Published online by Cambridge University Press: 10 November 2003
Although previous studies have highlighted the inflammatory responses of fish infected with parasites and exposed to pollutants, very little is known about how these two stressors interact within the fish. In this review, which also contains original data, the effect of these two parameters on the fish inflammatory response is assessed and, in particular, the role of apoptosis and the acute phase protein, C reactive protein, is evaluated. In Cyprinus carpio exposed to 0·5 mg NH4+ l−1 or 0·1 mg Cd2+ l−1 and experimentally infected with the blood fluke, Sanguinicola inermis, the pollutant type and the order in which the fish experiences the parasite and toxicant, significantly affects the ultrastructural appearance and cellular content of the pronephros and thymus. This is reflected in the intensity of infection where the pollutant appears to have less effect on an established infection. Both stressors, pollutant and infection, may mediate their effects via the endocrine system. Studies have revealed that cortisol at 100 ng ml−1 is able to induce apoptosis in pronephric cells of carp and that an increase in apoptosis is associated with an increase in phagocytosis in this immune organ. In addition, C reactive protein, which is used as a biomarker of the inflammatory response in humans and other mammals, is evaluated as a possible indicator of physiological states in fish exposed to pathogens and pollutants.
Fish in natural and aquaculture freshwater and marine systems are usually exposed to multiple stressors, the two most common being pathogens and xenobiotic bioaccumulation. Other additional stress factors, such as starvation, environmental parameters e.g. temperature, pH and, particularly in pisciculture, various husbandry procedures e.g. handling during grading and transport, can affect the pathological and immunological homeostasis of the fish. The complicated interaction between all these parameters has meant that a detailed understanding of the pathological and immunological responses of fish to known multiple stressors has eluded biologists. Recently however, there has been considerable interest in how two of the major stressors, infection and water quality, interact to affect the inflammatory response produced against parasites. This interest may have been stimulated by the proposal made by several authors that parasites may be useful monitors of fish migration (e.g. Moser, 1991) and pollution (e.g. Poulin, 1992; Vethaak & Rheinallt, 1992). Indeed, others workers (e.g. Broeg et al. 1999; Diamant et al. 1999) have suggested that the pathological interactions between the parasite and the host and, in particular fish metabolic, pathological and parasitological indices, may be useful in monitoring pollution. In addition, several immune parameters (Hoole, 1997; Wester, Vethaak & Van Muiswinkel, 1994) have been proposed as possible ‘biomarkers’ for monitoring the effect of pollutants on feral fish. The usefulness of immune assays developed in laboratory fish models as monitors of pollution effects on wild fish has recently been validated by Zelikoff et al. (2002). A further recent development of note is the observation by Shim et al. (2002) that the phagocytic abilities of macrophages of Sprague-Dawley rats and fish (Cyprinus carpio and Carassius auratus) respond similarly to exposure to polluted water. This led the authors to propose that the greater availability of rat macrophages would make them more useful than fish macrophages as a monitor of water pollution.
Previous studies have highlighted the occurrence of an inflammatory response in fish exposed to parasitic infection and pollution. There have been several field investigations that have related parasitic burdens to water quality; for example Valtonen, Holmes & Koskivaara (1997) noted that pollution in Finland induced an increase or decrease in the prevalence of parasitic species in roach (Rutilus rutilus) and perch (Perca fluviatilis). The mechanisms by which these changes are bought about have not been extensively studied. Studies by several workers (e.g. Holliman & Esham, 1977; Evans, 1982 a,b; Morley, Crane & Lewis, 2001 a,b, 2002a,b,c,d) have revealed that the pollutant can affect the host/parasite interaction by directly affecting those stages of the parasite life cycle that are exposed to water. In contrast, there have been few detailed studies on how both parasites and pollutants interact to affect the immunopathological status of the fish. This lack of knowledge is primarily due to the technical difficulties of infecting fish in the laboratory and simultaneously exposing them to known pollutants. Associated with this is the absence of detailed knowledge of how the various immune parameters in a fish are affected by parasitic infection. In most cases therefore the basic requirements to study the simultaneous effects of parasitisation and pollution on fish immunopathology are absent.
The authors in association with several co-workers have carried out extensive studies on the immunopathological interactions between the common carp, Cyprinus carpio, and the blood fluke, Sanguinicola inermis. This fluke, which is an important pathogen (Iqbal & Sommerville, 1986; Lee, 1990; Sommerville & Iqbal, 1991; Kirk & Lewis, 1993), resides in the vascular system of its fish host, adult parasites usually being located in the heart and associated vessels. In the gills, the presence of entrapped parasite eggs or migrating miracidia results in extensive damage to host tissue leading to granuloma formation and fusion between the primary and secondary gill lamellae (Lee, 1990). In addition, eggs become entrapped in a range of other organs where they form the foci for the deposition of collagenous connective tissue and the accumulation of leucocytes (Lee, 1990). Richards et al. (1994 a) revealed that parasite eggs in the mesonephros of carp are encapsulated by eosinophils, neutrophils and macrophages. This effect on the leucocyte component of the host response was reflected in changes in the cellular composition of the lymphoid organs of carp exposed to 500 cercariae of S. inermis which induced a significant increase in splenic and pronephric macrophages and a decrease in splenic neutrophils, pronephric lymphocytes and splenic and pronephric eosinophils (Richards et al. 1994 b). In addition, further investigations revealed that both the adult and cercarial stage of S. inermis induced blastogenesis in pronephric and splenic cells (Richards et al. 1996 a) and migration of pronephric leucocytes (Richards et al. 1996 b). Leucocytes also adhered to the surface of the cercarial and post-cercarial stages in in vitro culture (Richards et al. 1996 c). This immunopathological response is reflected in a significant reduction in the establishment of S. inermis in challenge infections (Roberts, 1997; Fig. 1).

Fig. 1. Recovery of adult flukes of Sanguinicola inermis in Cyprinus carpio exposed to 500 cercariae in primary and challenge infections. Challenge infection given 8 months post-primary infection and flukes recovered 35 days post-challenge. n=25; ±S.E.
The above studies have thus established the necessary controls and form the basis of a recent investigation to ascertain the effect of pollutants on the S. inermis/Cyprinus carpio interaction. In an experimental protocol described by the authors in Schuwerack et al. (2001, 2003), carp, 3·5 g mean weight, were exposed to 500 cercariae of S. inermis and subsequently, 30 days post-infection, placed in water containing 0·5 mg NH4+ l−1 or 0·1 mg Cd2+ l−1 for 48 h or 168 h. Ultrastructural observations revealed that there were greater morphological and cellular changes in the thymus and pronephros of carp exposed to both parasite and pollutant in comparison to infected fish kept in clean water. In fish exposed to both parasite and pollutant, vacuolation and cellular disintegration of lymphoid organs was noted, particularly in the thymus. In addition, the cellular composition of the pronephros and thymus was differentially affected by the presence of the pollutants and parasite. In infected fish exposed to NH4+ there was a significant decrease in pronephric and thymic lymphocytes and in the number of pronephric neutrophils and thrombocytes. In contrast, there were far fewer changes in the cellular composition in infected carp exposed to cadmium e.g. significant increase in thymic neutrophils and thrombocytes; significant decrease in pronephric neutrophils. In addition, there were differential effects on the blastogenic response of pronephric cells obtained from infected and pollutant treated carp exposed to mitogens (ConA, PWM) and cercarial antigens.
In a natural situation there are several scenarios in which a fish can be exposed to both parasitisation and pollutant. It is probably very unlikely that the host will be infected and exposed to a pollutant and parasite at the same time and that two alternative interaction strategies would occur. An infected fish is exposed to a pollutant, as in the case above, or alternatively, a fish that has been exposed to a pollutant is subsequently infected. The immunopathological response induced in both scenarios may be different. To investigate this possibility we have modified the experimental protocol described above and have exposed carp to either untreated water or water containing 0·5 mg NH4+ l−1 or 0·1 mg Cd2+ l−1 for 48 h or 168 h and then infected them with S. inermis. Various immunopathological parameters were monitored 30 days post-infection.
Ultrastructural observations on the thymus and pronephros of fish exposed to cadmium or ammonium prior to infection with S. inermis revealed a change in the morphological appearance of both organs. Both pollutants with subsequent infection induced cellular disruption in the thymus (Fig. 2) similar to that recorded by Schuwerack et al. (2001, 2003) in which exposure to the pollutant occurred after the fish were infected. In contrast, the morphology of pronephros did not appear to be so adversely affected although there was an increase in vacuolation (Fig. 3) in fish exposed to pollutant and S. inermis in comparison to carp exposed to infection alone.

Fig. 2. Thymus of Cyprinus carpio exposed to 0·1 mg Cd2+ l−1 for 48 h and subsequently exposed to 500 cercariae of Sanguinicola inermis for 30 days. Note presence of extensive cellular disruption. Scale bar=3 μm.

Fig. 3. Pronephros of Cyprinus carpio exposed to 0·5 mg NH4+ l−1 for 48 h (a) 168 h (b) and subsequently exposed to 500 cercariae of Sanguinicola inermis for 30 days. Vacuoles (v) some of which contain myelinated structures (m) occur and increase in size with time. Scale bars=3 μm.
Statistical analysis (Repeated Measure ANOVA) also revealed a change in the leucocyte populations of the two organs analysed in fish exposed to the pollutant prior to infection compared with those infected fish not exposed to the toxicant. In fish exposed to cadmium prior to parasitisation (Fig. 4) the number of neutrophils changed significantly over time (P=0·008), showed organ-specific differences (P=0·0009) and an interaction between organ and pollutant (P=0·01). Perhaps the most obvious effect was the significant increase (P<0·005) in eosinophils in both organs in fish exposed to cadmium and then the parasite. This contrasts with the changes noted in the effects that cadmium has on the thymic and pronephric leucocyte population in carp that have been exposed to the pollutant after the infection. It would also suggest that the immunopathological response of a carp to a pollutant and parasitisation might depend on the order that the fish experiences these stressors. This is substantiated by the effect of NH4+ and S. inermis on the pronephric and thymic leucocyte population in carp (Fig. 5). In fish exposed to the pollutant prior to infection neutrophils (P=0·001) and eosinophils (P=0·007) were more abundant in the pronephros than the thymus, and eosinophils, in particular, were significantly increased in number after 168 exposure to the pollutant and subsequent infection with S. inermis for 30 days. Although thrombocytes were present in both organs, a significant increase only occurred in the pronephros in fish that had been exposed to NH4+ for 168 h prior to infection with the parasite compared with infected carp not exposed to the pollutant (P=0·01) and fish exposed to NH4+ for 48 h with subsequent parasitic infection.

Fig. 4. Mean number of differential leucocyte counts in the thymus (a) and the pronephros (b) of Cyprinus carpio either unexposed or exposed to 0·1 mg Cd2+ l−1 for 48 h or 168 h prior to exposure to 500 cercariae of Sanguinicola inermis for 30 days. Cells were counted within 3 randomly chosen areas (550 μm2) in 5 pronephric or thymic tissue sections in individual carp. n=6; ±S.E. N=neutrophils, L=lymphocytes, M=macrophages, Eo=eosinophils, T=thrombocytes.

Fig. 5. Mean number of differential leucocyte counts in the thymus (a) and the pronephros (b) of Cyprinus carpio either unexposed or exposed to 0·5 mg NH4+ l−1 for 48 h or 168 h prior to exposure to 500 cercariae of Sanguinicola inermis for 30 days. Cells were counted within 3 randomly chosen areas (550 μm2) in 5 pronephric or thymic tissue sections in individual carp. n=6; ±S.E. N=neutrophils, L=lymphocytes, M=macrophages, Eo=eosinophils, T=thrombocytes.
It is of interest that, if the proposed hypothesis that the order in which the two stressors (parasitisation and pollutant) are applied affects the pathoimmunological response in the pronephros and thymus of carp is correct, then it may affect parasite burden in the hosts. Indeed, preliminary data based on the number of adult S. inermis obtained from the heart of carp in the above experimental protocol suggests that whilst there was no apparent effect on parasite burden in fish that had been infected with the parasite for 30 days prior to exposure to 0·5 mg NH4+ l−1 or 0·1 mg Cd2+ l−1 there were changes in intensity and prevalence of infection in carp exposed to the pollutant prior to infection. In fish that had been exposed to cadmium for 48 h prior to infection a lower prevalence (40%) and intensity of infection (mean 0·6; range 0–2) occurred compared with fish not exposed to the pollutant, where both a higher prevalence (100%) and intensity (mean 1·7; range 1–5) were recorded. However, after 168 h exposure to cadmium both prevalence and intensity of infection increased in cadmium exposed (100%; mean 2·4; range 2–6) and unexposed carp (100%; mean 4·0; range 2–5). Carp exposed to NH4+ for 48 h prior to infection with S. inermis had a prevalence of 90% and a mean intensity of 5·63 (range 0–20) after 30 days post-infection. However, after 168 h exposure the prevalence increased to 100% and the mean intensity decreased (2·5; range 0–6). This would suggest that the pollutant may have a more profound effect on parasite establishment rather than on an existing parasite burden, particularly when those established parasites have matured.
The mechanisms by which the pollutant and parasites, either individually or simultaneously, affect the immunopathological response in fish which then may subsequently affect parasite burden have not been elucidated. Undoubtedly the interactions are complex and involve both cellular and humoral factors. The observations that in the pollutant/S. inermis/carp interaction, cellular disruption occurs in the pronephros and thymus of the host would suggest that induction of cell death may be an integral component of the immunopathological response.
Cell death can be mediated by a necrotic mechanism or by induction of apoptosis. Necrosis is usually associated with an early loss of membrane integrity which results in leakage of the cell contents into the immediate tissue environment and the induction of inflammation. Necrosis has thus been recognized as a pathological form of cell death which is primarily associated with acute forms of tissue damage. In contrast, over the last two decades it as been realised that cell death can be mediated by a far more rigorously controlled system which involves expression of a variety of gene families. This process, apoptosis, is characterized by changes in both the nuclear and cytoplasmic contents of the cell and is thus associated with cell shrinkage, membrane blebbing and cytoskeletal disruption. Within the nucleus, endonucleases are activated which results in the fragmentation of DNA into oligonucleosomes and the formation of the characteristic ‘apoptotic ladder’ in DNA electrophoresis. Mitochondria also play an integral role in the apoptotic cascade. They release cytochrome c into the cytosol and their outer membrane contains several proteins of the Bcl-2 family which act as regulators of the apoptotic process i.e. Bcl-2, Bclxl (anti-apoptotic); Bax, Bak, Bik (pro-apoptotic). Cytochrome c is thought to interact with APAF1 (Apoptosis Protease Activating Factor 1) to induce the caspase cascade. The initiation of the apoptotic pathway is mediated via a variety of signals (1.) Genotoxic damage, (2.) Activation of the Fas or TNF receptor by the appropriate ligand and (3.) Cytokine deprivation. Apoptosis is now recognized as an important biological phenomenon that occurs in both invertebrates and vertebrates where it serves to eliminate cells during the development of several biological systems in multicellular animals e.g. immune system, nervous system, and removes tumours or damaged cells.
Apoptosis plays a critical role in the immune response and is important for modulating inflammation and infections. Recent reviews (Barcinski & DosReis, 1999; Luder, Gross & Lopez, 2001; Heussler, Knenzi & Rottenberg, 2001) have highlighted the interaction between intracellular protozoan parasites and apoptosis in mammalian cells. Several workers (e.g. Gon et al. 1997; Starke & Oaks, 1999) have noted an association between metazoan parasites and apoptosis in the host. Apoptosis (programmed cell death) is also an integral component of the homeostasis mechanism in several fish tissues for example, in the retina of Salmo salar (Kunz et al. 1994), the gills of Oreochromis mossambicus (Wendelaar Bonga & van de Meij, 1989) and reproductive organs of fish (Janz et al. 2001). In addition, it has been proposed that fish leucocytes are able to induce death of a target organism by apoptosis. Meseguer, Esteban & Malero (1996) noted that leucocytes from gilthead seabream (Sparus autata) and sea bass (Dicentrarchus labrax) are able to destroy tumour cells by necrosis and apoptosis, and Greenlee, Brown & Ristow (1991) observed that nonspecific cytotoxic cells can induce apoptosis in rainbow trout (Oncorhynchus mykiss).
The expression of apoptosis in a cell can be influenced by the action of stressors for example pollutants and infection. This area has been extensively studied in mammalian systems for example, Raffray et al. (1993) noted that tributyltin induced apoptosis in the thymocytes of rats and several investigations (see Thompson, 1995 for review) have correlated apoptosis with disease and infection. In fish there are several studies that indicate that pollutants can induce apoptosis in a range of fish cells. For example, Julliard, Saucier & Astic (1993) suggested that copper induces apoptosis in the olfactory system of rainbow trout (O. mykiss) and Risso-de Faverney et al. (2001) noted that cadmium stimulated apoptosis in the hepatocytes of rainbow trout and suggested that this was mediated by the production of reactive oxygen species. In contrast, there have been relatively few studies that have investigated the association between parasitism and apoptosis in fish. In studies from our laboratories (Roberts, 1997), cells obtained from the pronephros of carp (Cyprinus carpio) infected with 500 cercariae of S. inermis, 32 days previously, displayed significantly higher levels of apoptosis (P<0·05), as determined by acridine orange staining, than cells from uninfected fish (Fig. 6). Recently, Jung and co-workers (Jung et al. 2000; Murakawa, Jung & Yonehara, 2001) have cloned a novel apoptosis-inducing protein from chub mackerel infected with the larval nematode Anisakis simplex, which induces apoptosis in various mammalian cells. It was suggested that this programmed cell death was mediated by two mechanisms; one rapid and associated with the production of hydrogen peroxide and another which was delayed and involved withdrawal of L-lysine. However, whether the infection or pollutant has a direct effect on apoptosis or is mediated by a stress response has proven more difficult to elucidate. Whilst some authors such as Xiang, Shao & Meng (2001) have noted that heavy metal ions can induce apoptosis directly in fish cells in vitro, others such as Wendelaar Bonga and co-workers (Wendelaar Bonga et al. 1990; Iger et al. 1994 a,b,c) have recorded an increase in the rate of necrosis and apoptosis in the skin and gills of fish exposed to several toxic stressors such as acid water containing aluminium, copper and cadmium, the implication being that there may be some commonly mediated responses involved to the range of stressors used. In vivo, stress is associated with several hormonal changes within the vertebrate, perhaps the most important being an increase in the corticosteriods (see Wendelaar Bonga, 1997 for review). Bury et al. (1998) suggested that copper directly induced necrosis in the chloride cells in the gills of tilapia (Oreochromis mossambicus) and proposed that at low concentrations cortisol protected against copper toxicity whilst at higher concentrations (0·83 μM) this corticosteriod induced apoptosis. Recently there has been interest in the relationship between cortisol and apoptosis in the immune response of fish. Several studies have highlighted the relationship between the effect of stressors e.g. pollution on the immune response of fish (see Hoole, 1997 for review), several parameters of which have been associated with an alteration in serum cortisol levels (e.g. Bennet & Wolke, 1987). Alford et al. (1994) noted that stress, induced by confinement in a net, reduced rates of apoptosis in the peripheral leucocytes of channel catfish (Ictalurus punctatus) when compared to unstressed individuals. The role played by cortisol in this phenomenon was unclear, since in vitro experiments did not demonstrate any affects of cortisol on apoptosis in leucocytes. Since these initial studies however there have been several workers who have proposed an interaction between cortisol and fish immune cells. Espelid et al. (1996) noted that cortisol induced a suppressive effect on LPS-induced mitogenesis on the peripheral blood cells of Atlantic salmon (Salmo salar) although the response was both dose and time dependent. In 1997 Weyts and co-workers observed that cortisol-induced apoptosis in the peripheral blood lymphocytes of carp (C. carpio) was dependent on whether the cells were stimulated. In non-stimulated cultures apoptosis appeared to result from the absence of appropriate signals whilst LPS- and PHA-stimulated cells were sensitive to cortisol-induced apoptosis. Further studies carried out by Verburg-van Kemenade et al. (1999) noted that B lymphocytes of carp differed in their sensitivity to cortisol-induced apoptosis based on their organ of origin and state of activation. Work carried out at Keele University, UK has implicated apoptosis in pronephric cells of carp with exposure of the fish to cadmium (Hoole, 1997). When C. carpio (mean weight 5·67 g) were exposed to a single dose of cadmium at a concentration of 100 μg l−1 it was noted that the number of pronephric cells undergoing apoptosis or phagocytosis, as judged using ultrastructural analysis, changed over the following 90 day period (Fig. 7). Statistical analysis (ANOVA) revealed that there was a significant increase (P<0·001) in programmed cell death in fish exposed to cadmium compared to that occurring in unexposed carp. In addition, regression analysis showed that whilst there was no significant relationship between apoptosis and phagocytosis in control fish (r=0·307; P>0·05), a highly significant positive correlation occurred between the two parameters in cadmium exposed carp (r=0·78; P<0·001).

Fig. 6. Apoptosis in pronephric leucocytes of Cyprinus carpio exposed to 500 cercariae of Sanguinicola inermis for 32 days. n=6; ±S.E., *P<0·01.

Fig. 7. Effect of exposure to 0·1 mg Cd2+ l−1 on percentage of cells undergoing apoptosis or phagocytosis in the pronephric cells of Cyprinus carpio. n=6; ±S.E. Apoptosis 0·1 mg Cd2+ l−1; Apoptosis control; Phagocytosis 0·1 mg Cd2+ l−1; Phagocytosis control.
The possibility that the observed affects of cadmium on apoptosis in the immune system of fish may be mediated by cortisol has also been evaluated. In vitro studies have revealed that pronephric and thymic cells from C. carpio (8–12 cm in length) were susceptible to apoptosis-induced death when exposured to 1 μg ml−1 dexamethasone, a corticosteriod analogue, and visualised using acridine orange staining. Further analysis revealed that B lymphocytes and cortical thymocytes were particularly sensitive to dexamethasone-induced apoptosis. Using the above model system it was also revealed that cortisol at 10 ng ml−1 and 100 ng ml−1 induced a significant increase (P<0·001) in apoptosis in thymic and pronephric cells within the first 6 h of exposure compared to cells not exposed to cortisol (Fig. 8). There was no significant difference in the percentage of cells undergoing apoptosis in the pronephros and thymus. Further studies also revealed that when pronephric and thymic cells were exposed to 100 ng ml−1 for 1 h there was a significant increase (P<0·001) in apoptosis which continued to increase in the absence of the corticosteriod and above background levels over the following 12 h (Fig. 9). Analysis of the utilisation of cortisol in the in vitro system revealed that all detectable cortisol monitored utilising the technique as described in Balm et al. (1994) was absent within 10 min of being placed with the pronephric cells. Studies carried out by Weyts, Verburg-Van Kemenade & Flik (1998) also revealed that the binding of cortisol to carp peripheral blood leucocytes exhibited saturation kinetics and that the maximum binding was obtained within 10 min. Other studies on the interaction between the glucocorticoid receptor (GR) and cortisol or cortisol analogues in fish have revealed that the hormone down-regulates GR numbers in the gill (Shrimpton & Randall, 1994), liver (Pottinger, 1990; Lee et al. 1992), brain (Lee et al. 1992) and erythrocytes (Pottinger & Brierley, 1997). In contrast, there is an increase in GR numbers in leucocytes obtained from the spleen and pronephros of coho salmon that have been exposed to chronic stress or received in vivo cortisol treatment (Maule & Schreck, 1991). Weyts et al. (1998) noted that in C. carpio that received a single meal of cortisol-containing food, elevated blood cortisol levels induced a decrease in GR density in peripheral blood leucocytes. They suggested that the change in the number of these receptors in this leucocyte population might result from a down-regulation of receptor numbers per cell or a re-distribution of B lymphocytes. In recent years there has been increased interest in the relationship between parasitism and the cortisol response in both mammalian (e.g. Fleming, 1997, 1998; Sures et al. 2002) and fish (e.g. Ruanne et al. 1999; Nolan, Van der Salem & Wendelaar Bonga, 2000; Poole, Noland & Tully, 2000; van der Salem et al. 2000; Harris, Soleng & Bakke, 2000; Grutter & Pankhurst, 2000) hosts. However detailed investigation on the effects of other stressors on this relationship and the cellular components of the immunopathological response is required.

Fig. 8. Effect of cortisol on apoptosis in pronephric (a) and thymic (b) cells of Cyprinus carpio. p.i. control represents the number of cells undergoing apoptosis prior to incubation; p.e. control represents the number of cells undergoing apoptosis in the absence of cortisol. ([squf ]); 10 ng ml−1 cortisol (□); 100 ng ml−1 cortisol (). ±S.E.

Fig. 9. Effect on apoptosis in the pronephric (a) and thymic (b) cells of Cyprinus carpio after 1 h exposure to 100 ng cortisol/ml () or not exposed to the hormone () and its subsequent removal.
Although there has been extensive research on the immunopathological response in fish, one aspect that has received relatively little attention, with the exception of the complement system, is the importance of the humoral non-specific response. This is somewhat surprising given the fact that some of these proteins, for example C Reactive Protein (CRP), due to their acute phase nature during inflammatory episodes, are used as a monitor of tissue injury and disease activity in medicine (Pepys, 2001). In humans, CRP is the classical acute-phase reactant produced in response to tissue damage, infection or inflammation, circulating levels rising by up to 100-fold, from normal levels of less than 1 μg ml−1, within hours of infection or injury (Volanakis, 2001). The serum pentraxins CRP and amyloid P component (SAP) are part of a conserved family of cyclic oligomeric proteins, homologues of which have been found in mammals, amphibians, fish and the horseshoe crab, Limulus polyphemus (see Gewurz, Zhong & Lint, 1995). CRP is characterized by its Ca2+-dependent binding affinity for phosphocholine (Volanakis & Kaplan, 1971), a component which in vivo appears as a common component of fungal and bacterial polysaccharides and cell membranes. In the majority of species, mouse (Pepys et al. 1979) and the Atlantic Salmon, Salmo salar (Lund & Olafsen, 1999) being examples of notable exceptions, SAP-like pentraxins are often unresponsive during the acute phase response. In common with CRP in many species however, SAP-like pentraxins bind to the membrane phospholipid, phosphotidylethanolamine. The majority of the information relating to the function of the pentraxins, particularly CRP, has been acquired from mammals, in particular humans. The pentraxins have a multifactorial role in the non-specific host response, for example CRP activates complement via the complement component C1q (Volanakis & Kaplan, 1974), opsonises bacteria and other pathogens (Edwards et al. 1982), binds to phagocytes that bear the Fc receptor for IgG (Mold, Gresham & Du Clos, 2001), and interacts with nuclear debris (Pepys et al. 1994; Du Clos, 1997) and the cell membrane of damaged cells (Volanakis & Wirtz, 1979; Li, Mold & Du Clos, 1994). It is of interest that during the apoptotic process alterations occur in the phospholipid of the cell membrane. In particular the aminophospholipids, for example phosphatidylethanolamine and phosphatidylserine, that normally occur on the inner aspect of the plasma membrane, undergo ‘membrane flip-flop’ and occur on the outer aspects of the membrane in an apoptotic cell (see Sweet et al. 1999 for review). Unfortunately the details of the relationship between apoptosis and pentraxins in the inflammatory response have not yet been elucidated.
Pentraxin homologues have been reported from a range of fish species, for example Pleuronectes platessa (Pepys et al. 1982), Mustelus canis (Robey, Tanaka & Lui, 1983) and Oncorhynchus mykiss (Murata, Kodama & Onuma, 1995). However, there appears to be a wide variation in the levels of these pentraxins recorded in ‘normal’ fish serum ranging from a few μg ml−1 to over 1 mg ml−1. This variation may be related to the difficulties encountered in isolating and characterizing pure CRP or SAP, a necessity for producing accurate systems of measurement. There have, however, been several studies which have noted a change in serum pentraxin levels in fish exposed to temperature and chemical insults, pollutants and infection (see Table 1). Of particular note regarding the context of this review is the association between pollutants and an increase in the levels of CRP or CRP-like proteins although in many cases it is not clear that the pentraxin alone has been isolated. Indeed, the amino acid sequence of CRP or SAP has been obtained from very few fish species (see Table 2) and both the identity and the true acute phase response of reported pentraxin homologues within some fish species remains in doubt. Ghosh & Bhattacharya (1992) noted that the ‘normal’ levels of CRP in the murrel (Channa punctatus) were 220±12·06 μg ml−1 and that a range of pollutants induced a 2·9–3·9 fold increase in concentration at the peak of the response. A similar magnitude of increase also occurred in base levels (208–230 μg ml−1) of CRP in the rohu (Labeo rohita) exposed to 60 ppm of cadmium or 0·11 ppm of mercury (Sinha & Mandal, 1996) and in the normal concentration (180 μg ml−1) of CRP in major carp (Catla catla) exposed to the same pollutants (Paul, Mandal & Mandal, 1998). There have been very few studies on how the levels of serum pentraxins are affected in fish exposed to infection. Szalai, Bly & Clem (1994) observed a reduction in ‘phosphorylcholine-reactive protein’ in channel catfish (Ictalurus punctatus) infected with the fungal pathogen, Saprolegnia sp. However, Lund & Olafsen (1999) have noted minor but sometimes significant increases in Atlantic salmon SAP during exposure to LPS and Aeromonas salmonicida, suggesting that fish pentraxins may display acute phase changes during infection. In addition, rainbow trout pentraxins have been reported to bind A. salmonicida LPS in vitro, suggesting that they may be directly involved in attempts by the humoral immune system to clear this bacterial pathogen (Hoover et al. 1998). Studies carried out at Keele University include the development of a series of affinity chromatography protocols to purify a serum CRP homologue from C. carpio and to elucidate its response to parasitic infection and pollutants.


We are grateful to several colleagues who have contributed to the original studies described above. In particular, Professor G. Williams (Keele University) for assistance with apoptosis investigations, Drs J. Rombout and G. F. Wiegertjes (Wageningen University, The Netherlands) for supplying monoclonal antibodies to a range of leucocytes of Cyprinus carpio, Professor Wendelaar Bonga and Dr D. Nolan (Catholic University of Nijmegen, The Netherlands) for cortisol measurements and Dr A. Polwart (Keele University) for assistance in statistical analysis.

Fig. 1. Recovery of adult flukes of Sanguinicola inermis in Cyprinus carpio exposed to 500 cercariae in primary and challenge infections. Challenge infection given 8 months post-primary infection and flukes recovered 35 days post-challenge. n=25; ±S.E.

Fig. 2. Thymus of Cyprinus carpio exposed to 0·1 mg Cd2+ l−1 for 48 h and subsequently exposed to 500 cercariae of Sanguinicola inermis for 30 days. Note presence of extensive cellular disruption. Scale bar=3 μm.

Fig. 3. Pronephros of Cyprinus carpio exposed to 0·5 mg NH4+ l−1 for 48 h (a) 168 h (b) and subsequently exposed to 500 cercariae of Sanguinicola inermis for 30 days. Vacuoles (v) some of which contain myelinated structures (m) occur and increase in size with time. Scale bars=3 μm.

Fig. 4. Mean number of differential leucocyte counts in the thymus (a) and the pronephros (b) of Cyprinus carpio either unexposed or exposed to 0·1 mg Cd2+ l−1 for 48 h or 168 h prior to exposure to 500 cercariae of Sanguinicola inermis for 30 days. Cells were counted within 3 randomly chosen areas (550 μm2) in 5 pronephric or thymic tissue sections in individual carp. n=6; ±S.E. N=neutrophils, L=lymphocytes, M=macrophages, Eo=eosinophils, T=thrombocytes.

Fig. 5. Mean number of differential leucocyte counts in the thymus (a) and the pronephros (b) of Cyprinus carpio either unexposed or exposed to 0·5 mg NH4+ l−1 for 48 h or 168 h prior to exposure to 500 cercariae of Sanguinicola inermis for 30 days. Cells were counted within 3 randomly chosen areas (550 μm2) in 5 pronephric or thymic tissue sections in individual carp. n=6; ±S.E. N=neutrophils, L=lymphocytes, M=macrophages, Eo=eosinophils, T=thrombocytes.

Fig. 6. Apoptosis in pronephric leucocytes of Cyprinus carpio exposed to 500 cercariae of Sanguinicola inermis for 32 days. n=6; ±S.E., *P<0·01.

Fig. 7. Effect of exposure to 0·1 mg Cd2+ l−1 on percentage of cells undergoing apoptosis or phagocytosis in the pronephric cells of Cyprinus carpio. n=6; ±S.E. Apoptosis 0·1 mg Cd2+ l−1; Apoptosis control; Phagocytosis 0·1 mg Cd2+ l−1; Phagocytosis control.

Fig. 8. Effect of cortisol on apoptosis in pronephric (a) and thymic (b) cells of Cyprinus carpio. p.i. control represents the number of cells undergoing apoptosis prior to incubation; p.e. control represents the number of cells undergoing apoptosis in the absence of cortisol. ([squf ]); 10 ng ml−1 cortisol (□); 100 ng ml−1 cortisol (). ±S.E.
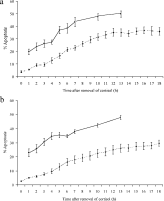
Fig. 9. Effect on apoptosis in the pronephric (a) and thymic (b) cells of Cyprinus carpio after 1 h exposure to 100 ng cortisol/ml () or not exposed to the hormone () and its subsequent removal.

Table 1. Acute phase characteristics of fish C-reactive protein (CRP) and CRP-like proteins

Table 2. Fish pentraxins. N-terminal amino acid sequence aligned based on homology with conserved pentraxin motifs