Introduction
Species within the genus Oropogon (Fée) Th. Fr. 1861 (Parmeliaceae) produce white-grey to brown-black fruticose thalli, which range from caespitose to pendent, and frequently branch isodichotomously (Esslinger Reference Esslinger1989). Oropogon species occur on trees, shrubs, soil and stone from the mid to high elevations of Central and South America (Esslinger Reference Esslinger1989; Sipman Reference Sipman1989, Reference Sipman, Balslev and Luteyn1992, Reference Sipman, Churchill, Balslev, Forero and Luteyn1995, Reference Sipman2002), and East and South Asia (Esslinger Reference Esslinger1989; Chen Reference Chen1996; Harada & Wang Reference Harada and Wang2008; Singh & Sinha Reference Singh and Sinha2010). Species diversity within Oropogon has received relatively little attention since the genus was first proposed. However, the number of species was dramatically and somewhat controversially increased with the recognition of 30 Oropogon species in the New World, where previously only three had been known (Esslinger Reference Esslinger1989). At present, eight species are known from Asia, with only two species, O. barbaticus Essl. and O. formosanus Asahina, appearing to occur in both Asia and the New World (Esslinger Reference Esslinger1989).
Oropogon is most diverse in northern South America where c. 90% of the currently known species occur, including several apparent endemics (Esslinger Reference Esslinger1989; Sipman Reference Sipman, Balslev and Luteyn1992, Reference Sipman2002). Consequently, montane regions of the Neotropics appear to be centres of diversification for Oropogon (Sipman Reference Sipman, Balslev and Luteyn1992, Reference Sipman, Churchill, Balslev, Forero and Luteyn1995). Warming and cooling periods during the Quaternary have been suggested as a possible catalyst for speciation in Oropogon in the páramos of northern South America (Esslinger Reference Esslinger1989). However, molecular dating analyses have suggested the Neogene as an important period for the diversification of extant Oropogon lineages, and the Pleistocene as important for the generation of intraspecific variation in extant species (Leavitt et al. Reference Leavitt, Esslinger and Lumbsch2012a ).
The cortex of Oropogon species is similar to that produced by Bryoria, and is composed of periclinally arranged hyphae forming a layer of prosoplectenchymatous tissue (Hawksworth Reference Hawksworth1969; Brodo & Hawksworth Reference Brodo and Hawksworth1977; Esslinger Reference Esslinger1989). Medullary tissue consists of loosely arranged, interwoven hyphae similar to that of other alectorioid genera (Brodo & Hawksworth Reference Brodo and Hawksworth1977; Esslinger Reference Esslinger1989). However, the form of the medulla, ranging from mostly hollow to filled, has proved useful for discriminating among Oropogon species (Esslinger Reference Esslinger1989). In addition, the presence or absence, and type of pseudocyphellae (shape and perforation) play an important role in distinguishing species (Esslinger Reference Esslinger1989).
Approximately half of the described Oropogon species regularly produce lateral, thalline apothecia (Esslinger Reference Esslinger1989, Reference Esslinger, Nash, Ryan, Gries and Bungartz2002). These apothecia contain large, broadly clavate, thick-walled asci, consistent with the Alectoria-form of Lecanora-type of ascus (Esslinger Reference Esslinger1989, Reference Esslinger, Nash, Ryan, Gries and Bungartz2002; Thell et al. Reference Thell, Mattsson and Kärnefelt1995). Oropogon species typically produce a single large, broadly ellipsoid, brown muriform spore per ascus (Esslinger Reference Esslinger1989). Spore size is quite variable within the genus; however, intraspecific variation renders this character of little use for segregating species from one another (Esslinger Reference Esslinger1989). Additionally, pycnidia have been found in a few Oropogon species, but do not appear useful for distinguishing among Oropogon species (Esslinger Reference Esslinger1989; Harada & Wang Reference Harada and Wang2008). The production of asexual propagules appears rare in Oropogon, with only about two species regularly forming soredia (O. aliphaticus) or spinules (O. loxensis); consequently the presence or absence of varying asexually-derived reproductive structures is not widely used for taxonomic purposes in Oropogon (Esslinger Reference Esslinger1989).
Oropogon is a chemically diverse genus, producing a greater number of substances than other alectorioid genera (Culberson & Culberson Reference Culberson and Culberson1978; Esslinger Reference Esslinger1980, Reference Esslinger1989). This chemical diversity, coupled with high intraspecific variation in numerous morphological characters, has resulted in secondary chemistry playing an important role in the delimitation of species (Esslinger Reference Esslinger1989). Most extrolites are restricted to medullary tissue, though some cortical substances are also known (Esslinger Reference Esslinger1989). In addition, all Oropogon species examined are known to produce the cell wall polysaccharide lichenan (Cetraria-type), while only one species also synthesized isolichenan (O. caespitosus); these findings are consistent with other Parmeliaceae genera where the production of lichenan is typically conserved at the genus level, while the synthesis of isolichenan often appears variable within genera (Common Reference Common1991).
Based on collections made largely from the Oaxacan Highlands in southern Mexico, Leavitt et al. (Reference Leavitt, Esslinger and Lumbsch2012a ) demonstrated that species boundaries developed by Esslinger (Reference Esslinger1989) for Oropogon, based on morphological, anatomical and chemical data, are largely supported by molecular sequence data, with the exception of O. caespitosus Essl. Oropogon caespitosus isolates could be placed into three putative species-level lineages, two of which (‘O. caespitosus A’ and ‘O. caespitosus B’), were closely related to one another and to O. mexicanus Essl. However, samples from the third lineage, ‘O. caespitosus C’, were distantly related to other O. caespitosus samples, forming a separate clade with O. loxensis, O. atranorinus and O. bicolor (Leavitt et al. Reference Leavitt, Esslinger and Lumbsch2012a ). The separation of ‘O. caespitosus C’ as a distinct species was corroborated by the subsequent discovery of differences in their production of extrolites, ‘O. caespitosus C’ having protocetraric acid as the major extrolite, in contrast to fumaroprotocetraric acid found in O. caespitosus s. str. (Esslinger Reference Esslinger1989; Leavitt et al. Reference Leavitt, Esslinger and Lumbsch2012a ).
Esslinger (Reference Esslinger1989) had previously noted that O. americanus Essl. specimens from the northern part of its range differed in their type of pseudocyphellae from specimens in the southern part of its distribution. In contrast to the pseudocyphellae that are open to a hollow thallus centre in O. americanus s. str., Oropogon aff. americanus from Mexico and Guatemala produce pseudocyphellae that perforate the cortex but are closed by a thin medullary layer which is continuous beneath them (Fig. 1; Esslinger Reference Esslinger1989). Recently, Oropogon aff. americanus specimens from the Oaxacan Highlands were included in a phylogenetic study of Oropogon diversity in Mexico (Leavitt et al. Reference Leavitt, Esslinger and Lumbsch2012a ). However, due to a lack of fresh specimens of southern O. americanus s. str. (with pseudocyphellae that open to a hollow thallus centre), the relationship of the northern and southern elements has not yet been assessed within a molecular phylogenetic framework.
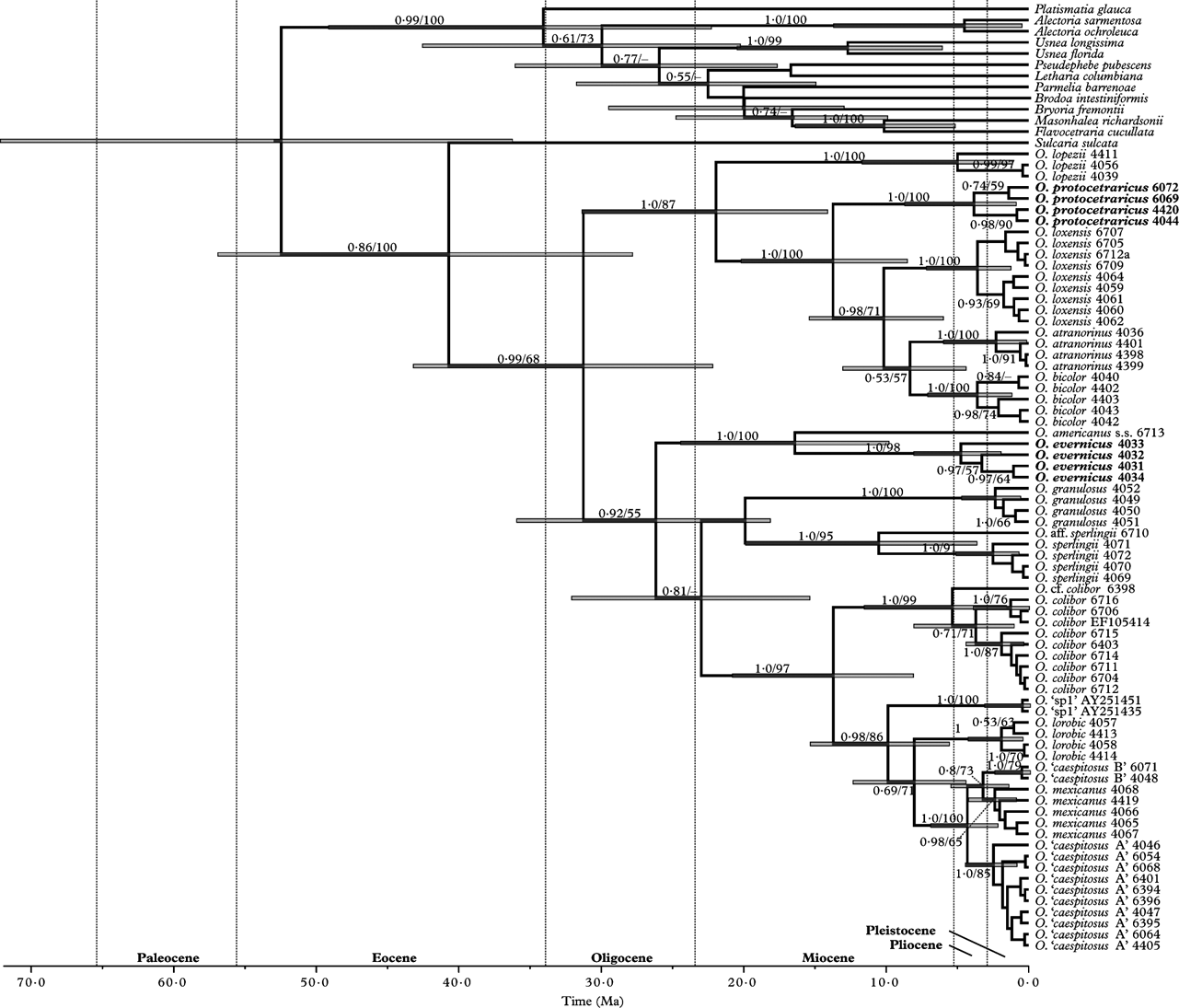
Fig. 1. Oropogon chronogram estimated from a partitioned dataset consisting of three loci (ITS, nuLSU and β-tubulin) under a relaxed molecular clock. The divergence times correspond to the mean posterior estimate of their age (in millions of years). The bars indicate the 95% highest posterior density interval for the estimated divergence times. Values on branches indicate posterior probabilities from a Bayesian analysis using the program BEAST and bootstrap values from a ML analysis using the program RAxML.
In the present study, we assessed the morphology and chemistry of O. caespitosus s. lat. collections from southern Mexico, together with representatives of O. americanus s. str. and O. aff. americanus, and inferred phylogenetic relationships among these groups using two nuclear ribosomal markers (ITS and partial nuLSU) and a fragment of the protein-coding β-tubulin gene. Here, we formally describe Oropogon protocetraricus as a new species, which is distinguished from O. caespitosus by its secondary chemistry as well as molecular data. Furthermore, molecular data confirm that Oropogon aff. americanus from Mexico and Guatemala, with pseudocyphellae only opening to the medullary layer, is a species distinct from O. americanus s. str., and it is here described as O. evernicus.
Materials and Methods
A total of 73 Oropogon specimens were included in the molecular dataset (Table 1), including all Oropogon specimens from Leavitt et al. (Reference Leavitt, Esslinger and Lumbsch2012a ). In the present study, we generated sequence data from additional O. caespitosus s. lat. specimens collected from the Oaxacan Highlands and collections made from Cerro de la Muerte, Talamanca Range, Costa Rica. The specimens collected from Cerro de la Muerte included a single collection of O. americanus s. str. Overall, our sampling included the following species: O. americanus s. lat., O. atranorinus, O. bicolor, O. caespitosus s. lat., O. colibor, O. granulosus, O. lopezii, O. lorobic, O. loxensis, O. mexicanus, and O. sperlingii. The position of Oropogon within Parmeliaceae remains unresolved, and we selected Alectoria ochroleuca, A. sarmentosa, Brodoa intestiniformis, Bryoria fremontii, Flavocetraria cucullata, Letharia columbiana, Masonhalea richardsonii, Parmelia barrenoae, Platismatia glauca, Pseudephebe pubescens, Usnea florida, and U. longissima as outgroups (Blanco et al. Reference Blanco, Crespo, Elix, Hawksworth and Lumbsch2004, Reference Blanco, Crespo, Ree and Lumbsch2006; Crespo et al. Reference Crespo, Lumbsch, Mattsson, Blanco, Divakar, Articus, Wiklund, Bawingan and Wedin2007, Reference Crespo, Kauff, Divakar, del-Prado, Pérez-Ortega, Amo de Paz, Ferencova, Blanco, Roca-Valiente and Nunez-Zapata2010). For some outgroup taxa, sequences were combined from different individuals representing the same species using available sequences from GenBank, in order to maximize the number of loci represented for each taxon (see Table 1). Although some GenBank accessions represent misidentified taxa, we assumed that sequences from distinct individuals in chimeric operational taxonomic units (OTUs) were at least correctly identified to genus.
Table 1. Voucher information for material and NCBI GenBank accession numbers for all sequences included in the present study.

Laboratory methods
All Oropogon specimens were studied using standard techniques of light microscopy and extrolites were identified by thin-layer chromatography (TLC; Orange et al. Reference Orange, James and White2001; Lumbsch Reference Lumbsch, Kranner, Beckett and Varma2002). DNA extraction, polymerase chain reactions (PCR), and sequencing reactions followed Leavitt et al. (Reference Leavitt, Esslinger and Lumbsch2012a ). In short, total genomic DNA was extracted from a small section of thallus material using the Prepease DNA Isolation Kit (USB, Cleveland, Ohio, USA), and the plant leaf extraction protocol. The ITS and nuLSU markers were amplified from new specimens via PCR using the primers ITS1F (Gardes & Bruns Reference Gardes and Bruns1993) with either ITS4 (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990) or ITS4a (Kroken & Taylor Reference Kroken and Taylor2001), and LROR (Cubeta et al. Reference Cubeta, Echandi, Abernethy and Vilgalys1991) with LR3 (Vilgalys & Hester Reference Vilgalys and Hester1990), respectively. PCR products were quantified on 1% agarose gel and stained with ethidium bromide. Amplification products were cleaned using ExoSAP-IT (USB, Cleveland, Ohio, USA), following the manufacturer's instructions. Complementary strands were sequenced from cleaned PCR products using BigDye v3.1 (Applied Biosystems, Foster City, CA, USA). Products were run on an ABI 3730 automated sequencer according to recommended protocols (Applied Biosystems) at the Pritzker Laboratory for Molecular Systematics and Evolution at The Field Museum (Chicago, IL, USA).
Sequence alignment
We assembled and edited sequences using the program Sequencher version 4.10.1 (Gene Codes Corporation, Ann Arbor, MI). Sequence identity was confirmed using the ‘megaBLAST’ search function in GenBank (Wheeler et al. Reference Wheeler, Barrett, Benson, Bryant, Canese, Chetvernin, Church, DiCuccio, Edgar and Federhen2006).
Although aligning the β-tubulin sequences was straightforward, both nuclear ribosomal markers (ITS and LSU) contained a number of difficult-to-align regions (see Leavitt et al. Reference Leavitt, Esslinger and Lumbsch2012a ). Regions that are difficult to align may carry substantial phylogenetic signal, and excluding gaps and variable regions has been shown to be detrimental in some cases (Lee Reference Lee2001; Liu et al. Reference Liu, Raghavan, Nelesen, Linder and Warnow2009; Dessimoz & Gil Reference Dessimoz and Gil2010; Lücking et al. Reference Lücking, Hodkinson, Stamatakis and Cartwright2011), including a previous study of Oropogon (Leavitt et al. Reference Leavitt, Esslinger and Lumbsch2012a ). In order to include variable regions in the ITS and LSU markers, we used the program SATé version 2.2.5 (Liu et al. Reference Liu, Raghavan, Nelesen, Linder and Warnow2009). SATé has been shown to improve alignment accuracy, compared to other currently available programs, making possible the use of otherwise difficult to align regions (Liu et al. Reference Liu, Raghavan, Nelesen, Linder and Warnow2009, Reference Liu, Warnow, Holder, Nelesen, Yu, Stamatakis and Linder2012). We aligned the ITS and LSU sequences in SATé using the following options: ‘Aligner’=MAFFT, ‘Merger’=MUSCLE, ‘Tree Estimator’ = RAxML, and ‘RAxML Model’ = GTRGAMMA. Each alignment was run for 24 h (>10 000 iterations) under the remaining default SATé settings.
Phylogenetic analyses
In order to assess the relationships of the new specimens included in this study, we estimated phylogenetic relationships from the concatenated three-gene dataset (ITS, nuLSU, and β-tubulin) (see justification in Leavitt et al. Reference Leavitt, Esslinger and Lumbsch2012a ). We performed a maximum likelihood analysis of the concatenated three-gene dataset in RAxML v7.3.2 (Stamatakis Reference Stamatakis2006; Stamatakis et al. Reference Stamatakis, Hoover and Rougemont2008). We used the GTRGAMMA model, which includes a parameter (Γ) for rate heterogeneity among sites, and chose not to include a parameter for estimating the proportion of invariable sites (Stamatakis Reference Stamatakis2006; Stamatakis et al. Reference Stamatakis, Hoover and Rougemont2008). A search combining 200 separate maximum likelihood searches (to find the optimal tree) and 1000 ‘fast bootstrap’ replicates (Stamatakis et al. Reference Stamatakis, Hoover and Rougemont2008) to evaluate support for each node was conducted. Relationships were considered supported if they had ML bootstrap support (BS) values of ≥70%.
We also estimated relationships and divergence times from the concatenated data matrices using a Bayesian approach implemented in the program BEAST version 1.7.4 (Drummond & Rambaut Reference Drummond and Rambaut2007), following methods described in Leavitt et al. (Reference Leavitt, Esslinger and Lumbsch2012a ). In short, the concatenated data matrix was analyzed using a relaxed clock model (uncorrelated lognormal), with a birth-death model prior for the node heights and unlinked substitutions models across the loci. Models of DNA sequence evolution for each marker were selected with the program jModeltest v0.1 (Posada Reference Posada2008), using the Akaike Information Criterion (AIC). To estimate the time to the most recent common ancestor (MRCA) for all clades, we used the LSU rate of 0·70×10–9 s/s/y estimated for Parmeliaceae (Protoparmelia excluded; Amo de Paz et al. Reference Amo de Paz, Cubas, Divakar, Lumbsch and Crespo2011), and for the ITS, we used the rate estimated for the parmelioid genus Melanohalea (3·30 × 10–9 s/s/y; Leavitt et al. Reference Leavitt, Esslinger, Divakar and Lumbsch2012b ). Branch rates were drawn from a lognormal distribution. Two independent analyses were run for 50 million generations and parameter values were sampled every 1000 generations. The output from each analysis was visualized using Tracer version 1.5 (Rambaut & Drummond Reference Rambaut and Drummond2003) to assess convergence and effective sampling size (ESS), and we also compared summarized tree topologies from separate runs. Based on these results, the first 12·5 million generations from each run were discarded as burn-in, and remaining samples were summarized as a maximum clade credibility tree with mean divergence times and highest posterior density intervals (HPD) of age estimates using the program TreeAnnotator version 1.7.4 (Rambaut & Drummond Reference Rambaut and Drummond2009).
Results and Discussion
The complete three-locus data matrix included 85 samples (including outgroups), consisting of 1740 aligned nucleotide position characters (ITS: 549; nuLSU: 537; β-tubulin: 654; TreeBase ID: 13959). All new sequences generated for this study have been deposited in GenBank under accession nos. KC667030–KC667052 (Table 1).
The partitioned ML analysis and Bayesian analysis yielded identical topologies and we present the Bayesian phylogeny in Fig. 1 (the ML topology is shown in Appendix, Fig. A1). Well-supported relationships among Oropogon lineages were similar to those presented in Leavitt et al. (Reference Leavitt, Esslinger and Lumbsch2012a ), with well-supported monophyletic clades generally corresponding to traditionally circumscribed species. All O. caespitosus s. lat. specimens containing protocetraric acid as the major extrolite were recovered in a well-supported monophyletic clade (labelled as ‘O. protocetraricus’) (Fig. 1). The single specimen representing O. americanus s. str. was recovered with strong support as sister to Oropogon aff. americanus from the Oaxacan Highlands, Mexico, with pseudocyphellae only open to the medullary layer (labelled here as ‘O. evernicus’) (Fig. 1).
The new species Oropogon protocetraricus was recovered in a well-supported clade along with O. atranorinus, O. bicolor, O. lopezii, and O. loxesnsis (Fig. 1). Species within this clade are all known to produce varying levels of protocetraric acid, although protocetraric acid is not consistently found in O. bicolor. In contrast, protocetraric acid is not present in the clade containing O. caespitosus, including lineages identified previously as ‘O. caespitosus A’ and ‘O. caespitosus B’, O. lorobic, and O. mexicanus (Fig. 1). Apart from differences in the production of extrolites, no morphological characters were observed corroborating the separation of O. caespitosus and O. protocetraricus. Within the O. caespitosus/O. mexicanus clade, O. caespitosus was not recovered as monophyletic (Fig. 2). The two species-level lineages representing O. caespitosus have been previously recognized as ‘O. caespitosus A’ and ‘O. caespitosus B’ (Leavitt et al. Reference Leavitt, Esslinger and Lumbsch2012a ). However, diagnostic morphological/chemical characters have not been observed corroborating the distinction of these two lineages as separate species. Improved molecular sampling and additional morphological investigations will be required to confirm the independence of these lineages and relationships within the O. caespitosus/O. mexicanus clade.
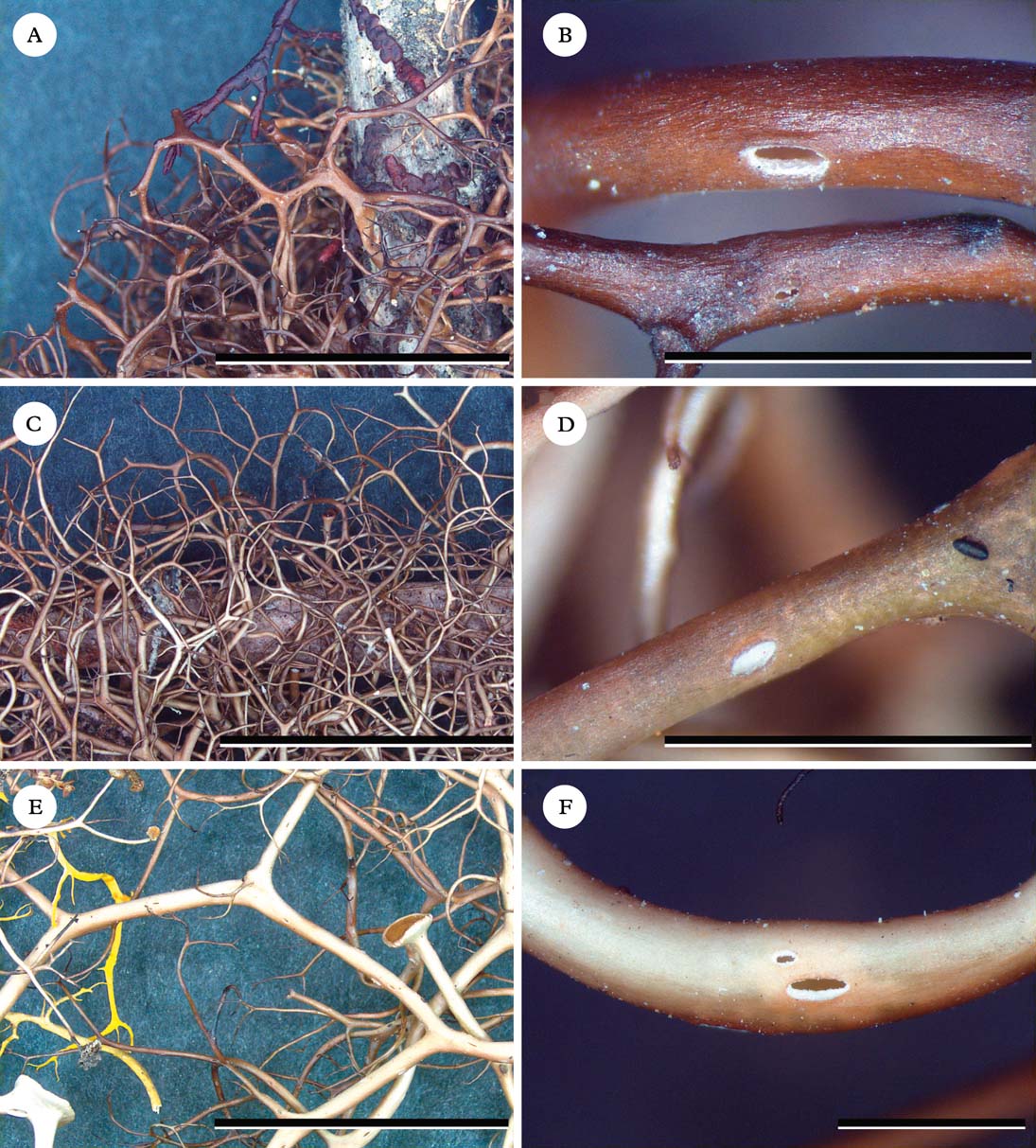
Fig. 2. Oropogon species, habit and close-ups of pseudocyphellae. A & B, Oropogon americanus (Arvidsson et al. 5898, TLE); C & D, O. evernicus (Esslinger 18591 – holotype, F); E & F, O. protocetraricus (Esslinger 18590 – holotype, F). Scales: A, C & E=1 cm; B, D & F=1 mm. In colour online.
Based on these data, the initial radiation of Oropogon is estimated to have occurred c. 31·4 Ma (HPD=22·3–43·3), as illustrated in Fig. 1. The split between O. americanus and ‘O. evernicus’ is estimated to have occurred c. 16·5 Ma (HPD=9·9–24·5), and the separation of ‘O. protocetraricus’ and its sister clade c. 13·9 Ma (HPD=8·6–20·3) (Fig. 1). Our estimates of the timing of diversification events in Oropogon support a Miocene-dominated diversification scenario in neotropical Oropogon species in Central America (Leavitt et al. Reference Leavitt, Esslinger and Lumbsch2012a ).
Taxonomy. The results of this study (and Leavitt et al. Reference Leavitt, Esslinger and Lumbsch2012a ) necessitate the formal description of these two species, which is made below.
Oropogon evernicus Essl. & S. Leavitt sp. nov.
MycoBank No.: MB 803457
Morphologically similar to Oropogon americanus Essl. but differs by having pseudocyphellae that open only to the medulla and not all the way to the hollow thallus centre.
Type: Mexico, Estado de Oaxaca: c. 65 km (by air) east of Oaxaca City, off Hwy 179 along secondary road to Mixistlan, 17° 09·473′N, 96° 04·138′W, c. 2640 m elev., remnant oak–madrone cloud forest on mountain ridge, with deforested strip on top of ridge, on bark, Esslinger 18591 (F—holotype; B, DUKE, MEXU, TLE— isotypes).
(Fig. 2)
Thallus caespitose, up to 10 or occasionally 12 cm long; branching mostly isodichotomous to rarely anisodichotomous, internodes mostly 2–8 (–12) mm long, main branches mostly up to 0·6 mm or rarely 0·8 mm in diam., pale tan to tan-brown or brown; pseudocyphellae frequent to often rather infrequent, rather narrow and sometimes nearly fissural, open only to the medulla layer. Thallus interior hollow, usually with a thin medulla, but occasionally narrower segments appearing loosely filled; medulla hyphal but soon becoming somewhat granular, off-white to pale yellow or dingy orange.
Apothecia frequent, up to 3 mm diam.; disc more or less flat to weakly concave; margin entire to weakly crenate; hymenium 148–157 µm thick, subhymenium 50–77 µm thick; spores muriform, 1 per ascus, 97–112×35–42 µm.
Pycnidia rare; conidia 4–5×1 µm, weakly and more or less unequally bifusiform.
Chemistry
Thallus reactions: cortex all spot tests negative; medulla PD−, K+ yellow-orange, C+ yellow-orange. Constituents: evernic acid (+++), usually with unknown yellow pigment.
Etymology
The epithet evernicus refers to the major extrolite, evernic acid.
Notes
In the monograph of New World Oropogon (Esslinger Reference Esslinger1989), this taxon was included within the concept of species O. americanus, considered to be distributed from southern Mexico into South America and down the Andes to Peru. It was noted there, however, that the small number of specimens seen from Mexico and Guatemala differ from those further south in the range by having pseudocyphellae that do not open all the way to the hollow thallus centre, but only to the medulla. The additional material now available from Mexico has confirmed that these distinctions are consistent, and that the northern specimens should be recognized as a distinct species. This decision was further confirmed when we had the opportunity to sequence a specimen of O. americanus s. str. from the northern part of its range [Costa Rica, Cerro de la Muerte, Nelsen 4186G (F)].
Additional specimens examined. Mexico: no locality, Muller s. n. (BM, M). Estado de Oaxaca: along Hwy 175 between Tuxtepec and Oaxaca City, c. 25 km north of Ixtlán de Juárez, north of Cerro Machin, 17° 33·154′N, 96° 30·674′W, c. 2745 m elev., pine-oak forest, Esslinger 17912, 17923 (hb. Esslinger); mountain tops c. 6 km (by air) north/north-east of Ixtlán de Juárez, 17° 23·217′N, 96° 28·109′W, c. 2865 m elev., pine-oak-madrone forest, Esslinger 18208A, 18219, 18225B, 18233, 18235, 18241C, 18244 (hb. Esslinger); mountains c. 1·7 km (by air) north-east of Ixtlán de Juárez, 17° 21·424′N, 96° 28·236′W, c. 2660 m elev., pine-oak-madrone forest, Esslinger 18286 (hb. Esslinger); c. 7·7 km (by air) north of Ixtlán de Juárez, off Hwy 175 c. 1·5 km along secondary road to San Pedro Yaneri, 17° 24·416′N, 96° 30·037′W, c. 2890 m elev., pines and hardwoods in partly logged area with many downed branches, Esslinger 18350A, 18356, 18357 (hb. Esslinger); along Hwy 175 between Tuxtepec and Oaxaca City, c. 25 km north of Ixtlán de Juárez, north of Cerro Machin, 17° 33·154′N, 96° 30·674′W, c. 2745 m elev., c. 2840 m elev., pine-oak forest, Esslinger 18387 (hb. Esslinger); mountains N of the city of Oaxaca, La Cumbre, 3150 m, on madrone, Beharrell 855A (hb. Esslinger); 2900 m, on top of a felled oak, Beharrell 895 (ASU), 948 (hb. Esslinger), 1119 (US), 1182A (hb. Esslinger), 1246 (DUKE). Estado de Chiapas: Rancho El Pervenir, c. 3 km adelante de El Escalon., mpio. Huixtán, 16°42′39″N, 92° 30′26″W, c. 2400 m elev., epifita sobre tronco de Pinus Wolf & Sipman 1979 (B).—Guatemala: San Marcos: Volcan Tajumulco, between Las Canojas and top of ridge, 7 mi from San Sebastian, 3300–3900 m, 1940, Steyermark (US).
Oropogon protocetraricus S. Leavitt & Essl. sp. nov.
MycoBank No.: MB 803458
Morphologically similar to Oropogon caespitosus Essl. but differs by the presence of protocetraric acid rather than fumarprotocetraric acid.
Type: Mexico, Estado de Oaxaca, c. 65 km (by air) east of Oaxaca City, off Hwy 179 along secondary road to Mixistlan, 17° 09·473′N, 96° 04·138′W, c. 2640 m elev., remnant oak-madrone cloud forest on mountain ridge, with deforested strip on top of ridge, on bark, Esslinger 18590 (F—holotype; B, DUKE, MEXU, TLE— isotypes).
(Fig. 2)
Thallus caespitose, up to 9 or 10 cm long; branching mostly isodichotomous, internodes mostly 6–14 (–18) mm long, main branches up to 0·8 mm or rarely 1 mm in diam., pale tan to tan-brown or brown, only occasionally with blackened areas; pseudocyphellae usually frequent and conspicuous, open to the hollow thallus centre. Thallus interior hollow, with a thin medulla; medulla hyphal to somewhat granular, white to off-white.
Apothecia frequent, up to 3 or 4 mm diam.; disc more or less flat to weakly concave; margin entire to weakly crenate; hymenium 150–170 µm thick, subhymenium 51–80 µm thick; spores muriform, 1 per ascus, 114–128×38–48 µm.
Pycnidia not seen.
Chemistry
Thallus reactions: cortex all spot tests negative; medulla PD+ orange or red-orange, K− or often K+ yellow-orange, C−, KC+ fleeting pale rose or occasionally KC−. Constituents: protocetraric acid (+++), occasional trace unknowns.
Etymology
The epithet protocetraricus refers to the diagnostic extrolite, protocetraric acid, separating O. caespitosus from O. protocetraricus.
Notes
Oropogon protocetraricus is morphologically indistinguishable from O. caespitosus, and in fact was first recognized as a unique genetic entity during a molecular study of O. caespitosus and other common Mexican species of the genus (Leavitt et al. Reference Leavitt, Esslinger and Lumbsch2012a ). Subsequent study by thin-layer chromatography showed that this new entity is chemically distinct, producing protocetraric acid as its major secondary compound, whereas the major compound produced by O. caespitosus s. str. is the related depsidone fumarprotocetraric acid, occasionally accompanied by trace amounts of protocetraric acid. The new species is known from four localities in Oaxaca State, southern Mexico, where it is sympatric with O. caespitosus and often grows with that species. In fact, the type specimen of O. protocetraricus was segregated from a large mixed collection of the two species.
Additional specimens examined. Mexico: Estado de Oaxaca: mountain tops c. 6 km (by air) north/north-east of Ixtlán de Juárez, 17° 23·217′N, 96° 28·109′W, c. 2865 m elev., pine-oak-madrone forest, Esslinger 18230A, 18241A, 18269 (hb. Esslinger); along Hwy 175 between Tuxtepec and Oaxaca City, c. 25 km north of Ixtlán de Juárez, north of Cerro Machin, 17° 33·154'N, 96° 30·674′W, c. 2745 m elev., pine-oak forest, Esslinger 17898 (hb. Esslinger); 17° 33·065′N, 96° 30·695′W, c. 2840 m elev., pine-oak forest, Esslinger 18387A/2 (hb. Esslinger).
The authors thank Kevin Feldheim (Pritzker Laboratory for Molecular Systematics and Evolution at the Field Museum), Sergio Garcia, Warren Chatwin, and Nicolas Koutsoubelis for valuable contributions in the laboratory. They also thank anonymous reviewers who provided valuable comments that improved this manuscript. Support from the National Science Foundation is gratefully acknowledged (“Hidden diversity in parmelioid lichens”, DEB-0949147, awarded to HTL and TLE). The collections by Esslinger were made under the auspices of NSF grant DEB-0614578 to Arizona State University, and Thomas Nash III of that institution and Dra. M. Herrera of UNAM are thanked for arranging the fieldwork. Collections by MPN were made thanks to funding from the Committee on Evolutionary Biology (University of Chicago) to attend an Organization for Tropical Studies course in Costa Rica. MPN was also supported by the Brown Family Fellowship through the Field Museum.
Appendix

Figure A1. Maximum likelihood phylogenetic analysis for sampled Oropogon estimated from a partitioned dataset consisting of three loci (ITS, nuLSU and β-tubulin) in RAxML. Values on branches indicate bootstrap values.






