Introduction
Grain hardness (kernel texture) is one of the most important end-use quality characteristics of cultivated common wheat (Triticum aestivum L.), as it has a profound effect on milling, processing and utilization. Based on the texture of the mature kernel, common wheat varieties are typically classified as ‘hard’ or ‘soft’. Soft wheat varieties are used for cakes, cookies, pastries and some types of noodles, whereas hard wheat varieties are used for breads and other yeast-leavened foods (Morris and Rose, Reference Morris, Rose, Henry and Kettlewell1996).
Much of the variation in wheat grain hardness is controlled by a single locus, called hardness (Ha) (Symes, Reference Symes1965; Baker, Reference Baker1977), which is located on the short arm of chromosome 5D (Mattern et al., Reference Mattern, Morris, Schmidt, Johnson, Sears and Sears1973; Law et al., Reference Law, Young, Brown, Snape and Worland1978). Greenwell and Schofield (Reference Greenwell and Schofield1986) reported the occurrence of ‘friabilin’, a small group of Mr 15k proteins that are abundant on the surface of water-washed starch granules from soft wheat, scarce on the surface of hard wheat starch granules, and absent in durum wheat. The genes encoding friabilin were tightly linked to the Ha locus (Jolly et al., Reference Jolly, Rahman, Korrt and Higgins1993; Sourdille et al., Reference Sourdille, Perretant, Charmet, Leroy, Gautier, Joudrier, Nelson, Sorrells and Bernard1996; Campbell et al., Reference Campbell, Bergman, Gualberto, Anderson, Giroux, Hareland, Fulcher, Sorrells and Finney1999). Friabilin is composed mainly of two proteins, puroindolines a and b, and the respective genes are Pina-D1 and Pinb-D1 (Morris, Reference Morris2002). These puroindoline proteins contain unique tryptophan-rich domains with apparent affinity for phospholipids on the surface of starch granules (Gautier et al., Reference Gautier, Aleman, Guirao, Marion and Joudrier1994; Greenblatt et al., Reference Greenblatt, Bettge and Morris1995; Bhave and Morris, Reference Bhave and Morris2008a, Reference Bhave and Morrisb).
To investigate a direct causal relationship between puroindolines and grain hardness, rice, which has no homologues to the puroindolines (Gautier et al., Reference Gautier, Cosson, Guirao, Alary and Joudrier2000), was transformed with wild-type puroindoline genes (Krishnamurthy and Giroux, Reference Krishnamurthy and Giroux2001). Textural analysis of the transgenic rice seeds indicated that expression of wild-type puroindoline genes reduced rice grain hardness. These data supported the hypothesis that puroindolines play an important role in controlling kernel hardness.
As mentioned previously, durum and related tetraploid wheat varieties lack the genes for puroindolines and consequently have very hard kernels. Upon allopolyploidization, wherein T. turgidum ssp. dicoccoides (Körn. ex Asch & Graebn.) hybridized with Aegilops tauschii (Coss.) forming T. aestivum, the puroindolines contributed by Ae. tauschii restored kernel softness. All soft wheat varieties examined so far carry these ancestral ‘wild-type’ puroindoline gene alleles that have been designated Pina-D1a and Pinb-D1a (Giroux and Morris, Reference Giroux and Morris1997, Reference Giroux and Morris1998; Morris, Reference Morris2002; Bhave and Morris, Reference Bhave and Morris2008a, Reference Bhave and Morrisb). Hard wheat varieties have specific mutations in either of the Pina-D1 or Pinb-D1 genes (Morris, Reference Morris2002; Morris and Bhave, Reference Morris and Bhave2007).
Subsequent to its formation about 7000 years ago in the south-east coastlands of the Caspian Sea (Tsunewaki, Reference Tsunewaki1966, Nishikawa et al., Reference Nishikawa, Furuta and Wada1980), common wheat spread to nearly every corner of the arable earth. During its transmission to Europe, Africa and southern and eastern Asia, mutations were selected, which conferred better adaptation to a wide range of climates. For instance, in China, there are areas of high mountains and severe arid deserts. Thus, the earliest eastward spread of wheat from its centre of origin was not a simple expansion of an ecological continuum. In the process of transmission to eastern Asia, the food products of wheat consumption changed from baked breads (generally flat breads) to steamed bread and noodles.
The genetic composition of wheat varieties reveals important information as to the movement and utilization of germplasm. We previously reported that limited but specific variation of seed storage proteins occur in Japanese common wheat varieties (Tanaka et al., Reference Tanaka, Tomita, Tsujimoto and Yasumuro2003). We also investigated the diversity of the low-molecular-weight glutenin subunit genes in Asian common wheat varieties (Tanaka et al., Reference Tanaka, Toyoda and Tsujimoto2005). The puroindolines are especially useful in this regard and show clear differences in allele/haplotype frequency based on region (Giroux and Morris, Reference Giroux and Morris1998; Lillemo and Morris, Reference Lillemo and Morris2000; Corona et al., Reference Corona, Gazza, Boggini and Pogna2001; Morris et al., Reference Morris, Lillemo, Simeone, Giroux, Babb and Kidwell2001; Pogna et al., Reference Pogna, Gazza, Corona, Zanier, Niglio, Mei, Palumbo, Boggini, Ng and Wrigley2002; Ram et al., Reference Ram, Boyko, Giroux and Gill2002; Bagulho et al., Reference Bagulho, Muacho, Carrillo, Brites, Lafiandra, Masci and D'Ovidio2003; Cane et al., Reference Cane, Spackman and Eagles2004; Chen et al., Reference Chen, He, Xia, Lillemo and Morris2005; Gazza et al., Reference Gazza, Nocente, Ng and Pogna2005; Huang and Röder, Reference Huang and Röder2005; Ikeda et al., Reference Ikeda, Ohnishi, Nagamine, Oda, Hisatomi and Yano2005; Xia et al., Reference Xia, Chen, He, Chen and Morris2005; Chang et al., Reference Chang, Zhang, Xu, Li, Liu, You and Li2006; Lillemo et al., Reference Lillemo, Chen, Xia, William, Pena, Trethowan and He2006; Ravel et al., Reference Ravel, Praud, Murigneux, Canaguier, Sapet, Samson, Balfourier, Dufour, Chalhoub, Brunel, Beckert and Charmet2006; Pickering and Bhave, Reference Pickering and Bhave2007). These surveys on wheat varieties have also been instrumental in discovering new mutations (alleles) in the puroindolines, thus expanding our understanding of these important genes as well as identifying valuable genetic resources for wheat improvement.
In the present study, we surveyed a large number of wheat varieties from eastern Asia for kernel texture and puroindoline haplotype. The frequency of previously known puroindoline haplotypes in Asian common wheat varieties is presented. Furthermore, we report the discovery of a new SNP in puroindoline b.
Materials and methods
Plant materials
We examined 246 varieties of common wheat from Asia (Tables 1 and 2). All of the varieties except those that are designated as ‘Norin’ in Japan are landraces. Japan was divided into two regions, north-eastern Japan (Hokkaido, Tohoku, Kanto and Hokuriku/Nagano) and south-western Japan (Tokai/Kinki, Chugoku and Shikoku/Kyushu; Nakamura et al., Reference Nakamura, Inazu and Hirano1999), based on climate. Many varieties within each region were bred using germplasm within the same region. Fukunaga and Inagaki (Reference Fukunaga and Inagaki1985) reported genealogical pedigrees of Japanese wheat lines and showed that the cultivars within each region were similar to each other, but those between the regions were differentiated. China was grouped into seven regions: eastern China (Shandong, Jiangsu, Anhui, Zhejiang and Fujian Provinces), north-western China (Shaanxi, Gansu and Ningxia Provinces), north-eastern China (Heilongjiang, Jilin and Liaoning Provinces), Sichuan, Inner Mongolia, Xinjiang and Tibet (Tsujimoto et al., Reference Tsujimoto, Yamada and Sasakuma1998). A few varieties were identified without a specific province and are indicated as only ‘China’. We also examined the AABB tetraploid durum (T. turgidum ssp. durum Desf. [Husn.]) variety, ‘Stewart’. The varieties were maintained at Tottori University as a part of the National Bioresources Project – Wheat, Japan.
Table 1 Soft wheat varieties (Pina-D1a/Pinb-D1a, SKCS hardness index <50, n=169) from eastern Asia classified according to geographic origin

Table 2 Hard wheat varieties (SKCS hardness index >50, n=77) from eastern Asia classified according to Puroindoline haplotype and geographic origin

Kernel texture and puroindoline analyses
Kernel texture of all 246 varieties was determined using the Perten Single Kernel Characterization System (SKCS) 4100 (Perten Instruments Inc., Springfield, IL) on a 50-kernel sample each of clean, unbroken wheat kernels, according to the manufacturer's instructions (see also AACC International, 2000).
Puroindoline gene sequence analysis was conducted by one of two procedures, depending on whether the analysis was performed in Japan or the United States. Total genomic DNA was extracted from young leaves (Japan) or dry mature seeds (USA) by the CTAB method (Murray and Thompson, Reference Murray and Thompson1980) and used as template for PCR. The PCR primers used to amplify the puroindoline genes were those reported by Gautier et al. (Reference Gautier, Aleman, Guirao, Marion and Joudrier1994) as follows: 5′-ATGAAGGCCCTCTTCCTCA-3′ and 5′-TCACCAGTAATAGCCAATAGTG-3′ for the amplification of the full-length (447 bp) puroindoline a gene, and 5′-ATGAAGACCTTATTCCTCCTA-3′ and 5′-TCACCAGTAATAGCCACTAGGGAA-3′ for the amplification of the full-length (447 bp) puroindoline b gene.
PCR amplification (Japan) was performed using TaKaRa Ex Taq DNA polymerase (2.5 U, TaKaRa) in 100 μl of reaction buffer (TaKaRa, 2 mM MgCl2) containing 200 ng of genomic DNA, 200 μM of each dNTP and 40 pmol of each primer. The PCR conditions were 93°C for 4 min followed by 35 cycles of 94°C for 60 s, 53°C for 90 s and 72°C for 120 s. A final cycle with an extension of 10 min at 72°C completed the reaction.
PCR amplification (USA) was performed using Taq DNA polymerase (0.5 U, Promega, Madison, WI) in 25 μl of 1 × Taq DNA polymerase reaction buffer (1.5 mM MgCl2) containing 100 ng of genomic DNA, 250 μM of each dNTP and 10 pmol of each primer. The PCR conditions were 94°C for 4 min followed by 35 cycles of 94°C for 60 s, 58°C for 90 s and 72°C for 120 s. A final cycle with an extension of 10 min at 72°C completed the reaction.
The products were separated in 1.5% (w/v) agarose gel, stained with ethidium bromide and visualized using UV light. Each amplified fragment was extracted from the agarose gel using QIAquick Gel Extraction Kit (QIAGEN) and inserted into the cloning site of the plasmid vector pGEM-T Easy (Promega, Japan), or purified from the dNTPs and oligonucleotide primers with exonuclease I and shrimp alkaline phosphatase (ExoSAP-IT, UBS, Cleveland, OH).
DNA sequences were determined on a DNA sequencer (ABI PRISM 3100 Genetic Analyzer, PerkinElmer Applied Biosystems Division, Foster City, CA) using the BigDye Terminator v3.0 (Japan) or v3.1 (USA) Cycle Sequencing Kit (Applied Biosystems). The sequencing primers were as follows: M13 (Japan) and those described above for primary gene amplification (Gautier et al., Reference Gautier, Aleman, Guirao, Marion and Joudrier1994). The sequence results were visualized using Sequencing Scanner v1.0 (Applied Biosystems) and analyzed using GENETYX v7.0 (GENETYX).
Isolation of Triton X-114-soluble proteins, which includes the puroindolines, and protein electrophoresis were carried out on single kernels according to the procedure of Giroux and Morris (Reference Giroux and Morris1998) (Japan and USA).
Results
Kernel texture
SKCS hardness indexes (HIs) for all the Asian common wheat varieties ranged from KU3333 (13.5) to KT020-584 (97.8) (Fig. 1), with a corresponding range in standard deviation (50 kernels in each variety) from 7.5 to 17.2. Standard deviation values in this range are indicative of homogeneous samples. Generally, the arbitrary separation of kernel texture classes is set at 50, since the SKCS 4100 kernel texture algorithm was designed to emulate the forerunning ground-grain near-infrared reflectance (NIR) spectrometer system, which sets a mean value of soft wheat varieties at 25, a mean value of hard wheat varieties at 75 and a separation of classes at 50 (AACC International, 2000, Approved Method 39-70A) (Morris, Reference Morris2002). Using 50, then, as a delimiter for class boundaries, the ‘soft group’ comprised 169 varieties (SKCS HI < 50) and the ‘hard group’ comprised 77 varieties (SKCS HI>50). The soft wheat varieties (Pina-D1a/Pinb-D1a, HI < 50) are presented in Table 1 according to their geographic origin.
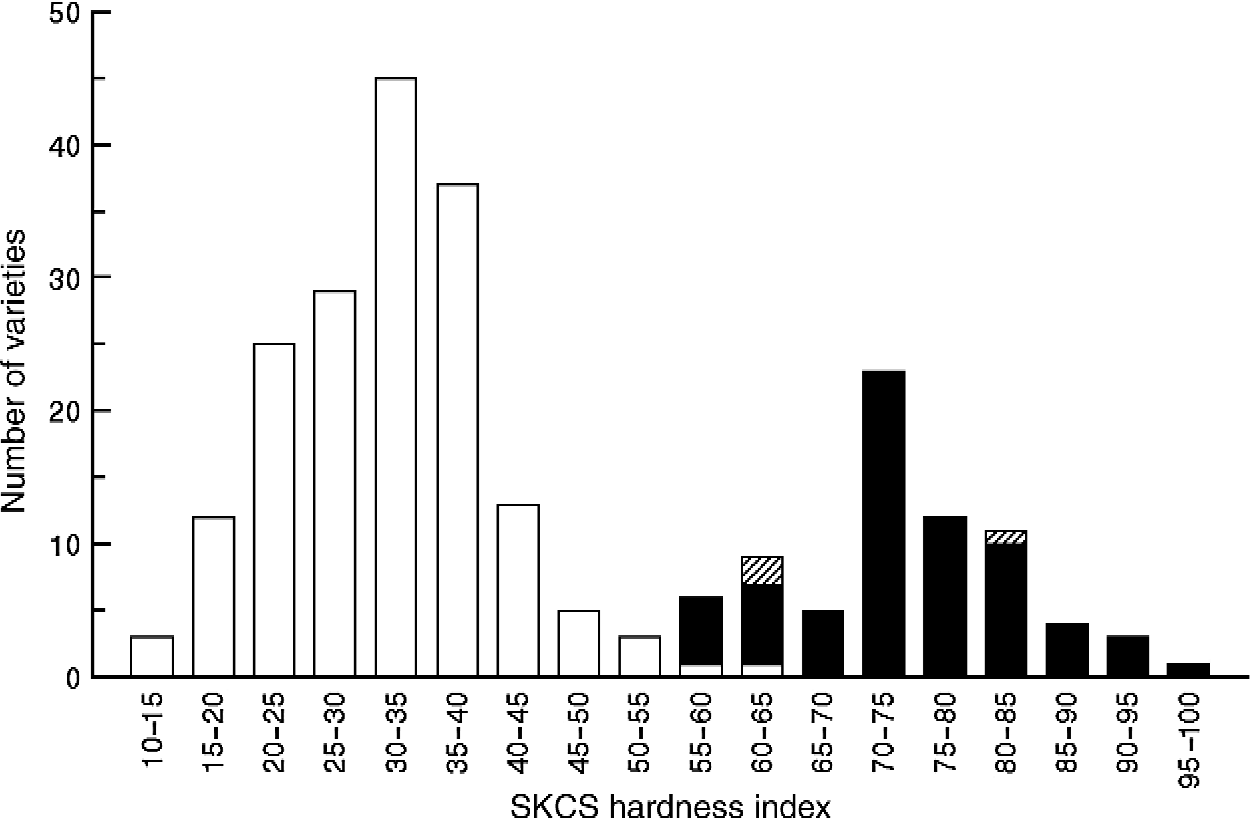
Fig. 1 Frequency distribution histogram of SKCS hardness index of 246 common wheat varieties from Asia. Varieties with the soft ‘wild-type’ puroindoline haplotype of Pina-D1a/Pinb-D1a are denoted by ‘open’ bars; varieties with the a characterized ‘hard’ puroindoline haplotype and the ‘double null’ variety KT020-584 are denoted by solid bars and three varieties with Pinb-D1a but unknown puroindoline a are denoted by ‘hatched’ bars.
Characterization of the puroindoline genes
We next sought to resolve the molecular genetic basis for the hard kernel phenotype exhibited by those varieties with HI>50. It is now well established that the presence/absence and sequence polymorphism of the puroindolines explains both the major classifications into ‘soft’ and ‘hard’, as well as some of the variation within the hard class (Morris, Reference Morris2002; Bhave and Morris, Reference Bhave and Morris2008a, Reference Bhave and Morrisb; Morris and Bhave, Reference Morris and Bhave2007). Since, it also seems, at present, that puroindoline b is more polymorphic than puroindoline a, at least in T. aestivum, we began our analysis by obtaining gene sequence for puroindoline b. To provide additional coverage of the germplasm, we included the first three lines with HI ranked just less than 50 (KT020-546, HI 48.8; KT020-529, HI 49.3; KU7070, HI 49.5).
Cloning and direct PCR sequencing of puroindoline b identified three of the known mutations associated with hard kernel trait in wheat. These were the G-to-A (designated hereafter ‘G/A’) SNP at 226 that confers the Gly-46 to Ser change, the T/C SNP at 266 that confers the Leu-60 to Pro change and the deletion of an A at 210–213. Because the A deletion occurs somewhere in the wild-type sequence of CAAAAT, we have assigned its location to the last of the A's, in other words, at position 213. This SNP produces an open reading frame shift after Thr-41. The current allele assignments for these mutations are Pinb-D1b, Pinb-D1c and Pinb-D1p, respectively (Morris and Bhave, Reference Morris and Bhave2007). Of the 80 varieties examined (i.e. 77 with HI>50, plus three with HI of 48.8–49.5), these three puroindoline b sequences accounted for 53 (Table 2).
Sequencing of puroindoline b led to the discovery of a new SNP that has not been previously described. It is a C/T SNP at position 382 and converts the CAG (Glu) codon to a TAG stop signal (Fig. 2). This SNP was provided the Pinb-D1ab allelic designation (Table 2) (Morris and Bhave, Reference Morris and Bhave2007). This novel sequence was present in accessions KU3062 and KU3069, both from Afghanistan, and was obtained via cloning (Japan) and from six individual kernels (USA). Its sequence has been entered into GenBank (AB302894).
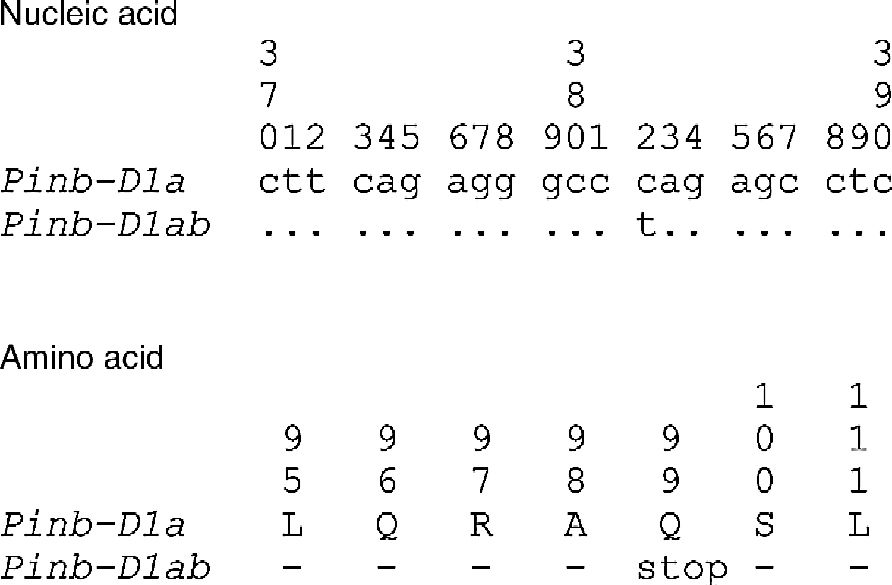
Fig. 2 Partial nucleic acid and amino-acid sequence of puroindoline b showing the wild-type (Pinb-D1a) and new SNP mutation (Pinb-D1ab). The C/T mutation at position 382 (numbering begins from the ATG start codon) produces a premature stop codon at processed protein position 99 (Glu-to-stop).
In all other varieties except one, sequencing produced the ‘wild-type’ puroindoline b sequence, which is designated Pinb-D1a. Repeated efforts to amplify puroindoline b from KT020-584 (HI = 97.8, China, Xinjiang) were unsuccessful.
Next, we characterized puroindoline a from the remaining hard varieties with HI>50 (17 that did not carry an identified mutation in puroindoline b, i.e. alleles Pinb-D1b, c, p and ab), plus the three varieties with HIs of 48.8–49.5. Analysis of puroindoline a is more problematic because applying the puroindoline a primers of Gautier et al. (Reference Gautier, Aleman, Guirao, Marion and Joudrier1994) do not produce a product in those varieties carrying the Pina-D1b mutation, a deletion of 15,380 bp (Giroux and Morris, Reference Giroux and Morris1998; Bhave and Morris, Reference Bhave and Morris2008a; Morris and Bhave, Reference Morris and Bhave2007). Nevertheless, wild-type puroindoline a sequence was obtained for KT020-529, KT020-546, KU4704, KU4705, KU4739, KU7001, KU7005 and KU7070 (Tables 1 and 2). With the exception of KU4739, these varieties represented seven of the eight softest varieties included in this set of 80 varieties with HI ≥ 48.8. The hardest of these eight lines, KU4739, had an HI of 61.8. In summary, these eight lines had the wild-type Pina-D1a/Pinb-D1a haplotype and HIs from 48.8 to 61.8 (Tables 1 and 2).
Three varieties were found to have a single-base-pair deletion of a C at 264–265 (assigned to 265) in puroindoline a, such that a frame shift in the open reading frame was expected from GGC CAA ATG to GGC AAA TG and a sequence change from Glu-61 to Lys, and thereafter. The varieties possessing this mutation were KT020-531, KT020-534 and KT020-535, with HIs of 71.2–86.0 (Table 2). This SNP has been designated Pina-D1l (Morris and Bhave, Reference Morris and Bhave2007).
Of the remaining varieties, 11 consistently produced no product with the puroindoline a primers, whereas three produced ambiguous or inconsistent results after repeated testing. Of the 11, one was KT020-584, which also produced no puroindoline b product and was the hardest of all 246 varieties. It was assumed to be potentially ‘null’ for both puroindoline genes. The other ten possessed wild-type puroindoline b sequence, and were thus assumed to be Pina-D1b/Pinb-D1a.
Puroindoline gene expression
We next analyzed puroindoline gene expression of all the varieties by analyzing for the presence of puroindoline a and b proteins in SDS–PAGE (Fig. 3). Generally, SDS–PAGE produced clear results and were consistent with the DNA results according to puroindoline haplotypes. Figure 3 provides examples of the typical results encountered: puroindolines were not present in the durum cv. Stewart, Norin 61 is typical of soft wheat varieties and expresses puroindolines a and b at similar relative levels, Haruyutaka is an ‘a-null’ (Pina-D1b) and lacks puroindoline a expression, Haruminori has the Pinb-D1b mutation, which expresses puroindoline b at a lower level relative to puroindoline a, and Haruhikari has the Pinb-D1c mutation, which apparently interferes with either the expression or the isolation of puroindoline b.

Fig. 3 Example of puroindoline gene expression analysis used to characterize 246 common wheat varieties from Asia. Puroindolines a and b were resolved using SDS–PAGE into two bands located around Mr 15k. Both puroindolines a and b are absent in the very hard durum variety, Stewart (lane 1), but present in the soft wheat variety, Norin 61 (lane 2). The hard wheat variety, Haruyutaka (Pina-D1b/Pinb-D1a), is an ‘a-null’ variety with no puroindoline a expression (lane 3). The hard wheat variety Haruminori (Pina-D1a/Pinb-D1b) has a mutation in puroindoline b, which generally reduces puroindoline b expression relative to puroindoline a (lane 4). The hard wheat variety Haruhikari (Pina-D1a/Pinb-D1c) has a mutation in puroindoline b, which either interferes with the expression or extraction such that puroindoline b is not detected (lane 5). Arrowheads indicate puroindolines a (upper) and b (lower).
Of the previously mentioned ten varieties that possessed the wild-type puroindoline b sequence but produced no puroindoline a PCR product, none exhibited puroindoline a on SDS–PAGE, therefore supporting their classification as having the Pina-D1b/Pinb-D1a haplotype (Table 2).
SDS–PAGE was used to attempt to ‘diagnose’ the four varieties (Norin 61, Haruyutaka, Haruminori and Haruhikari) that were still requiring puroindoline haplotype classification, and to provide further information on some of the varieties in the range of HI where the soft and hard classes may overlap. Figure 4 presents the results of this work and is worthy of a brief explanation. Alpowa near-isogenic lines (Morris and King, Reference Morris and King2007) and durum cv. Renville were used as controls. Alpowa is a soft variety and expresses puroindolines a and b. Two of its near-isogenic sister lines possess relevant puroindoline mutations [PI 644084 carries Pinb-D1e (lane 1) and PI 644080 carries Pina-D1b (lane 2)] representing ‘b-null’ and ‘a-null’ controls, respectively. The SDS–PAGE analysis supports the DNA sequence data and the designation of KU4706 (HI 74.9) as a puroindoline ‘a-null’ (Pina-D1b) (lane 3). The SNP deletion and ORF shift present in KT020-531 (HI 71.2, Pina-D1l) appears to interfere with the translation or extraction of puroindoline a (lane 4). KU4739 was the hardest ‘soft’ wheat (HI 61.8). Even though its puroindoline haplotype was determined to be Pina-D1a/Pinb-D1a, it appears to lack or have a very low relative level of puroindoline a expression (lane 5). Sadabouzu with hardness similar to KU4739 (HI 61.5) was one of three varieties for which repeated efforts at sequencing puroindoline a were unsuccessful or produced ambiguous results. Sadabouzu appeared to produce a heavy puroindoline b band relative to puroindoline a (lane 6). KU7001 with an HI of 55.4 and a puroindoline haplotype of Pina-D1a/Pinb-D1a expressed puroindoline b at a relatively higher abundance compared with puroindoline a (lane 7). Lastly, KT020-584 appeared to possess no puroindoline a or b, consistent with its failure to amplify either gene in PCR and its hardest HI (97.8) of any of the 246 varieties included here (lane 10). We refer to it simply as a ‘double null’ (Table 2).

Fig. 4 SDS–PAGE of Triton X-114 proteins from individual kernels of Alpowa near-isogenic line (NIL) PI 644084 puroindoline ‘b-null’ Pinb-D1e (lane 1), PI 644080 puroindoline ‘a-null’ Pina-D1b (lane 2), KU4706 (HI 74.9) puroindoline ‘a-null’ Pina-D1b (lane 3), KT020-531 (HI 71.2) Pina-D1l (lane 4), KU4739 (HI 61.8) Pina-D1a/Pinb-D1a (lane 5), Sadabouzu (HI 61.5) unknown/Pinb-D1a (lane 6), KU7001 (HI 55.4) Pina-D1a/Pinb-D1a (lane 7), Alpowa PI 566596 Pina-D1a/Pinb-D1a (lane 8), durum cv. Renville (lane 9) and KT020-584 (HI 97.8) (lane 10). Arrowheads indicate puroindoline a (upper) and puroindoline b (lower).
Geographical distribution of the puroindoline genotypes
All but three of the 246 varieties included in the study, Sadabouzu, KU4740 and KT020-560, were classified according to their puroindoline haplotypes (Tables 1 and 2). The remaining 243 varieties were summarized according to their puroindoline haplotypes and geographic origin (Table 3). The frequency of soft/hard kernel texture and puroindoline hardness haplotypes varied depending on the origin of the varieties. The lowest frequency of hard varieties occurred in Korea and south-western Japan. Tibet and Pakistan also had low frequencies of hard varieties. The highest frequency of hard varieties appeared in north-east China followed by Afghanistan and Xinjiang. It should be noted that the 13 varieties from China ‘region unknown’ could significantly shift these frequencies up or down. Also, there was no overt effort made to draw ‘representative’ samples of varieties from any of the regions, so that the results reported here are purely ‘observational’.
Table 3 Frequency of puroindoline haplotypes of 246 Asian common wheat varieties based on geographic origin, the total number of lines from each region and the percentage of hard kernel lines from each region

a KU3062 and KU3069 are Pinb-D1ab from Afghanistan; KT020-584 is a ‘double null’ from Xinjiang; KT020-560, Sadabouzu and KU4740 are unknown Pina/Pinb-D1a from east China, south-west Japan and Nepal, respectively.
b Based on SKCS HI delineation of 55.7 for soft and hard classes, KU4739 (Nepal) with an HI of 61.8 was the only hard wheat that possessed the Pina-D1a/Pinb-D1a haplotype.
Given those caveats, it is nevertheless of interest to examine the frequency of specific puroindoline hardness alleles due to their reflection of germplasm movement and utilization, as well as the interaction of wheat with human culture. The Pinb-D1b allele was the most prevalent across eastern Asia, being present in nearly half of all hard varieties (Table 3). Of the less frequent alleles, three of the four Pinb-D1c varieties were from north-east Japan. The Pinb-D1p allele was found predominantly in a geographic band ranging across northern China and Inner Mongolia. Although the numbers were not great, there seemed to be a notable occurrence of Pina-D1b among the few varieties from Nepal and Bhutan (two each, 31% of all varieties from these two countries). All three Pina-D1l varieties came from eastern China/China ‘unknown’. Lastly, the newly discovered SNP was associated with Afghanistan (n = 2) and the ‘double null’ with Xinjiang.
Discussion
Early work on wheat kernel texture and its relationship with puroindoline a and b gene sequence indicated that a limited number of puroindoline haplotypes accounted for the hard texture observed among wheat varieties of North America (Giroux and Morris, Reference Giroux and Morris1998; Morris et al., Reference Morris, Lillemo, Simeone, Giroux, Babb and Kidwell2001). Subsequent surveys conducted on populations from the United States, Canada and Australia would generally support this conclusion (Cane et al., Reference Cane, Spackman and Eagles2004; Lillemo et al., Reference Lillemo, Chen, Xia, William, Pena, Trethowan and He2006; Pickering and Bhave, Reference Pickering and Bhave2007). However, these results also largely reflect the ancestral wheat varieties introduced during European colonization, and germplasm introductions made during the early stages of wheat breeding and hybridization in these countries. Accordingly, these puroindoline haplotypes are quite similar to those encountered in Europe (Lillemo and Morris, Reference Lillemo and Morris2000; Corona et al., Reference Corona, Gazza, Boggini and Pogna2001; Pogna et al., Reference Pogna, Gazza, Corona, Zanier, Niglio, Mei, Palumbo, Boggini, Ng and Wrigley2002; Bagulho et al., Reference Bagulho, Muacho, Carrillo, Brites, Lafiandra, Masci and D'Ovidio2003; Gazza et al., Reference Gazza, Nocente, Ng and Pogna2005; Huang and Röder, Reference Huang and Röder2005; Ravel et al., Reference Ravel, Praud, Murigneux, Canaguier, Sapet, Samson, Balfourier, Dufour, Chalhoub, Brunel, Beckert and Charmet2006). Our survey and others (Chen et al., Reference Chen, He, Xia, Lillemo and Morris2005; Ikeda et al., Reference Ikeda, Ohnishi, Nagamine, Oda, Hisatomi and Yano2005; Xia et al., Reference Xia, Chen, He, Chen and Morris2005; Chang et al., Reference Chang, Zhang, Xu, Li, Liu, You and Li2006) conducted on wheat varieties of eastern Asia indicate some notable differences in both haplotype frequency and richer diversity in mutations/alleles. It is noteworthy that the survey of Ram et al. (Reference Ram, Boyko, Giroux and Gill2002) of Indian wheat varieties bears some relationship with the current puroindoline haplotype composition of the CIMMYT breeding programme (Lillemo et al., Reference Lillemo, Chen, Xia, William, Pena, Trethowan and He2006).
In the present study, most (72.5%) of the wheat varieties possessed the wild-type soft puroindoline haplotypes (Table 3). However, this frequency varied considerably depending on geographic origin. For example, of the seven regions with ten or more varieties, the frequency of hard varieties ranged from 2% (south-west Japan) to 60% in north-west China. Obviously, though reasonably close geographically, some other factor is likely involved in the separation of these gene pools. Other contrasts were between north-east China (78% hard) and Korea (0% hard). Naturally, more extensive and systematic surveys will be required to resolve these associations.
Among the ‘hard’ wheat varieties carrying an identified puroindoline haplotype (n = 74, Table 2), the most prevalent haplotype was Pina-D1a/Pinb-D1b (n = 30, Table 3). Interestingly, this haplotype was also often the most frequent among wheat varieties of the United States, Canada, Australia and Europe.
The second most prevalent haplotype was Pina-D1a/Pinb-D1p (n = 19, Table 3). This SNP deletion appears to be common in eastern Asia but not the Occident. Within Asia, the Pinb-D1p allele appears in a geographic region extending from north-eastern China through Inner Mongolia, north-western China, Xinjiang and Tibet, with the greatest frequency in north-western China (Table 3). This allele was also present in Pakistan and Afghanistan but was not found in Japan. As such, its distribution may be associated with the so-called ‘Silk Road’. Similarly, Nakamura (Reference Nakamura2002) reported that the Glu-D1f allele of high-molecular-weight glutenin might have been introduced along the Silk Road; it appeared frequently in Japanese varieties, especially those in the south-western part of the country. However, Pinb-D1p was not detected in south-western Japan. It may be that the population size and genetic diversity were reduced during the transmission of common wheat to Japan. Tsujimoto et al. (Reference Tsujimoto, Yamada and Sasakuma1998) argued that the low variation between Japanese and eastern Asian wheat varieties was due to a decreased population size during the transmission of common wheat to these areas, and that plants with less genetic variation became the founders of the present common wheat varieties in the region. Tanaka et al. (Reference Tanaka, Tomita, Tsujimoto and Yasumuro2003) also reported that the gliadin patterns of Japanese common wheat varieties differed greatly from those of European countries, and proposed that the limited and specific variation in seed storage protein composition of Japanese varieties may simply be attributable to the limited variability in germplasm used in breeding programmes.
A few specific varieties are worthy of further discussion. The first are KU3062 and KU3069 as they possess a new puroindoline mutation in puroindoline b, a C/T SNP at position 382 that confers a premature stop codon. At HIs of 71.0 and 76.5, this mutation is consistent with the ‘loss-of-function’ model for the kernel softening effect of the two puroindolines. The next variety worth discussing further is KT020-584, which appears to be a ‘double null’ (Table 2). Tranquilli et al. (Reference Tranquilli, Heaton, Chicaiza and Dubcovsky2002) first identified a puroindoline ‘double null’ in the substitution line Chinese Spring (Red Egyptian 5D) [CS(RE5D)]. Subsequently, Ikeda et al. (Reference Ikeda, Ohnishi, Nagamine, Oda, Hisatomi and Yano2005) discovered three Korean and two American varieties of this type. In the case of Tranquilli et al. (Reference Tranquilli, Heaton, Chicaiza and Dubcovsky2002), their adjusted HI for CS(RE5D) was 95.6, the hardest of all varieties tested. In our study, KT020-584 was also the hardest with an HI of 97.8. It seems that the complete absence of both puroindolines, as it occurs in these varieties and in durum, confers the hardest kernel texture. Lastly, even though KU4739, Sadabouzu and KU7001 expressed both puroindolines a and b, they were classified in the ‘hard group’. All of them had a low relative level of puroindoline a expression compared with puroindoline b. Capparelli et al. (Reference Capparelli, Borriello, Giroux and Amoroso2003) reported that puroindoline a expression and presence control the abundance of total puroindoline and its association with starch. Hogg et al. (Reference Hogg, Sripo, Beecher, Martin and Giroux2004) also reported that puroindolines a and b interact and together affect wheat grain texture.
In closing it is of interest to consider other possible factors affecting the frequencies of the puroindoline haplotypes. One of these is cultural preference for specific food products. In other words, the prevalence of some puroindoline haplotypes may be attributed to selective preferences for food quality/processing traits in a given region. We observed that the Pina-D1a/Pinb-D1p genotype appeared most frequently in China. Therefore, this haplotype may have been preferable with respect to Chinese food culture, typified by noodles and dumpling, as opposed to bread in Europe. Although almost all the varieties in south-west Japan are used for noodles, this genotype did not appear in Japan. However, the white firm noodles in China are quite different from the elastic soft udon noodles of Japan. In this regard, it is perhaps equally, if not more, probable that the genetic constitution of wheat in various regions largely reflects the early movement and adaptation to local environments, which then set the stage for influencing the quality attributes and styles of foods that evolved from those particular wheat varieties. The challenge for current wheat improvement is to maintain and improve the culinary end-use qualities of new hybridizations, while advancing yield and resistance to biotic and abiotic stresses. In this regard, wheat and humankind enjoy an interesting interspecific relationship.
Acknowledgements
This study was partly supported by the Elizabeth Arnold Fuji Foundation and Grant-in-Aid for Scientific Research (B) (19380006). The excellent technical assistance of Mishelle Freston, Art Bettge, Alicia Massa and the staff of the USDA ARS Western Wheat Quality Laboratory is warmly acknowledged.









