1. Introduction
The late Ediacaran fossil assemblages of Newfoundland, Canada preserve abundant, large, morphologically complex macro-organisms (Anderson & Misra, Reference Anderson and Misra1968; Narbonne, Reference Narbonne2011; Liu et al. Reference Liu2016) known as the Avalonian Assemblage (Waggoner, Reference Waggoner2003), predominantly found on coastal exposures of the Avalon and Bonavista peninsulas (e.g. Narbonne, Reference Narbonne2005; Liu et al. Reference Liu, Kenchington and Mitchell2015). The most famous of these sites is the Mistaken Point Ecological Reserve, a UNESCO World Heritage Site located on the southeastern portion of the island of Newfoundland (Fig. 1). The original discovery of the Mistaken Point biota occurred on the D and E surfaces of the Mistaken Point Formation at Mistaken Point itself (Anderson & Misra, Reference Anderson and Misra1968; Misra, Reference Misra1969). The fossil assemblage is interpreted to have been preserved in situ on the deep basin floor by smothering under an ash-laden turbidity current (Seilacher, Reference Seilacher1992; Clapham et al. Reference Clapham, Narbonne and Gehling2003; Wood et al. Reference Wood, Dalrymple, Narbonne, Gehling and Clapham2003; Ichaso et al. Reference Ichaso, Dalrymple and Narbonne2007), which has recently been dated at 565.00 ± 0.64 Ma (Matthews et al. Reference Matthews, Liu, Yang, McIlroy, Levell and Condon2020). The E Surface fossils are preserved as both positive and negative epireliefs cast by a fine silty mudstone that, in some cases, preserves very fine morphological details. The E Surface at Mistaken Point has the most abundant and diverse assemblage of Ediacaran macro-organisms found in Newfoundland, of which frondose taxa comprise approximately 50% of the total assemblage (Clapham, Reference Clapham, Laflamme, Schiffbauer and Dornbos2011). The most figured portion of the E Surface is known as ‘Seilacher’s Corner’, which includes a number of similarly orientated fronds of Charniodiscus procerus (Laflamme et al. Reference Laflamme, Narbonne and Anderson2004) as well as abundant negative epirelief casts of the lower surfaces of the fusiform epibenthic recliner Fractofusus (cf. Gehling & Narbonne, Reference Gehling and Narbonne2007; Mitchell et al. Reference Mitchell, Kenchington, Liu, Matthews and Butterfield2015; Dufour & McIlroy, Reference Dufour, McIlroy, Brasier, McIlroy and McLoughlin2017) and also the lower surface of Beothukis mistakensis (Brasier & Antcliffe, Reference Brasier and Antcliffe2009; Fig. 2a). Neither Fractofusus or Beothukis are consisitently orientated in the direction of the stems of C. procerus (Wood et al. Reference Wood, Dalrymple, Narbonne, Gehling and Clapham2003).

Fig. 1. Avalon and Bonavista peninsulas, southeastern Newfoundland, highlighting fossiliferous Ediacaran localities at Mistaken Point, Spaniard’s Bay and the Catalina Dome.

Fig. 2. (a) Holotype of Beothukis mistakensis from the ‘E Surface’ within the Mistaken Point Ecological Reserve. Scale bar, 5 cm. (b) Specimen previously attributed to Beothukis mistakensis from Spaniard’s Bay, showing that the inferred pedal disc and stalk are actually related to obstacle scour and lie within a flute mark. bp – primary branch; cos – crescentic obstacle scour; fl – erosion within flute; pd – pedal holdfast; st – stalk. The current was from left to right. Scale bar, 2 cm. (c) Specimen previously attributed to Beothukis mistakensis from Spaniard’s Bay showing a conical erosion on the up-current end of a shallow flute. fl – flute. The current was from left to right. Scale bar, 2 cm. (d) Holotype of Culmofrons plumosa from Lower Mistaken Point within the Mistaken Point Ecological Reserve. Scale bar, 3 cm.
The Ediacaran biota of Newfoundland was dominated by the Rangeomorpha, named for the first described member of this clade, Rangea schneiderhoehni (Gürich, Reference Gürich1930; Pflüg, Reference Pflüg1970, Reference Pflüg1972; Jenkins, Reference Jenkins1985; Grazhdankin & Seilacher, Reference Grazhdankin and Seilacher2005). This broad clade consists of millimetre- to metre-scale soft-bodied organisms that are characterized by a unipolar to multipolar frondose morphology composed of several orders of self-similar units that resemble Rangea fronds in a range of orientations (e.g. Jenkins, Reference Jenkins1985; Brasier & Antcliffe, Reference Brasier and Antcliffe2004, Reference Brasier and Antcliffe2009; Narbonne, Reference Narbonne2004; Brasier et al. Reference Brasier, Antcliffe and Liu2012). Some rangeomorphs have stems and/or basal discs and a range of growth programmes in the frondose portion that have created a diverse range of morphotaxa (Narbonne, Reference Narbonne2004; Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009; Brasier et al. Reference Brasier, Antcliffe and Liu2012; Laflamme et al. Reference Laflamme, Darroch, Tweedt, Peterson and Erwin2013; Liu et al. Reference Liu2016).
A wide variety of terminology has been used to describe rangeomorph architecture (Pflüg, Reference Pflüg1972; Jenkins, Reference Jenkins, Lipps and Signor1992; Laflamme & Narbonne, Reference Laflamme and Narbonne2008; Brasier & Antcliffe, Reference Brasier and Antcliffe2009; Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009; Brasier et al. Reference Brasier, Antcliffe and Liu2012). The self-similar architecture of the Rangeomorpha is built around the basic rangeomorph unit, or ‘branch’, which is repeated at a range of scales (orders) in the frondose portion of the organism (Narbonne, Reference Narbonne2004; Brasier et al. Reference Brasier, Antcliffe and Liu2012). The primary-order branches are here considered to be the largest rangeomorph units that compose the rows on either side of a central stalk or axis. Small (higher-order) rangeomorph units can be determined within larger (low-order) rangeomorph units (Jenkins, Reference Jenkins1985; Narbonne, Reference Narbonne2004; Brasier & Antcliffe, Reference Brasier and Antcliffe2009), although their precise three-dimensional organization is poorly known in many taxa.
It is generally accepted that all orders of rangeomorph units may be expressed in a number of different orientations and with variable degrees of rotation, openness and/or furling in different taxa (Brasier et al. Reference Brasier, Antcliffe and Liu2012). Rangeomorph taxa have been established based on combinations of: (1) the architecture of the rangeomorph elements; (2) the postulated growth program (insertion versus inflation); and (3) the number of growth tips or poles, plus other characters such as the presence or absence of a stem and basal disc (e.g. Brasier et al. Reference Brasier, Antcliffe and Liu2012). However, there is currently a lack of consensus concerning which morphological characters should be of significance at the genus and species level (e.g. continuous versus discrete branching characters; Liu et al. Reference Liu2016; Kenchington & Wilby, Reference Kenchington, Wilby, Brasier, McIlroy and McLoughlin2017). Our re-examination of the type material of Beothukis has the potential to inform that debate by assessing the validity of the emended diagnosis of Beothukis (Brasier et al. Reference Brasier, Antcliffe and Liu2012) that was broadened and inadvertently overlapped with the diagnosis of the simultaneously introduced genus Culmofrons (Laflamme et al. Reference Laflamme, Flude and Narbonne2012).
1.a. The rangeomorph Beothukis mistakensis
Beothukis is a uniterminal rangeomorph frond, with an oval to spatulate outline that has latterly been considered to have had a stem and holdfast (Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009; Brasier et al. Reference Brasier, Antcliffe and Liu2012; Liu et al. Reference Liu2016). The first mention of the taxon is under the moniker ‘Flat Recliner’ (Seilacher, Reference Seilacher1992), and later as the ‘Spatulate Rangeomorph’ (Laflamme & Narbonne Reference Laflamme and Narbonne2008). Formal description of Beothukis mistakensis (Brasier & Antcliffe, Reference Brasier and Antcliffe2009) noted similarities in its mode of growth to that of Charnia and Bradgatia, with which it is commonly reported in the Newfoundland sections (e.g. Laflamme et al. Reference Laflamme, Narbonne, Greentree, Anderson, Vickers-Rich and Komarower2007; Hofmann et al. Reference Hofmann, O’Brien and King2008; Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009; Liu et al. Reference Liu, Kenchington and Mitchell2015). The most spectacularly preserved material attributed to Beothukis comes from the Trepassey Formation, close to Spaniard’s Bay (Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009), which has a smooth, bulbous structure at the base of the frond that has been called a ‘pedal holdfast’ and separated from the frondose portion by a short stem or sheath (Fig. 2b, c). The resultant reconstruction of B. mistakensis as an erect frond with holdfast and a short stem (Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009) has been accepted in all recent literature on the Mistaken Point biota (Liu et al. Reference Liu, Kenchington and Mitchell2015, Reference Liu2016; Mitchell et al. Reference Mitchell, Kenchington, Harris and Wilby2018), despite the recognition that the Spaniard’s Bay fossils lie in flute marks and linear current scours (Brasier et al. Reference Brasier, Liu, Menon, Matthews, McIlroy and Wacey2013). The structures that resemble stems are likely to be current scours that originate from holdfasts that may not have been associated with the frond (see full discussion in Section 4.a).
1.b. The Beothukis/Culmofrons problem
The taxonomic status of the genus Beothukis is controversial due to an emendation of the original diagnosis (Brasier et al. Reference Brasier, Antcliffe and Liu2012) to include rangeomorphs with a basal disc (holdfast) and stem (Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009; Fig. 2b, c). The emendation occurred at around the same time as the creation of Culmofrons plumosa (Laflamme et al. Reference Laflamme, Flude and Narbonne2012), which has a similar spatulate frond to that of Beothukis, but was differentiated from it by having a larger number of primary-order branches, subparallel secondary branches, and the presence of both a basal disc and stem (Laflamme et al. Reference Laflamme, Flude and Narbonne2012; Fig. 2d). Subsequent review of this taxonomic issue concluded that stem length and number of branches might be ecophenotypic, characters that were inappropriate for differentiation of taxa at the generic level (Liu et al. Reference Liu2016).
While stem length is undoubtedly variable in Beothukis and Culmofrons, stem length is problematic due to all the short-stemmed forms considered being controversial specimens from Spaniard’s Bay (Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009; Fig. 3) discussed in detail in Section 4.a. The argument that Culmofrons should be considered a junior synonym of the emended concept of Beothukis – accepting that Beothukis could have a disk and stem – resulted in the creation of B. plumosa (Laflamme et al. Reference Laflamme, Flude and Narbonne2012). This assertion is based on comparisons of stem length, the number of rangeomorph units and taphonomy, as well as the relative importance of continuous versus discrete characters.

Fig. 3. Comparison of stem length versus total specimen length among specimens of Beothukis mistakensis (n = 10), Culmofrons plumosa (n = 13) and undetermined Beothukis-like spatulate rangeomorphs from Spaniard’s Bay (n = 6).
At the species level, Beothukis mistakensis is distinguished from B. plumosa (Laflamme et al. Reference Laflamme, Flude and Narbonne2012) (hereafter referred to simply as the species plumosa) on the basis of the latter having: (1) a zigzagged and concealed axis (despite this morphology being considered to be a possible taphonomic artefact; Laflamme et al. Reference Laflamme, Narbonne, Greentree, Anderson, Vickers-Rich and Komarower2007; Brasier et al. Reference Brasier, Antcliffe and Liu2012; Liu et al. Reference Liu2016); (2) a stem and disc; and (3) the fact that the more numerous (8–12) secondary-order rangeomorph units are commonly subparallel in the species plumosa, in contrast with the (5–8) radiating secondary-order rangeomorph units typical of B. mistakensis. However, this decision relies heavily on the validity of the emendation of the generic diagnosis to include the presence of a stem and disk in Beothukis (Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009), an assertion that is reassessed here.
The conclusion that continuous characters should not be included in generic diagnoses of the Rangeomorpha (Liu et al. Reference Liu2016) has been contested through statistical analysis of the architecture of the rangeomorph Primocandelabrum from the UK, which can apparently have a range of types of rangeomorph units in branches of the same order within the same fossil (Kenchington & Wilby, Reference Kenchington, Wilby, Brasier, McIlroy and McLoughlin2017). The current view is therefore that a combination of continuous and discrete characters is required to diagnose rangeomorph genera and species (Kenchington & Wilby, Reference Kenchington, Wilby, Brasier, McIlroy and McLoughlin2017).
2. Material
Careful photography of the holotype of Beothukis mistakensis (OUMNH ÁT.410p; Fig. 4a), and other published material, under controlled lighting, forms the main basis of this work. When the holotype of Beothukis mistakensis is retrodeformed with reference to the closest discs, the shape is notably more elongate than previous retrodeformations (cf. Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009, fig. 8.1; online Supplementary Fig. S1, available at http://journals.cambridge.org/geo), but the fundaments of the branching pattern remain broadly unchanged. Older field photographs were also studied in order to assess the possibility that some features have been lost to weathering, since the holotype was first exposed in 1990 by removal of the overlying crystal tuff (Seilacher, Reference Seilacher1992; Matthews et al. Reference Matthews, Liu, McIlroy, Brasier, McIlroy and McLoughlin2017; online Supplementary Fig. S1).

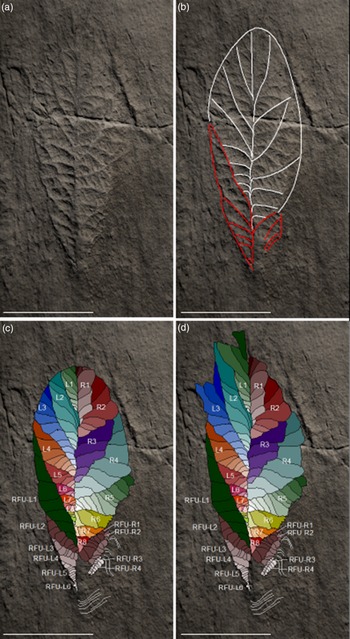
Fig. 4. Cast of the holotype of Beothukis mistakensis from the ‘E Surface’, Mistaken Point Ecological Reserve. (a) Complete holotype arc defines boundary between frond and zone of secondary growth (sg) in (c). (b) Fringe of secondary growth (f) at the margin of Beothukis mistakensis in a photograph of the holotype from around 2010 (courtesy of Liam Herringshaw). (c) Secondary growth at the tip of Beothukis mistakensis extending beyond what is normally considered to be margin of the spatulate frond. (d) Rotated, furled units (RFU) at the base of Beothukis mistakensis and the sharp proximal termination without evidence for a basal disc. Note the offset between the basal extent of the Rangeomorph units on each side of the centreline. Scale bars, 2 cm, except (a) which is 5 cm.
3. Results
3.a. Morphological analysis of the type material of Beothukis mistakensis
The holotype of B. mistakensis is beautifully preserved, and suitable for more detailed study than has been previously attempted. Previous illustrations have been tightly cropped and overlook some morphological elements as a result.
3.a.1. Primary-order units and frond outline
Of all of the fronds in the Mistaken Point type assemblages, Beothukis mistakensis is commonly considered to have some of the most complex fractal-like rangeomorph morphology. The type description of B. mistakensis focused on aspects of the self-similar rangeomorph elements without consideration of how those elements are distributed throughout the fossil (Brasier & Antcliffe, Reference Brasier and Antcliffe2009, fig. 17c). The holotype of Beothukis consists of two rows of primary-order rangeomorph elements (Fig. 5a). On each side of the fossil there are eight primary-order rangeomorph units (L1–8 and R1–8; Fig. 5b). L4–8 are bound on their distal margin by a row of units with rotated, furled rangeomorph primary-order units (RFU L1) and R6–8 abut against RFU R1–2 (see Section 3.a.3 below; Fig. 5c).

Fig. 5. Holotype of Beothukis mistakensis. (a) Cast of the holotype, carefully lit to show the maximum amount of morphological detail on the negative epirelief. (b) Holotype highlighting the margins of the primary-order rangeomorph units (white lines, excluding secondary growth at the tip, see (d)) and boundaries between rotated, furled primary-order units (red lines). (c) Interpretation showing the first-order units labelled L1–8 and R1–8; the second-order rangeomorph units within the first-order units are coloured to be progressively darker closer to the margin of the fossil. Tapering sets of rotated, furled primary-order rangeomorph units are present on each side of the basal region of the holotype (RFU L1–6 on the left and RFU R1–4 on the right; also Fig. 4d). (d) The holotype is prone to secondary growth from the tips of the first-order units (see L1–3, R1, R4; also Fig. 4c). Scale bars, 5 cm.
Determining which of the rangeomorph units are of primary order can be challenging in material that is composed of self-similar elements such as those that characterize Beothukis: units of different orders can appear to be similar. It is considered here that in the holotype of Beothukis all successive branches of the same order in a series have increasing angles relative to the axis (Fig. 5; i.e. radiating).
In the original diagnosis and type description, the authors grouped our R5–8 as a single primary-order rangeomorph unit (Brasier & Antcliffe, Reference Brasier and Antcliffe2009; Fig. 5b). We disagree with the original interpretation because their amalgamated primary-order unit: (1) does not clearly show the changing angle of sub-units within it that is characteristic of all the other primary-order units; and (2) would have an additional order of branching that is not seen in any of the other primary-order rangeomorph units. A consequence of this difference in interpretation is that the inferred glide-plane symmetry between the two rows of the holotype (Brasier & Antcliffe, Reference Brasier and Antcliffe2009) breaks down (Fig. 5a, b). This helps to distinguish Beothukis from taxa such as Charnia that has a simple alternating succession of primary branches that creates the glide-plane symmetry (cf. Laflamme et al. Reference Laflamme, Narbonne, Greentree, Anderson, Vickers-Rich and Komarower2007; Brasier et al. Reference Brasier, Antcliffe and Liu2012; Dunn et al. Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2018).
While the equivalent primary-order units on either side of the centreline, considered to be a concealed axis (sensu Brasier et al. Reference Brasier, Antcliffe and Liu2012), are somewhat similar in shape (compare outlines of L1 versus R1, L2 versus R2, etc.) and there is a degree of overlap onto the previous unit, their size and orientation are markedly different due to differing amounts of inflation of the primary-order units on the left- and right-hand side (Fig. 5b). The angle between the centreline and the boundary between the primary-order rangeomorph units – as measured in a series from the tip of the frond towards the base – is acute near the tip, becoming progressively steeper in angle in successive units in a series (Fig. 5a).
3.a.2. Secondary-order rangeomorph units in the holotype
The number of secondary-order rangeomorph units within the primary-order units of the holotype of Beothukis mistakensis varies from 3 to 11, with the primary-order units L3 and R5 containing the most secondary-order rangeomorph units (Fig. 5). It is noted that both L3 and R5 are at the maximum width of the holotype on their respective sides (Fig. 5). In all cases, secondary-order rangeomorph units systematically increase in angle from the frond margin to those closest to the centreline (Fig. 5a). Near the frond margin, the secondary rangeomorph units meet the boundary between primary-order units at an acute angle (c. 20°), whereas close to the centreline the secondary rangeomorph units are typically nearly perpendicular to the centreline of the frond (Fig. 5a). Diagnoses of the genus have all considered that the secondary-order units occur in radiating sets (Brasier & Antcliffe, Reference Brasier and Antcliffe2009; Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009; Brasier et al. Reference Brasier, Antcliffe and Liu2012; Liu et al. Reference Liu2016); however, this contradicts the inclusion of specimens formerly attributed to Culmofrons within the genus, as the secondary-order units in that genus are subparallel and not radiating (Laflamme et al. Reference Laflamme, Flude and Narbonne2012). The secondary-order rangeomorph units that make up a primary-order rangeomorph unit may have their maximum length and width either near the centre of the unit or near the margin of the frond.
The morphology of the secondary-order rangeomorph units is dependent upon their position in a series, with three distinct settings. (1) The smallest three to five secondary-order rangeomorph units closest to the centreline of the Beothukis frond are typically coincident with it, and have their distal margin constrained by the adjacent primary-order rangeomorph unit. Such secondary-order units are rotated and furled (sensu Brasier et al. Reference Brasier, Antcliffe and Liu2012; Fig. 5a). (2) Secondary-order units that are bound at their proximal and distal margins by the adjacent primary-order rangeomorph unit are commonly displayed and furled (Fig. 5b). (3) Rangeomorph units whose distal margin forms the outline of the Beothukis frond are variable in morphology, but are most commonly symmetrically displayed and furled. Secondary-order units with preservation beyond the ‘normal’ cuneate margin of the frond may also be unfurled (Fig. 5c), particularly in the paratype material.
3.a.3. Basal rotated, furled primary-order rangeomorph units
Tapering sets of progressively smaller primary-order units without clear rangeomorph architecture (cf. Jenkins, Reference Jenkins1985; Brasier et al. Reference Brasier, Antcliffe and Liu2012) are present on each side of the basal portion of the holotype of Beothukis (i.e. RFU L1–6 and RFU R1; Fig. 5c). It is unclear whether – in a more mature specimen – the component box-shaped (undivided) secondary-order rangeomorph units (sensu Brasier et al. Reference Brasier, Antcliffe and Liu2012) might develop into the typical rangeomorph morphologies (displayed versus rotated, and unfurled versus furled; Brasier et al. Reference Brasier, Antcliffe and Liu2012). Similar smooth second-order units have been proposed for Hapsidophyllas and Charniodiscus (possibly referring to C. procerus) by Brasier et al. (Reference Brasier, Antcliffe and Liu2012). The holotype of B. mistakensis is currently the only known specimen that shows such units at the base of a frond that has other more typical Rangea-like expressions (cf. Jenkins, Reference Jenkins1985; Brasier et al. Reference Brasier, Antcliffe and Liu2012). There is no indication of the likely presence of basal rotated, furled primary-order units in the material from Spaniard’s Bay (Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009) or any other figured material of Beothukis. The basal region of the holotype of Beothukis is the most topographically high part of the fossil, but shows no evidence for the rotated and undivided primary-order units arising from a stem. The addition of these rotated, furled primary-order units to the outline of the holotype of Beothukis mistakensis changes the gross outline of the frond from spatulate to cuneate.
3.a.4. Secondary growth
While the outline of the type specimen of Beothukis mistakensis – as defined by the margins of the primary-order branches – is spatulate to cuneate, the tip and the basal portion of the frond are prone to the development of secondary growth. The tip of the type specimen was not figured in the type description (Brasier & Antcliffe, Reference Brasier and Antcliffe2009), and is it not mentioned in the emended diagnosis (Brasier et al. Reference Brasier, Antcliffe and Liu2012). Careful photography of the tip region (Fig. 4c) demonstrates that secondary growth was present around the fringe of the frond, including portions that are clearly displayed and unfurled.
The presence of secondary growth in the basal region is hinted at by the camera lucida drawings that accompany the type description (Brasier & Antcliffe, Reference Brasier and Antcliffe2009, fig. 17b), but is not mentioned in the diagnosis. Photographs of the type specimen from around 2010 show the clear presence of a fringe of approximately heart-shaped elements adjacent to primary rangeomorph units R7–8 and RFU R1 (Fig. 4b, d).
3.a.5. Three-dimensional morphology of Beothukis and other reclining rangeomorphs
The Mistaken Point biota generally preserves only the lower surface of fronds and the upper surface of stems (e.g. Liu, Reference Liu2016; but see Seilacher, Reference Seilacher1992; Gehling & Narbonne, Reference Gehling and Narbonne2007). In our reconstruction of the morphology of Beothukis (Fig. 6), we are making the explicit assumption that the upper surface of reclining rangeomorph organisms was the same as the lower surface that is well preserved in the holotype. We are aware that need not be the case, and that the upper surface could have borne other tissues or even have been smooth, but we follow the current convention of assuming that the top is symmetrical with the lower surface for ease of illustration (Fig. 6) and to avoid needless conjecture.

Fig. 6. (a) Surficial and (b) lateral reconstruction of Beothukis mistakensis. This reconstruction assumes symmetry between the upper and lower surfaces of Beothukis mistakensis. (b) Our reconstructed morphology of Beothukis mistakensis highlighting its sediment-reclining mode of life on a matground-dominated seafloor.
3.b. Comparison of the holotype and paratypes of Beothukis
Superficially, the paratype material of Beothukis does not show close resemblance to the spatulate or cuneate holotype, being much more lanceolate without the same tightly constrained morphology, a greater abundance of displayed rangeomorph units and a very marked difference in the width of the two rows (Fig. 7). In the paratypes, the secondary-order rangeomorph units that are closest to the centreline show the displayed morphology, which is unlike the holotype. The more distal secondary-order rangeomorph units on the right-hand side of the paratype appear to be poorly organized, but clearly show the radiating pattern typical of the genus. We note that the paratypes are larger than the holotype (Figs 3, 7) and it is therefore possible that they show more unconstrained, eccentric growth during a senescent phase of their life cycle, giving them a more irregular outline and less-organized growth pattern, particularly the distal secondary-order branches that are unconstrained at the frond margin (Fig. 7b, c).

Fig. 7. Paratypes of Beothukis mistakensis. (a) Photograph of the ‘E Surface’ at Mistaken Point highlighting the locations of the Beothukis mistakensis paratypes (Bp1 and Bp2). (b) Beothukis mistakensis paratype 2. (c) Beothukis mistakensis paratype 1. (d) Beothukis mistakensis paratype 1, coloured to show primary branch structure. Note the eccentric, unconstrained distal growth, producing an erratic frond margin on the lower side of the image. Scale bars 5 cm, except (a) which is 20 cm.
4. Discussion
4.a. Spaniard’s Bay ‘beothukids’
The description and diagnosis of Beothukis from Mistaken Point (Brasier & Antcliffe, Reference Brasier and Antcliffe2009) shortly pre-dates the description of similar rangeomorphs from Spaniard’s Bay (Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009). While it was originally suggested that the Spaniard’s Bay assemblage was preserved within nodules (Narbonne, Reference Narbonne2004), it has subsequently been determined that the fossils lie within obstacle scours and flute marks, probably caused by the action of currents around the stems of erect frondose organisms (Brasier et al. Reference Brasier, Liu, Menon, Matthews, McIlroy and Wacey2013). The deepest part of horseshoe-shaped obstacle scours is found on the up-current end due to undercutting by eddying (cf. Fig. 2b; Dzulynski & Walton, Reference Dzulynski and Walton1963; Dzulynski & Simpson, Reference Dzulynski and Simpson1966; Sengupta, Reference Sengupta1966). Similar erosional morphologies are found on the up-current end of some of the fossiliferous scours at Spaniard’s Bay, including specimens attributed to Beothukis mistakensis (Fig. 2b). We therefore refute the assertion that the Spaniard’s Bay material has associated basal discs and short stems, which had previously given the impression that there was a continuum between the type material of Beothukis mistakensis and other spatulate fronds with stems and discs (Liu et al. Reference Liu2016). This is in agreement with Dececchi et al. (Reference Dececchi, Narbonne, Greentree and Laflamme2018), who came to the same conclusion about the importance of the stem as a taxonomic character, although that study appears to accept the Spaniard’s Bay material as Beothukis.
Some of the specimens identified as Beothukis mistakensis from Spaniard’s Bay differ from the holotype in outline, being lanceolate rather than spatulate, and by having displayed secondary-order rangeomorph units that are not demonstrably radiating (Fig. 2c), which does not fit the diagnosis of the genus. That specimen also has a previously unfigured conical erosional structure (Fig. 2c) that lies up-current of the previously inferred spade-shaped holdfast (Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009, fig. 6.1), which is suggestive of both features being current-generated rather than being morphological characters.
Some of the more spatulate material attributed to Beothukis mistakensis from Spaniard’s Bay shows alternate branching across the centreline of the frond, but has biserial primary-order branches and rather narrow, radiating uniserial secondary-order rangeomorph branches (Fig. 8). There are several such Beothukis-like forms from the same locality that show an en échelon stacking of clearly independent rangeomorph branches angled towards the base of the frond, along with a single specimen from Australia (Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009, figs 5, 6). This morphotype with its two rows of radiating rangeomorph fronds clearly had independent branches, which would imply that it is closer to Avalofractus than Beothukis although the material is in need of full taxonomic treatment. It is this morphotype that has been identified from the Rawnsley Quartzite in South Australia (Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009, fig. 8.6). It seems likely that the morphotype comprises a separate genus within the Rangida, or perhaps a separate species within Avalofractus. For the purpose of this work, it is sufficient to exclude it from Beothukis s.s.

Fig. 8. Small specimen previously attributed to Beothukis mistakensis from Spaniard’s Bay, excluded from the genus here. Note the presence of alternate branching across the frond centreline, with clear biserial (BS) primary-order branches on L6 (labelled following Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009) and the narrow, radiating uniserial secondary-order rangeomorph branches, some of which are also biserial (see L5). Note the marked erosion at the margins, particularly in the region of the frond tip. Scale bar, 1 cm.
The exclusion of the Spaniard’s Bay Beothukis-like taxa from the stem length data for Beothukis and Culmofrons demonstrates that there is a clear distinction between the stemless Beothukis mistakensis and the long-stemmed Culmofrons plumosa (Fig. 3). Further exploration of the idea that stem length may be an ecophenotyphic character in Culmofrons (Liu et al. Reference Liu2016) requires careful consideration on a bed-by-bed basis and a more robust sedimentological framework. We consider that the generic diagnosis of Beothukis can be emended to exclude reference to a stem and disc.
4.b. Comparison between Beothukis and the type material of Culmofrons
The type material of Beothukis plumosa (Laflamme et al. Reference Laflamme, Flude and Narbonne2012) must be reconsidered as a result of the redescription of the holotype of B. mistakensis in Section 3.a above, and the exclusion of the forms previously interpreted to be Beothukis mistakensis with short stems and discs (Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009; Brasier et al. Reference Brasier, Antcliffe and Liu2012; Liu et al. Reference Liu2016).
4.b.1. Primary-order rangeomorph units
The primary-order rangeomorph branches of Beothukis plumosa (Laflamme et al. Reference Laflamme, Flude and Narbonne2012) are alternate, describing the long-wavelength zigzagged axis to the frond (Laflamme et al. Reference Laflamme, Flude and Narbonne2012; Fig. 9a, b). There are up to five primary-order branches in each of the two rows (Laflamme et al. Reference Laflamme, Flude and Narbonne2012; Fig. 9a, b). The largest primary-order rangeomorph branches in plumosa are close to the frond–stem junction (Fig. 9a). These features are clearly different from the architecture of the holotype of B. mistakensis, which has a straight to slightly zigzagged axis, along which the primary-order rangeomorph units are arranged in a non-alternating manner (Fig. 5b, c). It is difficult to be sure of homologies between Culmofrons and Beothukis because of differences in their gross morphology. It could be argued that either the (up to) five primary-order units of Culmofrons are homologous with: (1) the more numerous primary-order rangeomorph units identified in the holotype here (i.e. L1–8 and R1–8; Fig. 5b, c); and (2) the two rows of rangeomorph elements in the holotype of Beothukis mistakensis (i.e. a single primary rangeomorph unit of Culmofrons would be equivalent to a complete row of rangeomorph units in Beothukis).

Fig. 9. Culmofrons plumosa. (a) Field photograph of Culmofrons plumosa holotype from Lower Mistaken Point, Mistaken Point Ecological Reserve, with alternating primary branches highlighted to emphasize the long-wavelength, low-amplitiude zigzagged medial axis. Scale bar, 5 cm. (b) Cast of Culmofrons plumosa from MUN surface, Catalina Dome, with primary branches artificially coloured to show the primary-order branching, revealing alternating branching including both displayed (labelled d) and proximal to the stem furled (labelled f) rangeomorph branching. Scale bar, 2 cm. (c) Close-up of secondary-order rangeomorph units close to the centreline of Culmofrons plumosa from MUN surface. Tertiary-order units within the secondary-order rangeomorph units are furled proximal to the centreline, but unfurled distally. Scale bar, 1 cm. (d) Close-up of the margin of Culmofrons plumosa from MUN surface, showing distally displayed second-order branch morphology not clearly seen in the type material. Scale bar, 1 cm. (e) Field photograph of Culmofrons plumosa from the MUN surface showing elongate subparallel second-order branches that appear to cross the boundaries between primary-order rangeomorph units and possible eccentric marginal growth extending beyond the frond margin on the right-hand side. Scale bar, 2 cm.
It is difficult to reconcile how the tightly constrained rangeomorph units that characterize Beothukis mistakensis might be modified during biostratinomy to create the distinctive strongly zigzagged axis diagnostic of Culmofrons (Laflamme et al. Reference Laflamme, Flude and Narbonne2012; Fig. 9b); we therefore retain it as a useful diagnostic character to distinguish between Beothukis and Culmofrons.
4.b.2. Secondary-order rangeomorph units
The secondary-order ‘branches’ of B. plumosa (Laflamme et al. Reference Laflamme, Flude and Narbonne2012) are numerous (8–12 in a series), subparallel to one another, subrectangular to trapezoidal in outline, and the largest in a series are either medial or distal (Fig. 9a, b). This contrasts with Beothukis, in which the largest secondary-order units are usually medial. Preservation in the type material of plumosa is comparatively poor at fine levels of detail, but many of the secondary-order branches would appear to be rotated and furled (Fig. 9a); the well-preserved MUN surface material (Liu et al. Reference Liu2016) shows proximally furled and distally displayed second-order morphology (Fig. 9b, c). The Culmofrons from the MUN Surface locality near Port Union (Hofmann et al. Reference Hofmann, O’Brien and King2008; Fig. 9d) have relatively well-preserved second-order morphology that appears to be predominantly furled, with some eccentric or ‘overcompensatory’ growth (Fig. 9d; cf. Kenchington et al. Reference Kenchington, Dunn and Wilby2018). Within the limitations imposed by the relatively low-relief preservation of the type material of plumosa, it seems that there are significant differences in the shape, size and distribution of secondary-order rangeomorph units between Beothukis mistakensis and plumosa. Additionally, no specimens of the species plumosa have been found without a stem and disk.
4.b.3. Significance of morphological differences between B. mistakensis and B. plumosa (Laflamme et al. Reference Laflamme, Flude and Narbonne2012)
There are a sufficient number of differences in the morphology and architecture of Beothukis mistakensis and the type material of B. plumosa (Laflamme et al. Reference Laflamme, Flude and Narbonne2012) to determine that the two species are not congeneric. (1) The regular alternation of primary-order units of plumosa, which produces the strongly zigzagged axis, is fundamentally different to the asymmetrical distribution of primary-order rangeomorph units along the straight centreline of B. mistakensis. (2) Notwithstanding considerations of homology discussed in Section 4.b.1 above, there are comparatively few primary-order rangeomorph units in each row of plumosa (five), and the primary-order units uniformly increase in length towards the stem and disc (suggesting a distal growth tip). That is unlike the more numerous rangeomorph branches per row in the type material of mistakensis. (3) There is no indication of rotated, furled primary-order rangeomorph units at the base of the frondose portion of plumosa as there is in the type material of B. mistakensis. (4) The secondary-order branches in the type material of plumosa are more commonly subparallel than radiating, and many are rotated and/or furled in the type material, but in other material (e.g. Liu et al. Reference Liu2016; Fig. 9a, b, e) they are commonly displayed and furled. (5) The fronds of the species plumosa are oval in outline, in contrast to the cuneate holotype of B. mistakensis. (6) All specimens of plumosa have a stem and disc (Laflamme et al. Reference Laflamme, Flude and Narbonne2012; Dececchi et al. Reference Dececchi, Narbonne, Greentree and Laflamme2018), whereas no authenticated mistakensis have either (accepting that the Spaniard’s Bay material is not attributable to Beothukis).
Notwithstanding differences in opinion regarding what should be considered to be genus- and species-level traits within the Rangeomorpha (Liu et al. Reference Liu2016; Kenchington & Wilby, Reference Kenchington, Wilby, Brasier, McIlroy and McLoughlin2017), it is clear that the species mistakensis and plumosa are not closely related in terms of their morphology, architecture or growth. It is therefore considered that plumosa should be excluded from the genus Beothukis. The status of Culmofrons as a valid genus, that is, distinct from Beothukis (Laflamme et al. Reference Laflamme, Flude and Narbonne2012), would appear to be justified at present and it should no longer be considered a junior synonym of Beothukis. The conclusion that stem length in Culmofrons plumosa may be an ecophenotypic character (Liu et al. Reference Liu2016) remains to be tested through careful integration of sedimentology and morphological variability on a bed-by-bed basis. Since rangeomorph architecture finer than second order is conventionally considered to be of species-level taxonomic significance (cf. Liu et al. Reference Liu2016), we do not currently distinguish between the displayed and undisplayed specimens of the genus Culmofrons.
4.c. Systematic palaeontology
4.c.1. Phylum Petalonamae Pflüg, Reference Pflüg1972
Class. Rangeomorpha Pflüg, Reference Pflüg1972
Order. Charnida Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009
Family. Charniidae Glaessner, Reference Glaessner, Robinson and Teichert1979
Genus. Beothukis Brasier & Antcliffe Reference Brasier and Antcliffe2009
Emended diagnosis. Unipolar spatulate to cuneate rangeomorph without visible stem and no basal disc, comprising two rows of undisplayed, radiating, uniserial primary-order rangeomorph units with medial inflation, arranged in a non-alternate manner across the weakly zigzagged centreline. Primary-order rangeomorph units comprise radiating uniserial secondary-order rangeomorph units with medial to distal inflation. Secondary-order rangeomorph units, that both abut against the axis and are constrained distally by an adjacent primary-order rangeomorph unit, are typically rotated and furled. Secondary-order rangeomorph units adjacent to the axis are typically displayed and furled, or may be displayed and unfurled where they extend beyond the – otherwise regular and smooth – frond margin. The narrow end of the frond may have radiating rows of primary-order box-like units without evidence of typical rangemomorph branching characteristics.
Species Beothukis mistakensis Brasier & Antcliffe, Reference Brasier and Antcliffe2009
1992 ‘Flat recliner’ Seilacher, pp. 608–9, figs 1–2 (partim).
1992 ‘Flat recliner’ Seilacher, p. 609, fig. 3 (partim).
1999 ‘Other form’ Seilacher, p. 98, fig. 3 (partim).
2004 Laflamme et al. p. 830, fig. 3.1 (partim).
[non] 2004 ‘Short stemmed rangeomorph frond’ Narbonne p. 1143, fig. 3B-C.
2008 ‘Spatulate rangeomorph’ Laflamme & Narbonne, p. 170, fig. 4.6–7.
2009 Beothukis mistakensis Brasier & Antcliffe pp. 383–3, fig. 17a–b; fig. 18a–b.
2009 Beothukis mistakensis Narbonne et al. pp. 507–15, fig. 8.1, 8.2, 8.4, 8.5.
[non] 2009 Beothukis mistakensis Narbonne et al. fig. 6–7, 8.3, 8.6.
[non] 2012 Beothukis mistakensis Dornbos et al. p. 58, fig. 5.2c.
2012 Beothukis mistakensis Brasier et al. p. 1116, fig. 5C–D.
2013 Beothukis Darroch et al. p. 596, fig. 2B.
2013 Beothukis mistakensis Laflamme et al. p. 562, fig. 2.2
[non] 2013 Beothukis mistakensis Laflamme et al. p. 562, fig. 2.1, 2.3, 2.4.
[?] 2013 Beothukis Macdonald et al. p. 257, fig. 6C.
2014 Beothukis mistakensis Hoyal Cuthill & Conway Morris p. 13 123, fig. 1.
2014 Beothukis Ghisalberti et al. p. 2, fig. 1e (partim).
[?] 2014 Beothukis cf. Beothukis mistakensis Narbonne et al. p. 215, fig. 6.
2015 Beothukis Zalasiewicz & Williams p. 144, fig. 13.
2015 Beothukis Liu et al. p. 1361, fig. 2B.
2015 Beothukis Burzynski & Narbonne, p. 37, fig. 4 A (partim).
[non] 2015 Beothukis Burzynski & Narbonne, fig. 5B (partim).
2016 Beothukis mistakensis, Liu et al. p. 9–10, fig. 4 A.
2019 Beothukis mistakensis, Matthews & McIlroy p. 2, fig. 2 (partim).
2017 Beothukis mistakensis, Matthews et al. p. 2, fig. 2c (partim).
Remarks. Previous works have assigned most foliose rangeomorphs with radiating displayed secondary-order ‘branches’ to Beothukis (Brasier & Antcliffe Reference Brasier and Antcliffe2009; Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009; Brasier et al. Reference Brasier, Antcliffe and Liu2012; Liu et al. Reference Liu2016). In doing so, there is the implicit assumption that branching characters are of greater taxonomic importance than the presence or absence of major organs such as the basal disc and stem (Kenchington & Wilby, Reference Kenchington, Wilby, Brasier, McIlroy and McLoughlin2017). Here, we restrict the genus Beothukis to rangeomorphs with uniserial primary- and secondary-order branching – comparable to the Charnida of Narbonne et al. (Reference Narbonne, Laflamme, Greentree and Trusler2009) – and do consider the absence of a stem and disc to be of taxonomic importance (contra Liu et al. Reference Liu2016). Superficially, Beothukis-like specimens with radiating, biserial, primary- and secondary-order rangeomorph (true) branches (e.g. Fig. 2b) should be considered to belong to the Rangida, probably within the genus Avalofractus of Narbonne et al. (Reference Narbonne, Laflamme, Greentree and Trusler2009). Material with uniserial primary- and secondary-order rangeomorph units, attached to a stem and basal disc, should be retained within the Charnida, specifically the genus Culmofrons since there is no demonstrable continuum of stem length between Beothukis and Culmofrons (Fig. 3; Dececchi et al. Reference Dececchi, Narbonne, Greentree and Laflamme2018).
Other elongate or filiform uniserial rangeomorphs with radiating primary- and secondary-order rangeomorph units that would seem to be taxonomically closer to Beothukis than Charnia include: Trepassia (Narbonne & Gehling, Reference Narbonne and Gehling2003; Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009; Fig. 10a), Vinlandia (Laflamme et al. Reference Laflamme, Narbonne, Greentree, Anderson, Vickers-Rich and Komarower2007; Hofmann et al. Reference Hofmann, O’Brien and King2008; Brasier & Antcliffe, Reference Brasier and Antcliffe2009; Fig. 10b) and a recently documented un-named Trepassia-like frond (Liu & Dunn, Reference Liu and Dunn2020; Fig. 10c). None of the well-preserved mature specimens of these taxa have a well-developed stem or basal disc (Fig. 10a–c, e). The recent redescription of the holotype of Charnia masoni and other material from the type locality (Antcliffe & Brasier, Reference Antcliffe and Brasier2008; Brasier & Antcliffe, Reference Brasier and Antcliffe2009; Dunn et al. Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2018) emphasizes the idea that Charnia is a rangeomorph with uniserial primary- and secondary-order rangeomorph units that are all rotated and furled (Jenkins, Reference Jenkins1985; Brasier et al. Reference Brasier, Antcliffe and Liu2012; Fig. 10d); however, even in large – presumably super-mature – ‘Charnia grandis’ specimens they are never displayed.

Fig. 10. Elongate and filiform rangeomorphs all with tightly constrained uniserial primary-order rangeomorph units (cf. the Charnida of Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009). (a) Field photograph of Trepassia wardae from the Drook Formation (MPER) showing radiating secondary-order rangeomorph units comparable to those of Beothukis. (b) Cast of an undescribed Beothukis- or Trepassia-like frond from Bonavista showing radiating secondary-order rangeomorph units. (c) Cast of Vinlandia antecedens from the Bonavista Peninsula showing tightly constrained, radiating, secondary-order rangeomorph units. (d) Cast of the holotype of Beothukis mistakensis artificially coloured to highlight evidence for arcs of secondary-order rangeomorph that cross both the boundaries between primary-order rangeomorph units. (e) Expanded portion of the holotype in Beothukis (boxed in (d)) showing three types of secondary-order rangeomorph units (Type 1, blue; Type 2, green; Type 3, purple; see text) separated by thin dashed lines that can be seen to cross the boundary between two primary-order units (heavier dashed line). (f) Cast of the holotype of Charnia masoni artificially coloured to highlight evidence for arcs of secondary-order rangeomorph that cross both the centreline and the boundaries between primary-order rangeomorph units. Scale bars 5 cm, except (a) which is 20 cm.
4.c.2. Phylum Petalonamae Pflüg, Reference Pflüg1972
Class. Rangeomorpha Pflüg, Reference Pflüg1972
Order. Charnida Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009
Genus. Culmofrons Laflamme et al. Reference Laflamme, Flude and Narbonne2012
Emended diagnosis. Unipolar ovate to obovate frond with stem and basal disc, comprising two rows of up to five undisplayed, radiating, uniserial primary-order rangeomorph units with medial to proximal inflation, arranged alternately across a strongly zigzagged axis. Primary-order rangeomorph units comprise subparallel to slightly radiating secondary-order rangeomorph units with medial to distal inflation that may be undisplayed or displayed.
Species Culmofrons plumosa Laflamme et al. Reference Laflamme, Flude and Narbonne2012
2007 ‘Frond’ Laflamme et al. p. 249, fig. 6d–e.
2012 Beothukis sp. Brasier et al. p. 1120, fig. 8b.
2012 Culmofrons plumosa gen et sp. nov. Laflamme et al. p. 196, figs 2.1–2.4.
[non] 2012 Culmofrons plumosa Laflamme et al. p. 196, figs 2.5–2.7.
2014 Culmofrons Kenchington & Wilby p. 105, fig. 2a.
2015 Culmofrons plumosa Liu et al. p. 1361, fig. 2e.
2016 Beothukis plumosa comb. nov. Liu et al. pp. 9–10, figs 2a, 4b–d.
Remarks. The re-establishment of Culmofrons as a valid genus is based upon reassessment of forms initially described as Beothukis (Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009) that appeared to bridge a gap between Beothukis and Culmofrons by having short, broad stems absent from the type material of Beothukis. That material is considered to belong to the Rangida rather than the Charnida, and additionally the stems and discs are considered to be erosional artefacts rather than real morphological features. The absence of a continuum of stem lengths between Beothukis and Culmofrons, along with the absence of specimens of stemless Culmofrons plumose, supports their separation into two genera within the Charnida of Narbonne et al. (Reference Narbonne, Laflamme, Greentree and Trusler2009), which encompasses taxa with uniserial secondary-order rangeomorph units.
Our field and laboratory observations of the type material of Culmofrons show that it is dominated by undisplayed secondary-order rangeomorph branching (Figs 2d, 9a). The material from the MUN surface of the Bonavista Peninsula (Liu et al. Reference Liu2016) shows a continuum between displayed rangeomorph units near the tip of obovate forms that become progressively undisplayed towards the stem (Fig. 9b). This suggests that secondary-order rangeomorph units change in morphology through ontogeny, and are not a reliable taxonomic character (cf. Liu et al. Reference Liu2016).
4.d. Palaeobiological implications
The basic building blocks of the Rangeomorpha (Pflüg, Reference Pflüg1972; Narbonne, Reference Narbonne2004; Brasier & Antcliffe, Reference Brasier and Antcliffe2004; Brasier et al. Reference Brasier, Antcliffe and Liu2012) are considered to be comparable in form to the gross-scale form of the eponymous Rangea (Pflüg, Reference Pflüg1970), arranged in different combinations of scales and in a range of orientations to create a diverse morphogroup. The strongly three-dimensional nature of Rangea includes three orders of self-similar branches (Grazhdankin & Seilacher, Reference Grazhdankin and Seilacher2005) and has been considered through detailed analysis of exceptionally preserved three-dimensional specimens to be multi-vaned (Dzik, Reference Dzik2002). Each vane comprised bilaminar sheets of frond-shaped elements, with the number of vanes now being demonstrated to be six (Vickers-Rich et al. Reference Vickers-Rich, Ivantsov, Trusler and Narbonne2013; Sharp et al. Reference Sharp, Wilson and Vickers-Rich2017). However, there do remain a number of important unresolved or poorly constrained gaps in our understanding of the palaeobiology of the Rangeomorpha.
4.d.1. Are rangeomorph fronds composed of truly fractal branches?
The architecture of the taxa now grouped under the Rangeomorpha has been a significant challenge to Ediacaran palaeontologists for many years (e.g. Narbonne, Reference Narbonne2005; Gehling & Narbonne, Reference Gehling and Narbonne2007; Brasier & Antcliffe, Reference Brasier and Antcliffe2009; Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009; Laflamme et al. Reference Laflamme, Flude and Narbonne2012; Brasier et al. Reference Brasier, Antcliffe and Liu2012). The model that the complex rangeomorphs of the deep-water palaeoenvironments of Avalonia were built of at least three orders of Rangea-like units in a range of orientations – which controls their appearance on bedding planes (Jenkins, Reference Jenkins1985; Brasier & Antcliffe, Reference Brasier and Antcliffe2009; Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009; Brasier et al. Reference Brasier, Antcliffe and Liu2012) – has been highly influential and is the principle paradigm used in interpreting their morphology and palaeobiology.
The lineations that demarcate the boundary between primary-order rangeomorph units have been called primary-order branch axes, and it has been considered that they are the locus of secondary-order ‘branch’ growth (Dunn et al. Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2018). It has been proposed that, in some taxa (e.g. Charnia), the secondary- and tertiary–order ‘branches’ were free to move relative to the point at which they are tethered to the primary-order branch axis (Brasier et al. Reference Brasier, Liu, Menon, Matthews, McIlroy and Wacey2013; Dunn et al. Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2018). This concept has been extrapolated to suggest that the modelled surface area of four orders of fractal branches might be sufficient to support an osmotrophic mode of feeding (Laflamme et al. Reference Laflamme, Xiao and Kowalewski2009; Hoyal Cuthill & Conway Morris, Reference Hoyal Cuthill and Conway Morris2014).
Contrary to the model of fully independent rangeomorph elements at all orders within the organism, the secondary-order rangeomorph units of Beothukis cross from one primary-order rangeomorph unit to the next (Fig. 10e), in the same way that the arcs of secondary-order rangeomorph units in Charnia masoni (Fig. 10d) cross the boundaries between primary-order branches. From this we infer that the secondary- and higher-order branches were not fully independent of one another, but were instead connected and closely juxtaposed. The lateral connectivity of the secondary-order branches, and the fact that units cross from one primary-order rangeomorph unit to another, opens up the possibility that the fronds of the Charnida may have grown from the tip towards the base (cf. Antcliffe & Brasier, Reference Antcliffe and Brasier2008). The proposed mode of growth is consistent with variations on the quilted ‘pneu construction’ (Seilacher, Reference Seilacher1989, Reference Seilacher1992; Seilacher et al. Reference Seilacher, Grazhdankin and Legouta2003; Buss & Seilacher, Reference Buss and Seilacher1994), and the conjugate branch margins of Beothukis noted by Brasier & Antcliffe (Reference Brasier and Antcliffe2009). This is at odds with the highly fractal and more independently branched plant or pennatulacean-like rangeomorph models of the Charnida (Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009; Hoyal Cuthill & Conway Morris, Reference Hoyal Cuthill and Conway Morris2014). That is not to say that all rangeomorphs conform to this more rigid inter-connected morphotype. It is clear that some of the Rangida, especially biserial forms, such as Avalofractus (Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009) and Fractofusus (Gehling & Narbonne, Reference Gehling and Narbonne2007), have clearly separated primary-order rangeomorph branches that were probably free from one another.
It seems unlikely that the tertiary-order rangeomorph units of Beothukis were three-dimensional independent tube-like branches, but are much more likely to simply constitute epithelial morphology. The rangeomorph morphology of the epithelium might therefore be an adaptation to increase the surface area of the Beothukis frond. While the increased surface area created by epithelial folds is significant, the surface area to volume ratio is not close to that of a truly three-dimensionally branching fractal frond (cf. Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009; Laflamme et al. Reference Laflamme, Xiao and Kowalewski2009; Hoyal Cuthill & Conway Morris, Reference Hoyal Cuthill and Conway Morris2014). Surface features, rather than constructional elements, are considered to be of low taxonomic importance (species-level) in many phyla. There is considerable potential for the surface texture to be generated in response to physical and chemical phenomena at the epithelium of Beothukis and potentially other rangeomorph organisms (cf. the tertiary-order rangeomorph units of Charnia; Dunn et al. Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2018), as has been recently suggested for Fractofusus (Dufour & McIlroy, Reference Dufour, McIlroy, Brasier, McIlroy and McLoughlin2017, Reference Dufour and McIlroy2018).
4.d.2. Was Beothukis an erect frond or a recliner?
Most of the frond-like rangeomorph Ediacaran organisms from the deep-marine Avalon assemblage are currently accepted to have had an erect mode of life (e.g. Clapham et al. Reference Clapham, Narbonne and Gehling2003; Narbonne, Reference Narbonne2005; Mitchell & Kenchington, Reference Mitchell and Kenchington2018; Kenchington et al. Reference Kenchington, Dunn and Wilby2018). These fronds are inferred to have had a basal attachment to a microbe-rich substrate (e.g. a holdfast or holdfast disc; mat-stickers sensu Seilacher, Reference Seilacher1999), many – but not all – of which are considered to have a stalk that held the frondose portion above the sediment–water interface (e.g. Jenkins, Reference Jenkins, Lipps and Signor1992; Seilacher, Reference Seilacher1992; Laflamme et al. Reference Laflamme, Flude and Narbonne2012; Liu et al. Reference Liu, Kenchington and Mitchell2015, Reference Liu2016; Dunn et al. Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2018). The generally accepted exception to this mode of life among the Mistaken Point biota is the abundant frond Fractofusus, which is inferred to be a recliner as evidenced by the lack of a disc and stalk as well as the absence of current orientation (e.g. Seilacher, Reference Seilacher1992; Gehling & Narbonne, Reference Gehling and Narbonne2007).
The lack of current orientation of the holotype of Beothukis in assemblages such as the E Surface of Mistaken Point provides strong evidence against an erect mode of life (e.g. Seilacher, Reference Seilacher1992). In an erect mode of life, the spatulate to cuneate shape of Beothukis would have provided a large surface area and be likely to have been re-orientated by the sediment-laden current, but would also be expected to occasionally produce Kullingia-type scratch circles (cf. Jensen et al. Reference Jensen, Högstrom, Almond, Taylor, Meinhold, Høyberget, Ebbestad, Agic and Palacios2018). The quality of preservation of the type material of Beothukis on the E surface is comparable only to that of the reclining taxon Fractofusus, in which the negative epirelief is inferred to be a cast of the lower surface that grew along and into the seafloor, below the ambient microbial mat (Seilacher, Reference Seilacher1999; Gehling & Narbonne, Reference Gehling and Narbonne2007; Dufour & McIlroy, Reference Dufour, McIlroy, Brasier, McIlroy and McLoughlin2017, Reference Dufour and McIlroy2018). The negative epirelief preservation of Beothukis is also consistent with it being the cast of the lower surface of a reclining organism (cf. Seilacher, Reference Seilacher1992; Fig. 6).
4.d.3. What is the purpose of the high-order self-similar units?
The highly divided, fractal-like, three-dimensionally branched model of Beothukis growth (e.g. Hoyal Cuthill & Conway Morris, Reference Hoyal Cuthill and Conway Morris2014) has been used to support the inference of an erect osmotrophic mode of life for Beothukis and other rangeomorphs (Laflamme et al. Reference Laflamme, Xiao and Kowalewski2009; Hoyal Cuthill & Conway Morris, Reference Hoyal Cuthill and Conway Morris2014). Our reconsideration of Beothukis as a reclining frond with closely bound primary- and secondary-order rangeomorph units – rather than true fractal branching – is consistent with the phagocytotic and/or chemosymbiotic mode of life inferred for Fractofusus, another reclining taxon in the Mistaken Point assemblage (Dufour & McIlroy, Reference Dufour, McIlroy, Brasier, McIlroy and McLoughlin2017, Reference Dufour and McIlroy2018). If that mode of life were extended to Beothukis, the three orders of invaginations on the lower surface could then have been used as a surface for the exchange of oxygen with the underlying sediment, restricting the build-up of hydrogen sulphide (which otherwise would kill the cells on the lower surface), simultaneously creating an ideal sub-organism microenvironment for the growth of sulphur-oxidizing bacteria (Fig. 11). The enhanced microbial productivity at the organism–sediment boundary is likely to be consumed by phagocytosis across the epithelial membrane, as part of an ectosymbiotic relationship between the rangeomorph and sulphur-oxidizing bacteria (Dufour & McIlroy, Reference Dufour, McIlroy, Brasier, McIlroy and McLoughlin2017, Reference Dufour and McIlroy2018). This increased microbial activity may also, incidentally, increase the preservation potential of high orders of rangeomorph units in the reclining rangeomorphs by biological or authigenic stabilization of the sub-organism surface, although that assertion would need to be biogeochemically tested – using stable sulphur isotopes – should appropriately preserved material be discovered.

Fig. 11. Diagrammatic reconstruction of cross-section through Beothukis mistakensis showing the means by which the organism might have interacted with the underlying substrate, and its impact on the microbiotic productivity at the organism–sediment interface (based on Dufour & McIlroy, Reference Dufour, McIlroy, Brasier, McIlroy and McLoughlin2017). The localized redox gradient is likely to have stimulated microbial productivity, possibly part of a simple ectosymbiosis or endosymbiosis if the organism was capable of phagocytosing microbes on its lower surface.
The systematically variable tertiary-order surface morphology of the lower surface of Beothukis has the highest surface area (displayed) secondary-order rangeomorph units closest to the margin, and the smaller, low-surface-area secondary-order rangeomorph units closest to the axis. We speculate that this variability in morphology might be an adaptation to reduce the surface area of the most central part of the frond (i.e. ‘rotated-furled’-type rangeomorph surface morphology, sensu Brasier et al. Reference Brasier, Antcliffe and Liu2012), whereas closer to the margin of the frond, the high-surface-area ‘displayed-unfurled’ rangeomorph morphologies are the norm. It also seems illogical for an erect organism with an osmotrophic or suspension-feeding lifestyle to have anything except a displayed-unfurled branch morphology. Furling would reduce feeding efficiency due to the decreased surface area to volume ratio; as such, future studies might consider that Charnia could also have been a Beothukis-like recliner. Reduction of rangeomorph surface area by furling might be an adaptation to reduce exposure to microbial hydrogen sulphide build-up. By decreasing the epithelial surface area, the amount of oxygen needed to prevent sulphide toxicity – which would otherwise cause cell death – is decreased. Oxygen could have been transported to the organism–sediment interface by either cilial irrigation or diffusion through the mesoglea or mesenchyme (Dufour & McIlroy, Reference Dufour, McIlroy, Brasier, McIlroy and McLoughlin2017; Fig. 11).
5. Conclusions
Through careful study of the type material of Beothukis mistakensis and B. plumosa (Laflamme et al. Reference Laflamme, Flude and Narbonne2012), we have concluded that the two species are not congeneric, and that Culmofrons plumosa should instead remain valid. We do however agree with the recent assertion that the morphology of secondary- and higher-order rangeomorph units is probably inappropriate for use as a genus-level taxonomic trait within the rangeomorphs of the Avalon Assemblage (Liu et al. Reference Liu2016; Dunn et al. Reference Dunn, Wilby, Kenchington, Grazhdankin, Donoghue and Liu2018). The key observation that tertiary-order rangeomorph architecture in Beothukis mistakensis is not in the form of three-dimensional branching calls into question the surface area estimates that underpin the inference of an osmotrophic mode of life for the erect Rangeomorpha (e.g. Laflamme et al. Reference Laflamme, Xiao and Kowalewski2009; Hoyal Cuthill & Conway Morris, Reference Hoyal Cuthill and Conway Morris2014).
The discovery that the second-order branches cross the boundaries between primary-order rangeomorph units is important in that it strongly suggests that Beothukis grew from the tip towards the base. That mode of growth might also explain the – centreline crossing – arcs of secondary rangeomorph units in Charnia. The resultant architecture of the Charnida (Narbonne et al. Reference Narbonne, Laflamme, Greentree and Trusler2009) is much more inflexible and much less pennatulacean-like in nature than previously considered, consistent with the rarity of non-idiomorphic specimens.
With our reassessment of the stems and discs of the Spaniard’s Bay material previously attributed to Beothukis mistakensis as being erosional artefacts, there is no positive evidence that Beothukis mistakensis had an erect mode of life. However, there is evidence to support a reclining mode of life comparable to that of Fractofusus (Seilacher, Reference Seilacher1992; Gehling & Narbonne, Reference Gehling and Narbonne2007; Dufour & McIlroy, Reference Dufour, McIlroy, Brasier, McIlroy and McLoughlin2017, Reference Dufour and McIlroy2018). These reinterpretations of the mode of life and classification of Beothukis and Culmofrons have knock-on implications for statistically based ecological, spatial and tiering analyses (e.g. Clapham et al. Reference Clapham, Narbonne and Gehling2003; Clapham, Reference Clapham, Laflamme, Schiffbauer and Dornbos2011; Mitchell et al. Reference Mitchell, Kenchington, Liu, Matthews and Butterfield2015, Reference Mitchell, Kenchington, Harris and Wilby2018; Mitchell & Butterfield, Reference Mitchell and Butterfield2018; Mitchell & Kenchington, Reference Mitchell and Kenchington2018) that are beyond the scope of this paper. The likelihood of some frondose taxa being recliners rather than having an erect pennatulacean-like mode of life (cf. Glaessner, Reference Glaessner1984) requires that the null hypothesis for interpreting Ediacaran fronds (and other taxa) must be that they lay on the seafloor, as we see them preserved in the field, until it is proven otherwise.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0016756820000941
Acknowledgements
Fieldwork at the Mistaken Point Ecological Reserve, Bonavista Peninsula and Spaniard’s Bay was conducted under permit from the Government of Newfoundland and Labrador. Funding was provided by Discovery Grant and Discovery Accelerator Supplement awards from the Natural Sciences and Engineering Research Council, which are acknowledged with thanks. Alex Liu kindly provided access to casts and reviewed an early version of the paper. This work has evolved from longstanding interactions with JJ Matthews, CG Kenchington, LG Herringshaw, PL Manning and JB Antcliffe, and through the mentorship of the late MD Brasier. The comments of S Jensen and an anonymous reviewer are greatly appreciated. Readers are advised that access to the fossil-bearing surfaces of Newfoundland requires a permit from the Government of Newfoundland and Labrador, in accordance with Regulation 67/11 of the Historic Resources Act 2011.
Conflict of interest
None.