Introduction
It is thought that the embryonic development of medaka eggs (Oryzias latipes) is unaffected by transplantation of embryonic cell nuclei as some transplants survive to reach the adult stage (Niwa et al., Reference Niwa, Ladygina, Kinoshita, Ozato and Wakamatsu1999; Wakamatsu et al., Reference Wakamatsu, Ju, Pristyaznhyuk, Niwa, Ladygina, Kinoshita, Araki and Ozato2001). In contrast, zebrafish (Danio rerio) and medaka eggs do not develop to a more advanced stage, even after reaching the larval stage, following transplantation of cultured somatic cell nuclei (Lee et al., Reference Lee, Huang, Ju, Yang and Lin2002; Ju et al., Reference Ju, Pristyazhnyuk, Ladygina, Kinoshita, Ozato and Wakamatsu2003).
At present, it is not possible to predict adult phenotypes during the embryonic stage. Thus, it is not until the fish have grown sufficiently that it becomes possible to select individuals that are suitable donors, based on their expression of desirable traits. The success of somatic cell nuclear transplantation is, therefore, essential to fish cloning.
Nuclear transplantation may be used to produce individuals that have rare traits. However, it is not currently possible to mass-produce a specific breed using this technique. Our objective was to evaluate whether somatic cell nuclear transplantation may be used to clone the highly valued Ranchu breed of goldfish (Carassius auratus auratus).
Material and methods
Fish
We used the goldfish breeds Wakin and Ranchu as sources of recipient eggs and donor cells, respectively. The Wakin breed represents the original and most common form of goldfish, having a body shape that resembles crucian carp (Carassius spp.) (Fig. 1A). Ranchu is a high-quality breed of goldfish that has a unique body shape with a cephalocele and lacking the dorsal fin, but having triple or quadruple caudal fins (Fig. 1B).

Figure 1 Recipient and donor fish. (A) Non-enucleated unfertilized eggs of ‘Wakin’ fish. (B) Somatic cells of ‘Ranchu’ fish. Bar = 50 mm.
Preparation of recipient eggs
We collected recipient eggs from previously unspawned Wakin breed females for each experiment. We induced ovulation by injecting each female intraperitoneally with 10 IU/g body weight human chorionic gonadotropin. The mature eggs were collected by gently massaging the abdomen. The eggs were placed in water for 3 min then treated with 0.25% trypsin solution to remove the egg membrane. Following this, the eggs were washed with Holtfreter's solution then incubated in this solution until the protoplasm accumulated at the animal pole.
Preparation of donor cells
We collected donor cells from one adult male Ranchu and one adult female Ranchu. Cultured cells from either individual were used in each nuclear transplant experiment. The primary cells were obtained by removing a section of the caudal fin. The caudal tissue was then washed in phosphate-buffered saline (PBS), sterilized with Dakin's solution, rewashed with PBS, and placed in a dish containing the Dakin's solution. A small amount of the epidermis was removed from the surface with a pair of tweezers. Each sample of caudal fin tissue was minced to yield approximately 3 mm2 fragments then fixed to the bottom of another dish. We added Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum Cellect™ and 60 mg/l kanamycin to the dish and incubated the cells for 1–2 weeks. We subcultured fibroblasts for three passages and collected the confluent state cells by centrifugation prior to nuclear transplantation.
Nuclear transplantation
The nuclear transplantation procedure is illustrated in Fig. 2. Nuclear transplantation was carried out under a stereoscopic microscope (SZX-12, Olympus) at room temperature (18°C). A 1.5 mm layer of agarose gel was laid on the bottom of a 90 mm petri dish. We excised and discarded half of the gel from the center of the plate. We then cut notches in the exposed cross section to hold the recipient eggs. We added Holtfreter's solution to the dish and placed the recipient eggs in their individual notch. The donor cells were floated in Holtfreter's solution, aspirated into a micropipette (15–20 μm internal diameter, Borosilicate Glass, Sutter Instruments) at a needle tip angle of 30°, and dispersed individually in a pipette using manipulator pressurization and depressurization to gently lyse the cell membrane. The treated eggs were then transferred to a glass dish filled with 80% Holtfreter's solution containing 0.01% kanamycin. Control eggs were obtained from matings between female fish that provided the recipient eggs and male fish that were used for the production of donor cells.
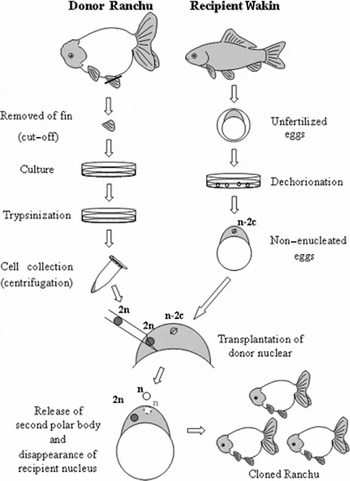
Figure 2 Schematic of the nuclear transplantation procedure for production of cloned Ranchu using cultured somatic cells.
Polyploidy determination
We analysed the ploidy of the nuclear transplanted embryos, the recipient parent fish fin tissue cells, and the cultured donor cells. We used round shiner (Gnathopogon elongates, Cyprinidae) erythrocytes as a control. The chromosome count of this species (2n = 50) is half that of the goldfish (2n = 100). We extracted and stained the cell nuclei using a Ploidy Analyzer kit (Partec). The ploidy was determined by measuring the amount of DNA in the cell nucleus using a flow cytometer.
RAPD and microsatellite analyses
We evaluated whether the nuclei of the nuclear transplants were derived from the recipient cells, donor cells, or both using the same tissues described in the previous section. Nuclear transplantation and control DNA samples (extracted as described above) were stored in TNES-urea buffer (Asahida et al., Reference Asahida, Kobayashi, Saitoh and Nakayama1996). The samples were digested with proteinase K and the DNA was extracted using phenol–chloroform. We used the random primer A-10 (5′-GTGATCGCAG-3′) from the Operon 10mer kit A (Operon Technologies Inc.) for RAPD analysis. RAPD PCR amplification was conducted in a total volume of 12.5 μl containing 50 ng of genomic DNA, 1.25 μl of 10× dNTP, 1.25 μl of the primer, 1.25 μl of 10× Taq buffer, and 0.125 μl of Taq DNA polymerase. We used the following thermal cycle: 5 min at 94°C, followed by 1 cycle of 1 min at 94°C and 1 min at 36°C, 45 cycles of 2 min at 94°C, and a final extension of 7 min at 72°C. The PCR amplification product was electrophoresed using a 2% agarose gel. We used Gf17 primers (forward primer, 5′-GGAACTAGAGCCCACTGACA-3′; reverse primer, 5′-TGCATTTGGGAGACGATA-3′) during the microsatellite analysis (Zheng et al., Reference Zheng, Stacey, Coffin and Strobeck1995). Microsatellite PCR amplification was conducted in a total volume of 25 μl containing 85 ng of genomic DNA, 1.2 μl of 10× dNTP, 0.85 μl of the primer, 1.5 μl of 10× Taq buffer, and 0.1 μl of Taq DNA polymerase. The thermal conditions were: 3 min at 94°C, followed by two cycles of 30 s at 94°C, 20 s at 58°C, and 1 s at 72°C, 35 cycles of 15 s at 94°C, 20 s at 58°C, and 1 s at 72°C. There was a final extension of 30 s at 72°C. The PCR amplification product was electrophoresed in a 7.5% polyacrylamide gel.
Results
Survival rates of nuclear transplants
All eight tests using non-enucleated, unfertilized eggs transplanted with fin cell nuclei yielded viable eggs. Of the 2323 eggs that received a transplant, 802 (34.4%) underwent cleavage, 321 (13.8%) survived to the blastula stage, and 51 (2.2%) reached the gastrula stage. A considerable number of the eggs that underwent cleavage exhibited abnormal division. However, division did appear to be normal in several of the eggs. Eleven (0.5%) of the gastrula developed up to the hatching stage (Table 1) and two transplants hatched successfully.
Table 1 Number and percentage (in brackets) of nuclear transplants and controls surviving to each stage.

a NT refers to individuals examined by nuclear transplantation. The numbers 1–8 denote recipient individual numbers; a, b denote donor individual numbers (a: male; b: female).
b Control is fertilized eggs of ‘Wakin’.
c No nuclear transplants survived.
d NM, not measured.
Polyploidy of nuclear transplants
We analysed the ploidy of the four nuclear transplants that reached the segmentation and hatching stages (Fig. 3). Three of these were diploid (Fig. 3C, Table 2) and the remaining individual could not be analyzed because of the limited amount of DNA in the sample. The recipient egg provider (Wakin, eight parents) and the donor cell provider (Ranchu, one male parent and one female parent) were diploid (Fig. 3A, B).

Figure 3 Histograms of the relative DNA content of somatic cells of the donor, recipient, and nuclear transplants when erythrocytes of Gnathopogon elongatus elongates (2n = 50) are used as control. The solid peak represents the control and the open peaks represent the donor, recipient, and nuclear transplants. (A) Donor diploid Ranchu (2n = 100). (B) Recipient diploid Wakin (2n = 100). (C) Diploid nuclear transplant.
Table 2 Polyploidy and possession states of nucleus in nuclear transplants.

a D denotes that nuclear transplants have a donor nucleus, R denotes that they have a recipient nucleus, and D + R denotes that they have a nucleus derived from both donor and recipient.
b Not measured.
Genome composition of nuclear transplants
The Wakin and Ranchu breeds were easily distinguished using both RAPD and microsatellite analyses. Both analyses yielded a band specific to Wakin and a band specific to Ranchu. Analysis of the DNA extracted from the recipient Wakin and the donor Ranchu and nuclear transplants (Figs. 4 and 5) revealed that, of the 18 nuclear transplants, 15 had only a donor nucleus, one had only the recipient nucleus, and the remaining two had both the donor and recipient nuclei (Table 2). There was no clear electrophoretic pattern for one individual in the gastrula stage.

Figure 4 RAPD analysis of nuclear transplants. (A) 100-bp DNA ladder; (B) recipient Wakin; (C) Ranchu donor cell; (D–F) nuclear transplants. All of the nuclear transplants had a donor nucleus. The white arrowheads indicate a band specific for the Ranchu nucleus.

Figure 5 Microsatellite analysis of nuclear transplants. (A) φX174 HaeIII digest; (B) recipient Wakin; (C) Ranchu donor cell; (D–G) nuclear transplants. Nuclear transplants of (G) had a nucleus derived from the recipient alone. The white arrowhead indicates a band specific for a donor nucleus.
Discussion
Generation of fin-cultured cell nuclear transplants
More than one-third of the nuclear transplanted eggs underwent cleavage. Unfortunately, the survival rate of the embryos decreased with development. However, a small number of hatched larvae were obtained in one of the experiments. Our results are consistent with those reported in zebrafish (Liu et al., Reference Liu, Yu, Zhou, Wang, Tong and Wu2002). In another study, Ju et al. (Reference Ju, Pristyazhnyuk, Ladygina, Kinoshita, Ozato and Wakamatsu2003) attempted the transplantation of caudal fin cultured cell nuclei from adult GFP transgenic medaka into the non-enucleated unfertilized eggs of orange-red medaka. The authors reported that embryonic development was generally successful, leading to the production of some larval fish. In this instance, it is thought that the nuclear division cycle of the donor nuclei was synchronized with that of unfertilized recipient eggs by endogenous factors. In general, the markedly decreased survival rate of late stage embryos is likely caused by insufficient reprogramming of the donor nuclei.
There have been attempts to improve the developmental success of nuclear transplanted eggs in a number of animal species. Cell cycle synchronization to the G0/G1 phase by serum starvation culture has improved the success rate of attempts to clone mammals (Wilmut et al., Reference Wilmut, Schnieke, McWhir, Kind and Campbell1997; Kato et al., 1998) and has also been trialed in fish cloning (Liu et al., Reference Liu, Yu, Zhou, Wang, Tong and Wu2002). In mammals, nuclear transplantation after treatment of donor cells with trichostatine A® (Kishigami et al., Reference Kishigami, Mizutani, Ohta, Hikichi, Thuan, Wakayama, Bui and Wakayama2006), a deacetylase inhibitor, and roscovitine® (Gibbons et al., Reference Gibbons, Arat, Rzucidlo, Miyoshi, Waltenburg, Respess, Venable and Stice2002), a cyclin-dependent kinase, has led to an improvement in the developmental ability of nuclear transplanted eggs. A recent study also suggested that the reprogramming of somatic cell nuclei could be induced by treatment with amphibian egg cell extract (Hansis et al., Reference Hansis, Barreto, Maltry and Niehrs2004). Attempts to reprogramme donor cells are also considered necessary for the success of nuclear transplantation using cultured somatic cells from teleosts.
Feasibility of cloning by somatic cell nuclear transplantation
The use of non-enucleated, unfertilized eggs as the recipients of nuclear material from fish embryos (Gasaryan et al., Reference Gasaryan, Hung, Neyfakh and Ivanenkov1979; Tanaka et al., Reference Tanaka, Takahashi and Ueno2009) and somatic cells (Liu et al., Reference Liu, Yu, Zhou, Wang, Tong and Wu2002) leads to an enhanced generation of nuclear transplants having only a 2n donor nucleus. Our results suggest that 80% of the nuclear transplants had only a donor nucleus. Among these transplants, three were diploid, two of which were produced by cultured cells from the same donor. The cytological mechanism underlying the formation of individuals having only a 2n donor nucleus is unclear. However, our results suggest that it is technically feasible to clone goldfish.
The use of non-enucleated unfertilized eggs has been associated with the formation of 100% triploids (Niwa et al., Reference Niwa, Ladygina, Kinoshita, Ozato and Wakamatsu1999) or with individuals that have chromosome counts that are not compatible with the formation of a normal diploid (Ju et al., Reference Ju, Pristyazhnyuk, Ladygina, Kinoshita, Ozato and Wakamatsu2003). Differences in the time course of recipient egg meiosis after nuclear transplantation and the phase of the cell cycle of donor cells for nuclear transplantation are thought to cause the various combinations between a recipient nucleus and a donor nucleus. Given this, successful cloning attempts will rely on optimization of the timing for nuclear transplantation and a behavioural analysis of the recipient and donor nuclei following nuclear transplantation.
Based on the current definition of cloned animals, it may be inappropriate to refer to transplants that have only a 2n donor nucleus obtained by nuclear transplantation to non-enucleated eggs as clones. However, if the recipient nucleus or chromosome is inactivated or removed following the transplantation of a donor nucleus by cytological mechanisms, the result will be the same as that of nuclear transplantation to enucleated eggs. Because the enucleation of recipient eggs requires much effort and may cause the physical impairment of fish eggs, the use of non-enucleated recipient eggs may be preferable.
Acknowledgements
The authors wish to thank Professor Yukio Tsunoda of the Faculty of Agriculture, Kinki University, for helpful suggestions and critical reading of the manuscript. We are also grateful to Mr Yoshio Koyanagi of the Ohno-Ranchu Fish Farm for providing the samples and to Drs Takashi Suzuki, Satoshi Otani, Naoki Yagishita and Masahiro Nakagawa for technical assistance. This study was supported in part by the Global COE programme of the Ministry of Education, Culture, Sports, Science, and Technology of Japan.









