Introduction
Arms of the Petalocrinidae are composed of only two functional brachial plates and have multiple ambulacral bifurcations on the second brachial plate. The first brachial plate is normal in appearance, but the second brachial plate is a compound plate that is either subtriangular or cylindrical. Eopetalocrinus Li, Reference Li1993 is the oldest crinoid with this unique arm morphology, and it originated during the Ordovician (Darriwilian; Dawan Formation) on the South China Block. During the early Silurian (Llandovery, Aeronian), the Petalocrinidae diversified, yielding three genera: Petalocrinus Weller and Davidson, Reference Weller and Davidson1896; Sinopetalocrinus Mu and Lin, Reference Mu and Lin1987; and Spirocrinus Mu and Wu, Reference Mu and Wu1974 (Mao et al., Reference Mao, Lin and Ausich2015, Reference Mao, Ausich, Li, Lin and Lin2017). Petalocrinus has ambulacra confined to the oral side of the second brachial plate (Fig. 1.1, 1.3), and ambulacra grow over the edges of the second brachial in Sinopetalocrinus so that ambulacra are present on both the oral and aboral side of this arm plate (Mao et al., Reference Mao, Ausich, Li, Lin and Lin2017, fig. 4). In Spirocrinus, ambulacra are either approximately straight or spiral around the cylindrical second brachial (Fig. 1.2). During the Silurian, only Petalocrinus dispersed beyond the South China Block, and this genus became a distinctive crinoid in reef-associated habitats in Laurentia, Avalonia, and Baltica. The final known occurrence of the Petalocrinidae is Vadarocrinus Prokop, Reference Prokop1983, which is from the Pragian of the Czech Republic.

Figure 1. Oral view of petalocrinid second brachial plates. (1) Petalocrinus inferior Bather, Reference Bather1898; OSU 54648; (2) Spirocrinus circularis Mao et al., Reference Mao, Ausich, Li, Lin and Lin2017; OSU 54650; (3) Petalocrinus stenopetalus Mao et al., Reference Mao, Ausich, Li, Lin and Lin2017; OSU 64649. Scale bar 5.0 mm.
The fundamental question surrounding the petalocrinids is the origin of their unique second brachial plate. Was this plate derived from a single, hypertrophied brachial plate exhibiting excessive growth, or did this plate form through fusion of multiple brachial plates? If they are composed of fused plates, how was this accomplished? In this paper, we use polarized light microscopy to address this question.
Background
Echinoderms have a modular skeleton composed of multiple plates. When formed, plates are high-magnesium calcite with a porous stereom microstructure that consists of a meshwork of calcite trabeculae surrounded by soft tissue in the living animal (Macurda and Meyer, Reference Macurda and Meyer1975). Each plate is the product of intracellular biomineralization (Okazaki, Reference Okazaki1960; Märkel, Reference Märkel1986; Gorzelack et al., Reference Gorzelack, Stolarski, Dubois, Kopp and Meibom2011, Reference Gorzelack, Dery, Dubois and Stolarski2017), and is a single calcite crystal in optical continuity (e.g., Jackson, Reference Jackson1912; Raup, Reference Raup1959; Towe, Reference Towe1967). During diagenesis, the original crystallinity of the plate is the seed for syntaxial cement, which occludes the pore spaces of the stereom. The result is one relatively large calcite crystal representing each plate, and the crystallinity of individual plates is typically maintained in fossil specimens. This unique skeletal construction has formed the basis by which to ask a variety of questions (e.g., Raup, Reference Raup1959, Reference Raup1960, Reference Raup1962, Reference Raup1965, Reference Raup1966; Emlet, Reference Emlet1985, Reference Emlet1989; Bodenbender, Reference Bodenbender1996, Reference Bodenbender1997; Bodenbender and Hiemstra, Reference Bodenbender and Hiemstra1998; Bodenbender and Ausich, Reference Bodenbender and Ausich2000; O'Malley et al., Reference O'Malley, Ausich and Chin2013, Reference O'Malley, Ausich and Chin2016).
In virtually all crinoids other than the Petalocrinidae, arms are composed of multiple plates (brachials). Ambulacra typically are located within a groove on the oral side of the brachials, and ambulacra commonly bifurcate on a specialized, pentagonal axillary brachial. New arm plates are added distally and grow through ontogeny. Juvenile brachials are typically much higher than wide and grow anisometrically to produce an adult brachial that is commonly as high as wide or wider than high (Brower, Reference Brower1973, Reference Brower1974, Reference Brower, Moore and Teichert1978; Ausich and Wood, Reference Ausich and Wood2012).
When Weller and Davidson (1896, p. 169) first described Petalocrinus, they referred to the second brachial plates as the following: “The plates composing them closely ankylosed, no sutures visible.” Subsequently, Thomas (Reference Thomas1916, p. 289) said the brachials above the first primibrachial were “…united into a solid fan-shaped piece.” Lane and Moore (1978, p. T594) indicated that the “… brachials of each ray completely fused into single, large, fan-shaped arm plate…”.
Terminology for naming crinoid arm plates is based on the divisions within the arms. Brachials from the radial plate to and including the first arm bifurcation (if present) are termed primibrachials and compose the primibrachitaxis; brachials after the first arm bifurcation to and including the second arm bifurcation (if present) are secundibrachials and compose the secundibrachitaxis; etc. In a simple view, arms of petalocrinids are atomous (nonbranching) and are composed of a first and second primibrachial. However, as discussed below, the large arm plate in petalocrinids is a compound plate and was formed by the fusion of numerous brachitaxes. Thus, the typical arm terminology is in some ways inaccurate. For the purposes of this paper, the first, small brachial in petalocrinids will be referred to as the first primibrachial; but the second, large arm plate will be referred to as the second brachial rather than the second primibrachial.
Materials and methods
Three petalocrinid second brachial plates were embedded in bioplastic, and thin sections were prepared along the long axis of the arm plate. Specimens studied include Petalocrinus inferior Bather, Reference Bather1898, Leijiatun Formation (Aeronian), Baisha Section, South China Block, China; Petalocrinus stenopetalus Mao et al., Reference Mao, Ausich, Li, Lin and Lin2017, Leijiatun Formation (Aeronian), Fengxiang Section, South China Block, China; and Spirocrinus circularis Mao et al., Reference Mao, Ausich, Li, Lin and Lin2017, Shihniulan Formation (Aeronian), Shuibatang Section, South China Block, China (Fig. 2; see Mao et al., Reference Mao, Ausich, Li, Lin and Lin2017 for stratigraphic and biogeographic details). These encompass most of the range of second brachial shapes present among Silurian petalocrinids, including the typical widely diverging, subtriangular second brachial of Petalocrinus inferior (Fig. 1.1), the narrow subtriangular second brachial of Petalocrinus stenopetalus (Fig. 1.3), and the cylindrical second brachial of Spirocrinus circularis (Fig. 1.2).

Figure 2. Geographic and stratigraphic occurrences of Llandovery petalocrinids in Guizhou Province, China. (1) Location of stratigraphic sections in Guizhou Province; (2) positions of the Shihniulan and Leijiatun formations relative to graptolite biozones; (3) stratigraphic columns with petalocrinid-yielding beds indicated (from Mao et al., Reference Mao, Ausich, Li, Lin and Lin2017).
Repository and institutional abbreviation.—Specimens are deposited in the Orton Geological Museum, Ohio State University (OSU).
Crystallinity of petalocrinid second brachial plates
In all three specimens studied, most of the second brachial plates are composed of one crystal of calcite with unit extinction in crossed polarized light. This region includes multiple ambulacral bifurcations.
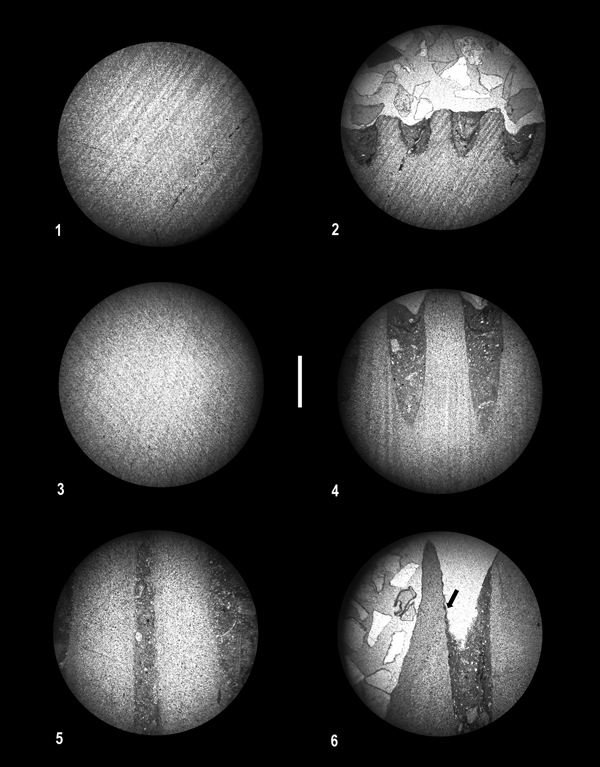
The Petalocrinus inferior second brachial plate was 18.0 mm in height (Fig. 1.1), and the prepared thin section was 17.5 mm in height and 20 mm in maximum width. As noted above, nearly the entire plate had unit extinction, indicating construction by a single crystal (Fig. 3.1; Supplemental Figs. 1.1, 2). In contrast, the distal 0.5 mm of this specimen is a thin zone with subtle, wavy extinction, indicating a crystallinity that contrasts with the majority of the plate (Fig. 3.2; Supplemental Figs. 1.2, 3). Prior to preparation, the Petalocrinus stenopetalus second brachial plate was 22.0 mm in height with a maximum width of 10.5 mm (Fig. 1.3), and the prepared thin section remained 22.0 mm in height. Similar to P. inferior, the majority of the plate was a single crystal (Fig. 3.3; Supplemental Figs. 1.3, 4), and the distal 0.5 mm of the P. stenopetalus second brachial plate has subtle, wavy extinction (Fig. 3.4; Supplemental Figs. 1.4, 5).

Figure 3. Photomicrographs of thin sections of petalocrinids in crossed-polarized light (scale bar 1.0 mm). (1, 2) Petalocrinus inferior Bather, Reference Bather1898; OSU 54648; (1) central portion of second brachial plate, (2) distal edge of second brachial plate; (3, 4) Petalocrinus stenopetalus Mao et al., Reference Mao, Ausich, Li, Lin and Lin2017; OSU 64649; (3) central portion of second brachial plate, (4) distal edge of second brachial plate; (5, 6) Spirocrinus circularis Mao et al., Reference Mao, Ausich, Li, Lin and Lin2017; OSU 54650; (5) central portion of second brachial plate, (6) distal edge of second brachial plate, arrow indicates boundary between adjoining crystals (see color images and videos in Supplemental Figures).
Prior to preparation, the second brachial plate of Spirocrinus circularis was 18.0 mm in height and 5.0 mm in diameter (Fig. 1.2), and the prepared thin section was 15.0 mm in height. As in Petalocrinus, the majority of the S. circularis second brachial plate is a single crystal in optical continuity (Fig. 3.5; Supplemental Figs. 1.5, 6), but the distal, narrow zone of wavy extinction is absent. In contrast, one small (3.4 mm in height and 0.5 mm in width) individual crystal is incorporated at the distal end of the second brachial plate (Fig. 3.6; Supplemental Figs. 1.6, 7). The crystallinity of this crystal is different from that of the remainder of the plate.
Interpretation
The large second brachial plate of petalocrinids was not formed by hypertrophy of a single, normal-sized, second primibrachial. Rather, this large brachial plate is interpreted to have been formed by the fusion of multiple plates, with or without merging of the crystal orientations of individual plates. This confirms the interpretations of previous workers (Weller and Davidson, Reference Weller and Davidson1896; Thomas, Reference Thomas1916; Lane and Moore, Reference Lane, Moore, Moore and Teichert1978). The wavy extinction of the distal portion of the Petalocrinus specimens is interpreted as fused plates that have incompletely merged their crystallographic orientations to that of the remainder of the second brachial plate. This implies that the second compound brachial plate was formed through progressive fusion of ever more distal brachial plates. Distal brachial plates fused to more proximal plates, and these plates also fused with laterally adjacent plates. Thus, this large plate is referred to as the second brachial plate because it is a combination of multiple brachitaxes. The plate illustrated in Figure 1.1 was formed through the fusion of the second primibrachial through several tertibrachitaxis plates.
The formation of the Spirocrinus second brachial plate is similar to that in Petalocrinus, in that the majority of the second brachial is one very large crystal. However, at least one additional large crystal is also present in the Spirocrinus second brachial plate (Fig. 3.6; Supplemental Figs. 1.6, 7). On the studied specimen, a distal zone of wavy extinction is absent. This second brachial plate was formed by fusion of brachial plates. Most of the fused brachial plates changed their crystallographic orientation to conform to the majority of this plate. However, at least one fused plate retained its original crystallinity. The size of this additional crystal far exceeds the size of a constituent brachial plate. We presume that this additional plate enlarged by fusion of multiple brachials that changed their crystallographic orientations, but we are unable to eliminate the possibility that this extra crystal is the product of a single, hypertrophied brachial plate incorporated into the second brachial plate.
Discussion
Despite the fact that initial biomineralization of each echinoderm plate occurred within a single mesodermal cell, it is well established that adjacent plates can merge. This can occur through stereomic interlocking of adjacent plates, as illustrated in clypeasteroid echinoids (e.g., Grun et al., Reference Grun, Mancosu, Belaústegui and Nebelsick2018), although in the case of clypeasteroids, most plates maintain their original crystallinity. Similarly, most camerate crinoids are thought to have ankylosed thecal plates that retain individual crystallographic orientations, although the exact mechanism for this fusion has not been documented.
Evolutionary reduction in the number of plates in the proximal circlet of crinoids (either infrabasal or basal circlet) has been demonstrated to be the product of plate fusion. Wilson (Reference Wilson1916) developed a rationale for recognizing this fusion, and Peter (Reference Peter2019) provided a modern treatment of plate fusion with the infrabasals of flexible crinoids. Fusion of separate plates also occurred in the transition from pentameric to holomeric columnals in crinoids (e.g., Warn and Strimple, Reference Warn and Strimple1977). Plate fusion is also recognized in other echinoderms. For example, it is widely recognized as a derived condition in ambulacral plates of crown-group echinoids (Smith, Reference Smith and Briggs2005; Gao et al., Reference Gao, Thompson, Petsios, Erkenbrack, Moats, Bottjer and Davidson2015), and Sprinkle and Sumrall (personal communication, 2018) recognized plate fusion in the formation of the deltoid plate in parablastoids.
Although the plate fusion described here is unusual (if not unique) for crinoid arms, this process of fusion occurs in the crown and column plates of other crinoids, the test of echinoids, and the theca of blastozoan echinoderms. Demonstrating plate fusion in pelatocrinids clarifies homology statements for this group and emphasizes the potential of this process for other studies in echinoderm disparity and phylogeny.
Acknowledgments
Discussion with J.R. Thompson and C.D. Sumrall expanded our perspective from which to understand fusion in petalocrinid arm plates. We acknowledge funding from the State Key Laboratory of Palaeobiology and Stratigraphy (Nanjing Institute of Geology and Palaeontology, CAS) (No. 20192105) and the Strategic Priority Research Program of Chinese Academy of Sciences Grant (No. XDB26000000). R. Mooi improved an earlier version of this manuscript.
Accessibility of supplemental data
Color images and videos of the petrographic study of petalocrinid specimens are available from the Dryad Digital Repository: https://doi:10.5061/dryad.9kj4ms0.