotic resistance among bacteria. In this regard, Antarctic marine invertebrates could constitute a reservoir for antibiotic-resistant bacteria, as occurs in environments highly impacted by human activities.
Acknowledgements
This investigation was funded by a grant from the Chilean Antarctic Scientific Program (PROCIEN) in the frame of FONDECYT grant number 11090265 and INACH grant T 17-08. We thank the reviewers for their thoughtful and constructive comments which improved the manuscript.
Author contribution
MGA & GGR designed the research. RU, KDC, PL & MGA performed the majority of microbiology analyses. CAC & PL undertook the statistical analyses. MGA, PL, CAC, CMVLW and GGR contributed to writing of the manuscript.
Supplementary Material
A supplemental table and figure will be found at http://dx.doi.org/10.1017/S0954102016000109.








