Introduction
The rostral cingulate cortex has a central role in the generation of affect (MacLean, Reference MacLean1955). Alterations in its activity are important for depression, which has been associated with structural, functional and biochemical changes in various cingulate subregions (Mayberg et al. Reference Mayberg, Liotti, Brannan, McGinnis, Mahurin, Jerabek, Silva, Tekell, Martin, Lancaster and Fox1999; Auer et al. Reference Auer, Pütz, Kraft, Lipinski, Schill and Holsboer2000; Caetano et al. Reference Caetano, Kaur, Brambilla, Nicoletti, Hatch, Sassi, Mallinger, Keshavan, Kupfer, Frank and Soares2006; Drevets et al. Reference Drevets, Savitz and Trimble2008b ; Gabbay et al. Reference Gabbay, Mao, Klein, Ely, Babb, Panzer, Alonso and Shungu2012). The rostral cingulate cortex can be divided into two regions on the basis of structure and function, the anterior cingulate cortexFootnote 1 Footnote † (ACC) and the mid-cingulate cortex (MCC), which can be further divided into subregions. Subgenual ACC mediates the relationship between affect and visceromotor function (Freedman et al. Reference Freedman, Insel and Smith2000) whereas pregenual ACC is important in reward-based learning and the assessment of affective valence (Phan et al. Reference Phan, Wager, Taylor and Liberzon2002; Rogers et al. Reference Rogers, Ramnani, Mackay, Wilson, Jezzard, Carter and Smith2004). Within the MCC, the anterior portion is activated by control processes associated with cognition, affect and nociception (Shackman et al. Reference Shackman, Salomons, Slagter, Fox, Winter and Davidson2011), whereas posterior MCC is more closely associated with skeletomotor function (Vogt & Vogt, Reference Vogt and Vogt2003). The relationship between the subregions is complex, and there are overlapping contributions from the subregions to many affective, cognitive and motor processes. Nonetheless, the rostral cingulate clearly subserves discrete functions along its axis (Vogt, Reference Vogt2005), and there is a need for more systematic investigation of cingulate dysfunction in depression to further our understanding of how it contributes to the illness.
The recent development of resting-state functional connectivity magnetic resonance imaging (fcMRI) has allowed an examination of disturbances in the functional organization of brain systems in psychiatric disorders (Fornito & Bullmore, Reference Fornito and Bullmore2010; Fox & Greicius, Reference Fox and Greicius2010; Zhang & Raichle, Reference Zhang and Raichle2010). Functional connectivity is defined by correlated spontaneous fluctuations of the blood oxygenation level-dependent (BOLD) signal between remote brain regions; with the observation that regions with similar functional properties exhibit strong connectivity under resting, or non-task, imaging conditions. These ‘functional networks’ closely parallel underlying axonal–anatomical connections, representing slow fluctuations of neuronal excitability that reflect both enduring and dynamic functional activities (Harrison et al. Reference Harrison, Pujol, Ortiz, Fornito, Pantelis and Yücel2008b ; Fair et al. Reference Fair, Cohen, Power, Dosenbach, Church, Miezin, Schlaggar and Petersen2009; Shehzad et al. Reference Shehzad, Kelly, Reiss, Gee, Gotimer, Uddin, Lee, Margulies, Roy, Biswal, Petkova, Castellanos and Milham2009). fcMRI has been used to successfully localize functional connectivity disturbances in several psychiatric disorders, including being able to demonstrate for some illnesses correlations between the magnitude of connectivity disturbances and measures of illness severity (Fornito & Bullmore, Reference Fornito and Bullmore2010; Fox & Greicius, Reference Fox and Greicius2010; Zhang & Raichle, Reference Zhang and Raichle2010).
Several fcMRI studies in depression have implicated alterations in connectivity with the rostral cingulate cortex. Subgenual ACC has been reported to show increased connectivity with the dorsomedial frontal cortex in two studies (Sheline et al. Reference Sheline, Price, Yan and Mintun2010; Hamilton et al. Reference Hamilton, Chen, Thomason, Schwartz and Gotlib2011), whereas another study reported reduced connectivity between subgenual ACC and an anterior midline region extending from pregenual ACC to MCC, including the medial frontal cortex (Cullen et al. Reference Cullen, Gee, Klimes-Dougan, Gabbay, Hulvershorn, Mueller, Camchong, Bell, Houri, Kumra, Lim, Castellanos and Milham2009). The reasons for the latter finding are unclear, although it is of note that participants were listening to music of their choice during the acquisition of the resting-state sequences (and the study was not, therefore, truly resting state). Alterations in connectivity with the anterior MCC have also been reported, with demonstration of reduced connectivity with striatum, amygdala and medial thalamus (Anand et al. Reference Anand, Li, Wang, Wu, Gao, Bukhari, Mathews, Kalnin and Lowe2005).
Although several studies have used fcMRI to examine alterations in functional connectivity of the cingulate cortex in depression, they have not examined how connectivity varies across the functionally diverse subregions of the cingulate cortex. In this study we undertook a systematic approach to assessing functional connectivity of multiple subregions of the rostral cingulate cortex in young depressed participants. We included participants from late adolescence and early adulthood, whose relatively young age and early stage of illness meant that their depressive symptomatology was less influenced by the accumulated effects of lifestyle factors and treatments, thereby potentially providing a clearer demonstration of the primary functional pathophysiology of depression. We predicted that we would confirm the role of subgenual ACC in depression, providing important confirmation of increased connectivity with the dorsomedial frontal cortex. In addition, we aimed to characterize the contributions of the other cingulate subregions, hypothesizing that connectivity differences would arise more often with ventral (ACC) compared to dorsal (MCC) regions.
Method
Participants
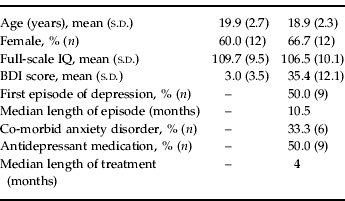
Depressed participants were recruited for the study from Orygen Youth Health, a public mental health service for young people in Melbourne, Australia. Patients were between 15 and 24 years of age, and had major depressive disorder determined by the Structured Clinical Interview for DSM-IV (SCID; First et al. Reference First, Spitzer, Gibbon and Williams1997). Patients were included from an age range that extended from the middle teenage years to early adulthood, which accords with a clinical focus on youth mental health (McGorry, Reference McGorry1998) and is consistent with our current understanding of the continuities in brain and social development through this period (Davey et al. Reference Davey, Yucel and Allen2008). Patients did not meet the criteria for psychotic disorder, substance dependence disorder, pervasive developmental disorder or intellectual disability, and were not excluded if they were taking antidepressant medication. The depressed participants were successfully matched with a group of control participants on age, gender and full-scale IQ (Table 1). The control participants were recruited through advertisement placed in a daily metropolitan newspaper, and had no history of mental illness, as determined by the SCID. The participants (and their parents if they were younger than 18 years of age) provided their informed consent to participate in the study, which was approved by the local research and ethics committees. Imaging data from one depressed participant were excluded because of excessive head movements during scanning, resulting in a control group of 20 participants and a depressed group of 18 participants.
Table 1. Characteristics of the control and depressed participants
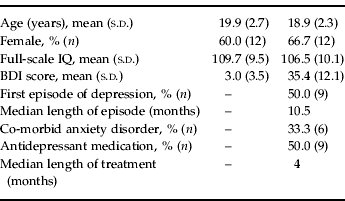
BDI, Beck Depression Inventory; s.d., standard deviation.
The illness characteristics of the depressed participants reflected their relatively young age and their recruitment through a public mental health service that treats young people with relatively severe illness. The mean Beck Depression Inventory (BDI-II; Beck et al. Reference Beck, Steer and Brown1996) score for the depressed participants of 35.4 indicates that their illnesses were at the severe end of the illness spectrum. Six of the participants had co-morbid anxiety disorders: these were panic disorder (n=2), social anxiety disorder (n=1), generalized anxiety disorder (n=1), and combined panic disorder and generalized anxiety disorder (n=2). Nine of the depressed participants were being treated with antidepressant medication: fluoxetine (n=4), citalopram (n=2) and venlafaxine (n=3). Using the categorization of dose strengths outlined by Hansen et al. (Reference Hansen, Moore, Dusetzina, Leinwand, Gartlehner and Gaynes2009), two patients were taking a low dose of medication, six patients a medium dose, and one patient a high dose. The BDI-II scores for the medicated (32.5) and unmedicated patients (38.3) were not significantly different (p=0.32).
Image acquisition and preprocessing
A 3-T Siemens Magnetom Trio magnetic resonance scanner (Erlangen, Germany) was used to acquire whole-brain functional T2*-weighted echo-planar images. Functional sequences consisted of gradient-recalled acquisition in the steady state (repetition time=2400 ms, echo time=40 ms, pulse angle=90°) within a field of view of 210 mm, with a 64×64-pixel matrix, and a slice thickness of 3 mm (no interslice gap). Thirty-six interleaved slices were acquired parallel to the anterior–posterior commissure line, with particular attention paid to ensuring good coverage of ventral brain areas. Fieldmaps were also acquired to correct for distortion caused by magnetic field inhomogeneities. For each participant, a single 12.3-min continuous functional sequence was acquired, generating 307 whole-brain echo-planar imaging volumes. In addition, high-resolution T1-weighted anatomical images were acquired to aid registration of the functional images to standard space. During scanning, participants were provided with earphones to reduce scanner noise, and foam rubber inserts were used to aid head stability. Participants were instructed to relax, stay awake, and lie still without moving while keeping their eyes closed throughout. In post-scan assessments, all participants reported remaining awake throughout the scan.
Imaging data were transferred and processed on an Apple OSX platform running MATLAB version 7 (MathWorks, USA) and used Statistical Parametric Mapping 8 (SPM8; www.fil.ion.ucl.ac.uk/spm/). Motion correction was performed by aligning each participant's time series to the first image using least-squares minimization and a six-parameter (rigid body) spatial transformation, and the fieldmaps were used to unwarp the images. Participants' data were excluded from analysis if translation and rotation estimates were greater than 2 mm or 2° respectively, and excessive movement in the z axis resulted in the exclusion of data from one depressed participant. The realigned and unwarped functional sequences were then co-registered to each participant's respective anatomical scan. Anatomical scans were segmented and spatially normalized to the International Consortium for Brain Mapping template using the unified segmentation approach, and the normalization parameters were applied to the co-registered functional images, which were resliced to 2-mm isotropic resolution. Functional images were smoothed with a 6-mm (full-width at half-maximum) Gaussian filter. With this preprocessing strategy we ensured that functional scans were in the same stereotaxic (Montreal Neurological Institute, MNI) space as the anatomical segments of grey matter, white matter and cerebrospinal fluid (CSF). All image sequences were routinely inspected for potential normalization artefacts.
Functional connectivity analyses
We performed a detailed seed-based functional connectivity analysis of the resting-state time-series to assess potential differences between depressed and control participants in the pattern of cortical and subcortical functional connectivity of specific cingulate subregions. Our approach was based on the method reported by Margulies et al. (Reference Margulies, Kelly, Uddin, Biswal, Castellanos and Milham2007), and focused on the segregation of functional connectivity maps between subregions of the ACC and MCC. Starting from the subgenual ACC (x=±5, y=25, z=−10), the seeds were located 5 mm from the corpus callosum, and were spaced 20 mm apart along a curve parallel to the corpus callosum. The additional seeds were in the pregenual ACC (x=±5, y=38, z=6), anterior MCC (x=±5, y=27, z=21) and posterior MCC (x=±5, y=10, z=33); see Fig. 1. In each location, spheres of radius 3.5 mm were created using the MarsBaR region-of-interest (ROI) toolbox (http://marsbar.sourceforge.net/). The time-courses were extracted for each of the four subregions in each hemisphere for each participant. In addition to our signals of interest, we derived estimates of white matter, CSF and global brain signal fluctuations to include in the regression analyses. The segmented white matter and CSF images were thresholded at 50% tissue-probability type and binarized to create nuisance masks, together with a binary mask of the global brain volume (summed from the grey matter, white matter and CSF segments). Nuisance signals were then extracted for each mask; they are included in resting-state functional connectivity analyses because they reflect global signal fluctuations of non-neuronal origin (for example, physiological artefacts associated with variables such as cardiac and respiratory cycles, CSF motion and scanner drift; Fox et al. Reference Fox, Corbetta, Snyder, Vincent and Raichle2006).

Fig. 1. The seed regions were placed in subregions of the cingulate cortex: (1) subgenual anterior cingulate cortex (ACC), (2) pregenual ACC, (3) anterior mid-cingulate cortex (MCC) and (4) posterior MCC. Their locations were adapted from Margulies et al. (Reference Margulies, Kelly, Uddin, Biswal, Castellanos and Milham2007): starting from the subgenual ACC seed, they were placed 5 mm from the corpus callosum, and were spaced 20 mm apart along a curve parallel to the callosum.
Statistical analysis
Functional connectivity maps were estimated for each cingulate subregion by including the seed and nuisance signals as predictors of interest and no interest respectively in whole-brain, linear regression analyses. Prior to model estimation, each of the three nuisance covariates were orthogonalized (using an iterative Gram–Schmidt method) and then removed from each seed's time-series by linear regression, resulting in a general linear model for each hemisphere that comprised the four ‘noise-cleaned’ seeds and three orthogonal nuisance variables (Harrison et al. Reference Harrison, Soriano-Mas, Pujol, Ortiz, López-Solà, Hernández-Ribas, Deus, Alonso, Yücel, Pantelis, Menchon and Cardoner2009). A high-pass filter set at 128 s was used to remove low-frequency drifts of less than approximately 0.008 Hz. Contrast images were generated for each participant by estimating the regression coefficient between all brain voxels and each seed's time-series, separately for each hemisphere. These images were then included in group (second-level) random-effects analyses, adopting a 2×2 factorial model [group (control, patient) by hemisphere (right seed, left seed)]. We included age, medication status and presence of anxiety disorder as covariates. To assess the magnitude and extent of functional connectivity for each pair of cingulate seeds (left and right) within each group, resulting z-transformed statistical maps were thresholded at p<0.001, with cluster-wise familywise error (FWE) correction of p FWE <0.05 for the whole-brain volume. We used the same correction for the analysis of between-group differences and for correlations with depression severity (measured with the BDI). For these analyses we report clusters whose peak voxels were within regions that were positively correlated with the relevant cingulate subregion for either group from the first analysis: our interest was in regions that were functionally connected with the rostral cingulate and not in regions that showed anticorrelated activity.
Additional analyses were performed to examine the effects of medication status, co-morbid anxiety and participant gender. The first eigenvariate was extracted from ROIs formed by spheres of radius 3.5 mm around the peak voxels in the significant between-group clusters. Strengths of connectivity were calculated for each ROI using independent-sample t tests for the non-medicated and medicated depressed participants compared to controls, for the depressed participants with and without co-morbid anxiety compared to controls, and for the depressed and control participants of each gender.
Results
Both groups exhibited robust patterns of connectivity for the four cingulate subregions that closely resembled the connectivity maps reported by Margulies et al. (Reference Margulies, Kelly, Uddin, Biswal, Castellanos and Milham2007). They revealed distinct differences in functional connectivity between the subregions. All showed strong connectivity with the medial frontal cortex, and in addition: subgenual ACC showed significant functional connectivity with the orbitofrontal cortex, medial parietal cortex, lateral parietal cortex, medial temporal lobe and striatum; pregenual ACC with the orbitofrontal cortex, medial parietal cortex, lateral parietal cortex and striatum; anterior MCC with the lateral frontal cortex, insular cortex, lateral parietal cortex and striatum; and posterior MCC with the primary sensorimotor cortex, insular cortex and striatum (see Fig. 2 and online Supplementary Table 1).
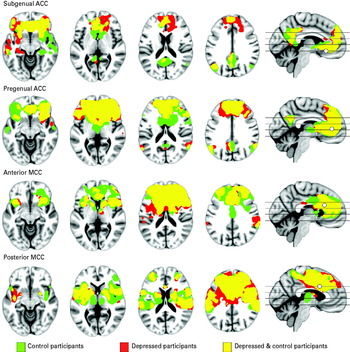
Fig. 2. Significant within-groups functional connectivity maps of connectivity with cingulate regions. Green indicates control participants; red depressed participants; and yellow the overlap of functional connectivity maps between the control and depressed participants. The results are thresholded at p<0.001, cluster-wise corrected (p FWE<0.05). ACC, anterior cingulate cortex; MCC, mid-cingulate cortex.
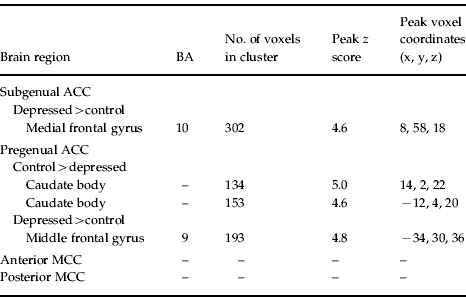
Comparison of the depressed and control participants revealed significant differences in cingulate functional connectivity (Table 2). Depressed participants showed increased connectivity between the subgenual ACC and dorsomedial frontal cortex. They also showed increased connectivity between the pregenual ACC and left dorsolateral prefrontal cortex, and reduced connectivity between the pregenual ACC and the caudate nucleus body bilaterally (Fig. 3). No connectivity differences were demonstrated for either the anterior or posterior MCC.

Fig. 3. (a) Depressed participants showed greater connectivity between subgenual anterior cingulate cortex (ACC) and dorsomedial frontal cortex (orange), and connectivity strength was correlated with depression severity in the depressed participants (green). (b) Correlation between connectivity strength and Beck Depression Inventory (BDI) score was investigated in a 3.5-mm sphere around the peak voxel [x=8, y=58, z=18] in the dorsomedial frontal region that showed greater connectivity in depressed compared to control participants. (c) Depressed participants showed increased connectivity between pregenual ACC and left dorsolateral frontal cortex; and (d) decreased connectivity between pregenual ACC and the caudate nucleus bilaterally.
Table 2. Between-group differences for functional correlations with each of the rostral cingulate subregions [thresholded at p <0.001, cluster-wise corrected (pFWE<0.05)]
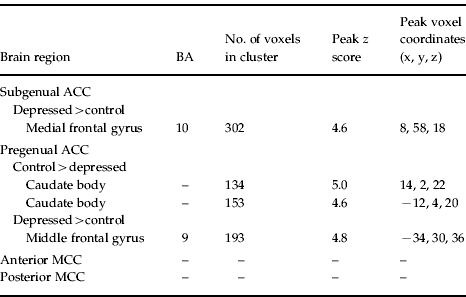
ACC, Anterior cingulate cortex; MCC, mid-cingulate cortex; BA, Brodmann area; FWE, familywise error.
The influence of symptom severity on connectivity was examined by correlating BDI scores with whole-brain connectivity for each of the cingulate subregions in the depressed participants. The results are summarized in Supplementary Table 2. Of note, depression severity exhibited a positive correlation with connectivity strength between the subgenual ACC and dorsomedial frontal cortex. As highlighted in Fig. 3, this region overlaps with the larger cluster identified as exhibiting greater connectivity with the subgenual ACC in depressed compared to control participants. Depression severity also exhibited a negative correlation with connectivity between the anterior MCC and the left dorsal caudate nucleus.
Analysis of strengths of connectivity within the significant between-group clusters demonstrated that medication status, co-morbid anxiety and participant gender had minimal influence on the reported results (Supplementary Fig. 1). Although the small number of participants in each of the subgroups reduced the statistical power, significant differences were demonstrated when non-medicated and non-anxious depressed subgroups were compared with control participants for all clusters except the medial frontal cortex, where non-medicated depressed participants showed an increase in connectivity compared to controls that reached trend-level significance (p=0.06). When analysis was restricted to females, the differences were maintained for all clusters, whereas for males, with only six participants included in each group, significant differences were demonstrated in both caudate clusters.
Discussion
In this study we examined whether alterations in the functional connectivity of subregions of the rostral cingulate cortex were present in young people with major depressive disorder. Our results show broadly similar patterns of connectivity in the depressed and control participants, with both groups demonstrating distinct functional networks for each cingulate subregion. There were, however, specific and robust differences in connectivity in young depressed patients that were confined to ventral cingulate subregions. Depressed participants showed increased cingulo-cortical connectivity, represented by increased connectivity between subgenual ACC and dorsomedial frontal cortex and between pregenual ACC and dorsolateral frontal cortex; and they showed decreased connectivity between pregenual ACC and the caudate nucleus bilaterally.
The results of this study provide further evidence for the role of the subgenual cingulate in depression, replicating previous findings that have shown increased connectivity with the dorsomedial frontal cortex (Sheline et al. Reference Sheline, Price, Yan and Mintun2010; Hamilton et al. Reference Hamilton, Chen, Thomason, Schwartz and Gotlib2011). The dorsomedial frontal cortex is a core component of the default-mode network, a set of brain regions that are active during introspective states, as demonstrated by imaging studies of self-referential tasks (Buckner et al. Reference Buckner, Andrews-Hanna and Schacter2008; Harrison et al. Reference Harrison, Pujol, López-Solà, Hernández-Ribas, Deus, Ortiz, Soriano-Mas, Yücel, Pantelis and Cardoner2008a ). The subgenual ACC has been reported to be abnormally coupled to the default-mode network in depressed participants (Greicius et al. Reference Greicius, Flores, Menon, Glover, Solvason, Kenna, Reiss and Schatzberg2007), which the results of our study suggest is mediated through the dorsomedial frontal cortex.
The dorsomedial frontal cortex is particularly activated by tasks that require a degree of self-analysis; for example, when participants judge the self-relatedness of words or pictures. Such processes are disturbed in depression (Grimm et al. Reference Grimm, Ernst, Boesiger, Schuepbach, Hell, Boeker and Northoff2009), reflected in symptoms such as self-reproach and guilty rumination, which a recent study has reported were correlated in depressed patients with connectivity between subgenual ACC and another core self-related region, the posterior cingulate cortex (Berman et al. Reference Berman, Peltier, Nee, Kross, Deldin and Jonides2010). The role of the subgenual ACC in visceromotor control suggests that these self-reflective processes may be more closely intertwined with visceromotor processes in depressed patients. There is a wealth of evidence that somatic processes are disturbed in depression: including well-described alterations in sleep, appetite, libido and energy levels; and more recent evidence that autonomic function is disrupted, including evidence of higher resting heart rate and reduced heart rate variability (Carney et al. Reference Carney, Freedland and Veith2005). The results of our study indicate that the disturbances in the self-reflective and visceromotor processes that underlie depressed mood might correspond with abnormally increased cingulo-cortical connectivity. The robust correlation between connectivity strength and depression severity provides further support for the relationship, and suggests that it may a candidate imaging marker for predictions of treatment response and prognosis.
Increased connectivity between pregenual ACC and dorsolateral frontal cortex in depressed patients has not previously been demonstrated by fcMRI. The dorsolateral frontal cortex has, however, frequently been implicated in depression, particularly on the left side. It has shown reduced resting-state activity in nuclear imaging studies (Bench et al. Reference Bench, Friston, Brown, Scott, Frackowiak and Dolan1992; Ito et al. Reference Ito, Kawashima, Awata, Ono, Sato, Goto, Koyama, Sato and Fukuda1996) and reduced activation to both cognitive and emotional stimuli in fMRI studies (Elliott et al. Reference Elliott, Rubinsztein, Sahakian and Dolan2002; Siegle et al. Reference Siegle, Thompson, Carter, Steinhauer and Thase2007). The dorsolateral cortex has a primary role in working memory, planning and attentional processes, and shows increased activity in paradigms that require cognitive regulation of emotion (Ochsner et al. Reference Ochsner, Bunge, Gross and Gabrieli2002, Reference Ochsner, Ray, Cooper, Robertson, Chopra, Gabrieli and Gross2004; Phan et al. Reference Phan, Fitzgerald, Nathan, Moore, Uhde and Tancer2005), including sad mood (Lévesque et al. Reference Lévesque, Eugène, Joanette, Paquette, Mensour, Beaudoin, Leroux, Bourgouin and Beauregard2003). Increased connectivity between the dorsolateral cortex and pregenual ACC might suggest, therefore, more effortful cognitive regulation of internally generated affective stimuli in the resting state in depression. Greater involvement of the dorsolateral frontal cortex in such resting-state processes may be in part responsible for its tendency to show, in depression, reduced activation to cognitively demanding external stimuli.
Depression has been considered to result from disturbances in circuits involving the striatum (Drevets et al. Reference Drevets, Price and Furey2008a ; Price & Drevets, Reference Price and Drevets2010), which the results of this study support. fcMRI studies have reported reduced connectivity in depressed participants between the caudate nucleus and anterior MCC (Anand et al. Reference Anand, Li, Wang, Wu, Gao, Bukhari, Mathews, Kalnin and Lowe2005), and also with the posterior cingulate cortex (Bluhm et al. Reference Bluhm, Williamson, Lanius, Théberge, Densmore, Bartha, Neufeld and Osuch2009). In this study, the pregenual ACC showed decreased connectivity with both the left and right caudate bodies in depressed participants; and connectivity between anterior MCC and the left caudal caudate showed negative correlation with symptom severity. Together the results suggest that altered connectivity between the rostral cingulate and caudate nucleus is implicated in depression, showing both reduced connectivity and decreasing connectivity with worsening symptom severity (albeit with neighbouring cingulate subregions). Dysfunction in networks involving the ACC and caudate nucleus has been proposed to underlie symptoms such as anhedonia, reduced motivation and psychomotor dysfunction (Price & Drevets, Reference Price and Drevets2010). Our analysis was not able to demonstrate correlations with these specific symptom domains (we do not have the relevant data), but suggests an interesting line for further study.
We found no evidence of significant alterations in functional connectivity with mid-cingulate regions in this study. Given that this is the first study to systematically compare connectivity across distinct subregions of the cingulate cortex, it suggests that altered MCC connectivity is not a prominent feature of depression, consistent with the fact that such findings are less frequently reported than for the ACC. This study included depressed youth with early depression, when cognitive impairment is not a significant component of the illness (Grant et al. Reference Grant, Thase and Sweeney2001; Kyte et al. Reference Kyte, Goodyer and Sahakian2005). The dysfunction of dorsal cognitive-attentional networks that has previously been reported in depression has typically involved adults with established illness (e.g. Wagner et al. Reference Wagner, Sinsel, Sobanski, Köhler, Marinou, Mentzel, Sauer and Schlösser2006; Siegle et al. Reference Siegle, Thompson, Carter, Steinhauer and Thase2007), suggesting that these alterations might occur later in the illness course, and perhaps secondarily to primary disturbance of affective networks.
The methodology adopted in this study, namely seed-based correlational analysis, was selected to answer a specific question about how connectivity in depressed participants might be altered along the axis of the rostral cingulate cortex. The methodology is similar to that used by other fcMRI studies in depression, and we selected a similar subgenual ACC seed, although we differed in the selection of other seed regions. Sheline et al. (Reference Sheline, Price, Yan and Mintun2010) examined connectivity with seeds in subgenual ACC, dorsolateral frontal cortex and precuneus. In addition to reporting an increase in connectivity between subgenual ACC and dorsomedial frontal cortex, they reported an increase in connectivity between their other seed regions and the dorsomedial frontal cortex (a region they subsequently labelled the ‘dorsal nexus’). Hamilton et al. (Reference Hamilton, Chen, Thomason, Schwartz and Gotlib2011) used Granger causality analysis to examine connectivity with a subgenual cingulate seed. Granger causality differs from traditional seed-based analysis in that it purports to additionally demonstrate the directional influence of changes in connectivity (although whether it achieves this for fMRI data is a matter of some debate; David, Reference David2011; Smith et al. Reference Smith, Bandettini, Miller, Behrens, Friston, David, Liu, Woolrich and Nichols2012). The authors reported that the subgenual ACC and dorsomedial frontal cortex were mutually reinforcing (activation in each region influenced the other), and that increased subgenual ACC activation was predicted by increased hippocampal activation. An alternative fcMRI methodology, independent component analysis, estimates maps of maximal spatial independence from the imaging data, and is predominantly used for characterizing large-scale cortical networks in a data-driven manner (Harrison et al. Reference Harrison, Pujol, Ortiz, Fornito, Pantelis and Yücel2008b ). It takes a more exploratory approach, and does not require hypothesis-driven selection of seed regions. It was used by Greicius et al. (Reference Greicius, Flores, Menon, Glover, Solvason, Kenna, Reiss and Schatzberg2007) to show that subgenual ACC was abnormally coupled to the default-mode network in depressed participants. That such a data-driven technique produced a result consistent with seed-based studies, including the current findings, provides important corroborative support. Our demonstration of increased connectivity between pregenual ACC and dorsolateral frontal cortex, and reduced connectivity between pregenual ACC and caudate nucleus, is a more novel finding, and one that requires replication.
The study has its limitations. Our study sample included young people ranging in age from late adolescence to early adulthood. Although the age range is relatively narrow, it does encompass a developmental period over which significant brain maturational processes occur, including changes in brain connectivity. These changes from childhood to adulthood include decreases in short-range connections and increases in longer-range connections (Fair et al. Reference Fair, Dosenbach, Church, Cohen, Brahmbhatt, Miezin, Barch, Raichle, Petersen and Schlaggar2007; Supekar et al. Reference Supekar, Musen and Menon2009; Dosenbach et al. Reference Dosenbach, Nardos, Cohen, Fair, Power, Church, Nelson, Wig, Vogel, Lessov-Schlaggar, Barnes, Dubis, Feczko, Coalson, Pruett, Barch, Petersen and Schlaggar2010), including with cingulate regions (Kelly et al. Reference Kelly, Di Martino, Uddin, Shehzad, Gee, Reiss, Margulies, Castellanos and Milham2009), with increasing coherence in connectivity of the default-mode network (Fair et al. Reference Fair, Cohen, Dosenbach, Church, Miezin, Barch, Raichle, Petersen and Schlaggar2008). Age was included as a covariate in our analyses and is therefore unlikely to have had a significant influence on the results, although further investigation of how maturation of functional connectivity networks contributes to vulnerability to depression is an important area for further research. We included participants who were taking medication and who had co-morbid anxiety disorders, reflective of the ‘real-world’ nature of the clinical group recruited for the study. Again, these variables were added as covariates to the analyses to mitigate their influence on the current results, and post-hoc analyses suggested they were not significant factors.
The study clarifies previous analyses of resting-state connectivity with the cingulate cortex in depression. The results demonstrate a pattern of increased cingulo-cortical connectivity and decreased cingulo-striatal connectivity. The demonstration of increased connectivity between subgenual ACC and dorsomedial frontal cortex in depressed participants supports previous findings, and is given further validity by the demonstration of a positive correlation between the strength of connectivity and depression severity. The systematic examination of cingulate connectivity differences in young depressed patients shows that they seem to be specific to ventral cingulate regions; and although alterations in correlated activity with both the subgenual and pregenual ACC are important for depression, their contributions seem to be distinct. The connectivity differences that we have demonstrated may help to explain the disturbances in autonomic, somatic and motivational processes observed in young depressed patients.
Supplementary material
For supplementary material accompanying this paper, please visit http://dx.doi.org/10.1017/S0033291712000323.
Acknowledgements
C. G. Davey is supported by a National Health and Medical Research Council (NHMRC) Training (Post-doctoral) Fellowship (ID No. 628922), B. J. Harrison (ID No. 628509) and M. Yücel (ID No. 509345) are supported by NHMRC Clinical Career Development Awards, and N. B. Allen is partially supported by a grant from the Colonial Foundation.
Declaration of Interest
None.