Introduction
Cannabis is the illicit drug most commonly abused by people with schizophrenia, and schizophrenia patients appear to use the drug more frequently than the populations from which they are drawn (Cantwell et al. Reference Cantwell, Brewin, Glazebrook, Dalkin, Fox, Medley and Harrison1999). Several well-designed cohort studies have shown that cannabis use increases the risk of developing schizophrenia (Andreasson et al. Reference Andreasson, Allebeck, Engstrom and Rydberg1987; Arsenault et al. Reference Arsenault, Cannon, Poulton, Murray, Caspi and Moffitt2002; Zammit et al. Reference Zammit, Allebeck, Andreasson, Lundberg and Lewis2002). Overall, according to published prospective studies, it has been reported that cannabis use significantly increases the likelihood of developing schizophrenia, with a pooled odds ratio of 2.1 (Henquet et al. Reference Henquet, Murray, Linszen and van Os2005). However, little is known about whether pre-illness cannabis abuse can aggravate cognitive deficits shown by schizophrenia patients.
There is substantial evidence of the cognitive deficits associated with cannabis abuse. Most studies agree that heavy cannabis users exhibit temporary cognitive deficits for hours or days after stopping cannabis (Pope et al. Reference Pope, Gruber, Hudson, Huestis and Yurgelun-Todd2001; Solowij et al. Reference Solowij, Stephens, Roffman, Babor, Kadden, Miller, Christiansen, McRee and Vendetti2002), although this might be attributable to withdrawal effects or to a residue of cannabinoids in the brain. However, there is less consensus about whether cannabis can produce cognitive deficits that persist after a longer period of abstinence. A meta-analysis (Grant et al. Reference Grant, Gonzalez, Carey and Natarajan2001) found no evidence of long-term cognitive decline, with the possible exception of slight decrements in the area of learning new information. A longitudinal population study also found no evidence of cognitive deficits 3 months after cessation of regular use (Fried et al. Reference Fried, Watkinson and Gray2005). Two studies have examined whether 28-day abstinent cannabis users have persistent cognitive deficits. Pope et al. (Reference Pope, Gruber, Hudson, Huestis and Yurgelun-Todd2001) found no differences between controls and abstinent heavy cannabis users in measures of attention, verbal learning and memory, visuospatial memory and executive function, whereas Bolla et al. (Reference Bolla, Brown, Eldreth, Tate and Cadet2002) found, in heavy cannabis users, persistent dose-related deficits in tests measuring verbal and visual memory, executive function, visuoperception, psychomotor speed and manual dexterity. Finally, Solowij et al. (Reference Solowij, Stephens, Roffman, Babor, Kadden, Miller, Christiansen, McRee and Vendetti2002) found that long-term cannabis users were more impaired than controls in measures of memory and attention, these impairments worsening with increasing years of regular cannabis use. Decision making, as assessed with the Iowa Gambling Task (GT), has been reported to be altered in current (Whitlow et al. Reference Whitlow, Liguori, Livengood, Hart, Mussat-Whitlow, Lamborn, Laurienti and Porrino2004) and 25-day abstinent (Bolla et al. Reference Bolla, Eldreth, Matochik and Cadet2005) heavy cannabis users.
Among patients with schizophrenia, concomitant substance misuse has been associated with better performance on a variety of cognitive tasks (Carey et al. Reference Carey, Carey and Simons2003; Potvin et al. Reference Potvin, Briand, Prouteau, Bouchard, Lipp, Lalonde, Nicole, Lesage and Stip2005; Jokers-Scherübl et al. Reference Jockers-Scherübl, Wolf, Radzei, Schlattmann, Rentzsch, Gomez-Carrillo de Castro and Kühl2007). Furthermore, two studies examining first-episode psychosis patients found that patients with substance misuse performed significantly better than non-user patients on a variety of cognitive tasks (Sevy et al. Reference Sevy, Robinson, Solloway, Alvir, Woerner, Bilder, Goldman, Lieberman and Kane2001; McCleery et al. Reference McCleery, Addington and Addington2006).
Among all brain areas, the prefrontal cortex (PFC) has a fundamental role in motivation, emotion and higher cognitive functioning. The primary functions of the PFC have been often dissociated into ‘emotional’ and ‘cognitive’ domains, with motivational/emotional processes attributed to the orbitofrontal region, and high-level cognitive processes to the dorsolateral region (Fuster, Reference Fuster1997). Thus, the orbitofrontal cortex (OFC) mediates aspects of motivational functioning, including emotional reactions and social behaviour (Stuss & Benson, Reference Stuss and Benson1986), decision making (Bechara et al. Reference Bechara, Damasio, Tranel and Anderson1998), and the process of addiction and reward (Grant et al. Reference Grant, London, Neulin, Villemagne, Liu, Contoreggi, Phillips, Kimes and Margolin1996). Anatomical studies indicate that the OFC is extensively interconnected with the amygdala, ventral striatum, hypothalamus, and other areas implicated in emotional processing (Öngür & Price, Reference Öngür and Price2000). The dorsolateral prefrontal cortex (DLPFC), however, is mainly involved in high-level cognition, especially working memory, short-term retention and executive functions (Baddeley, Reference Baddeley1986; Goldman-Rakic, Reference Goldman-Rakic1996). Contrary to the OFC, the DLPFC is sparsely connected with classic limbic regions but is interconnected with paralimbic structures such as the hippocampus and the anterior cingulated cortex (Goldman-Rakic, Reference Goldman-Rakic1996).
Prefrontal cortical cognitive dysfunctions have been described extensively in patients with schizophrenia, including first-episode patients (Saykin et al. Reference Saykin, Shtasel, Gur, Kester, Mozley, Stafiniak and Gur1994; Barch et al. Reference Barch, Carter, Braver, Sabb, MacDonald, Noll and Cohen2001). In addition, cognitive abnormalities represent the most disabling and persistent features of schizophrenia, and the degree of cognitive impairment may be the best predictor of long-term outcome in affected individuals (Green, Reference Green1996). DLPFC abnormalities in schizophrenia have attracted the greatest interest, and have been described in structural (Ohnuma et al. Reference Ohnuma, Kimura, Takahashi, Iwamoto and Arai1997; Buchanan et al. Reference Buchanan, Vladat, Barta and Pearlson1998) and functional (Ragland et al. Reference Ragland, Gur, Glahn, Censits, Smith, Lazareth, Alavi and Gur1998; Glahn et al. Reference Glahn, Ragland, Abramoff, Barrett, Laird, Bearden and Velligan2005) neuroimaging studies. However, abnormalities in the OFC have also been described in structural (Goldstein et al. Reference Goldstein, Goodman, Seidman, Kennedy, Makris, Lee, Tourville, Caviness, Faraone and Tsuang1999; Crespo-Facorro et al. Reference Crespo-Facorro, Kim, Andreasen, O'Leary and Magnotta2000) and functional (Andreasen et al. Reference Andreasen, O'Leary, Flaum, Nopoulos, Watkins and Hichwa1997; Crespo-Facorro et al. Reference Crespo-Facorro, Kim, Andreasen, O'Leary, Wiser, Bailey, Harris and Magnotta1999) studies.
Although there are no cognitive tasks that are distinctively sensitive to specific frontal regions, as mentioned previously, it appears that working memory and executive functions are associated with the DLPFC, while decision making is related more to OFC function (Bechara et al. Reference Bechara, Damasio, Tranel and Anderson1998). It is nowadays widely accepted that patients with schizophrenia, compared to control subjects, show impairments on working memory (Lee & Park, Reference Lee and Park2005) and executive functions (Hutton et al. Reference Hutton, Puri, Duncan, Robbins, Barnes and Joyce1998). However, studies examining the performance of patients with schizophrenia on decision-making tasks have produced inconsistent results. Hutton et al. (Reference Hutton, Murphy, Joyce, Rogers, Cuthbert, Barnes, McKenna, Sahakian and Robbins2002), Ritter et al. (Reference Ritter, Meador-Woodruff and Dalack2004) and Shurman et al. (Reference Shurman, Horan and Nuechterlein2005) reported dysfunction on this capacity in chronic schizophrenia patients; Beninger et al. (Reference Beninger, Wasserman, Zanibbi, Charbonneau, Mangels and Beninger2003) in those schizophrenia patients taking atypical but not typical antipsychotics; and Kester et al. (Reference Kester, Sevy, Yechiam, Burdick, Cervellione and Kumra2006) in adolescents with early-onset schizophrenia. On the contrary, several studies have reported no differences between schizophrenia patients and controls: Wilder et al. (Reference Wilder, Weinberger and Goldberg1998), Cavallaro et al. (Reference Cavallaro, Cavedini, Mistretta, Bassi, Angelone, Ubbiali and Bellodi2003) and Evans et al. (Reference Evans, Bowman and Turnbull2005) in chronic patients; Beninger et al. (Reference Beninger, Wasserman, Zanibbi, Charbonneau, Mangels and Beninger2003) in those chronic patients taking typical antipsychotics; and Hutton et al. (Reference Hutton, Murphy, Joyce, Rogers, Cuthbert, Barnes, McKenna, Sahakian and Robbins2002) and Rodriguez-Sanchez et al. (Reference Rodriguez-Sanchez, Crespo-Facorro, Perez-Iglesias, Gonzalez-Blanch, Alvarez, Llorca and Vazquez-Barquero2005) in first-episode patients.
The goals of our study were: (1) to investigate whether cannabis abuse produces prefrontal cognitive function abnormalities in cannabis abusers experiencing the early phases of their first episode of schizophrenia; and (2) to examine whether OFC (decision-making) and DLPFC (executive function and working memory) functions are distinctively affected in cannabis abusers versus no-abusers in the early phases of schizophrenia.
Method
Subjects
During the period from February 2001 to February 2005 a sample of 182 stable first-episode patients with a schizophrenia-spectrum disorder were recruited as part of an ongoing longitudinal study of first-episode psychosis, the clinical and research programme on early phases of psychosis (PAFIP), at the University of Cantabria, Santander, Spain (see Crespo-Facorro et al. Reference Crespo-Facorro, Perez-Inglesias, Ramirez-Bonilla, Martinez-Garcia, Llorca and Vazquez-Barquero2006 for further details), and were invited to participate in the study. Patients with a history of neurological disease, head injury, mental retardation or drug dependence, according to DSM-IV criteria after using the Structured Clinical Interview for DSM-IV (SCID) and reviewing past medical records, were excluded from this study. Diagnoses of schizophrenia, schizophreniform disorder, schizo-affective disorder, brief psychotic disorder, or psychosis not otherwise specified were established using the SCID 6 months after the first contact with the patient. All of the patients were in their first psychotic episode and had not previously received neuroleptic medication for more than 4 weeks.
One hundred and thirty-two of the above-mentioned patients (87 males and 45 females) completed a cognitive battery at baseline. Regarding their antipsychotic treatment, which was randomly assigned, 43 were receiving haloperidol, 41 olanzapine, and 48 risperidone. Anticholinergic medications were prescribed according to clinical judgement based on extrapyramidal side-effects. At the time of neuropsychological evaluation, 38.6% of patients were taking anticholinergic medication. The patients had a mean duration of untreated psychosis (DUP) of 12.04 (s.d.=23.79) weeks, and a mean of 19.76 (s.d.=11.14) days of hospitalization. For the purposes of this report, patients were grouped according to their cannabis abuse prior to illness (psychosis) onset. Cannabis abuse was considered positive if there had been at least weekly use during the previous year.
All subjects gave their written informed consent prior to participation in the study in accordance with the ethical committee of the Hospital Marques de Valdecilla, University of Cantabria.
Clinical evaluation
Clinical symptoms were rated by the same trained psychiatrist (B.C.-F.) after 6 weeks of programme entry, using the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, Reference Andreasen1984a) and the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, Reference Andreasen1984b).
Cognitive measures
All subjects completed the cognitive evaluation. For patients, the evaluation was completed following clinical stabilization of acute psychotic symptoms, between 2 and 22 weeks of programme entry and antipsychotic treatment initiation (mean of 10 weeks). Cognitive data were obtained after stabilization of acute psychotic symptoms to maximize collaboration and avoid state effects of the acute psychosis. Moreover, patients were asked to abstain from cannabis for at least 2 weeks.
Estimation of IQ
We obtained the Verbal Comprehension (VC) index from the Weschler Adult Intelligence Scale – III (WAIS-III). This index has a good correlation with global IQ (Weschler, Reference Wechsler1999) and provides an approach to general intellectual performance. It includes the scores of the Vocabulary, Information and Similarities subtests.
OFC task: the Iowa Gambling Task (GT)
We used a computerized version of the Iowa GT (Bechara et al. Reference Bechara, Damasio, Damasio and Anderson1994) to measure decision-making capacity. According to lesion (Bechara et al. Reference Bechara, Tranel and Damasio2000; Bechara, Reference Bechara2004) and neuroimaging (Rogers et al. Reference Rogers, Owen, Middleton, Williams, Pickard, Sahakian and Robbins1999a) studies, this task is thought to relate to OFC functioning. In this task, participants are told that they will be making a long series of selections, one at a time, from four decks of cards on a computer screen. They are informed that they can switch from one deck to another as often as they wish and that the overall goal of the game is to maximize profit on a loan of US$2000 of ‘play money’. Participants are also informed that they can make a profit and win money over time if they learn to select cards from the ‘good’ decks and avoid the ‘bad’ decks. After turning each card, the subject receives an amount of money. However, on some cards the subject receives money but also pays a penalty. Specifically, turning any card from deck A or B yields US$100 and turning any card from deck C or D yields US$50. In deck A, the penalties are frequent and range from US$100 to US$350, while in deck B the penalties are infrequent but larger (US$1250). Thus, decks A and B are considered disadvantageous, as subjects incur a net loss over time. In deck C the penalties are frequent and range from US$25 to US$75, while in deck D the penalties are infrequent but larger (US$250). By picking preferentially from decks C and D subjects incur a net gain over time, so these decks are considered advantageous. A total of 100 trials are completed, although subjects are not informed of the exact number of trials. The variable that measures total performance on this task is the difference between choices in advantageous decks (C and D) minus choices in disadvantageous decks (A and B). To study the evolution of the performance along the test, total performance was divided into five periods of 20 choices each. Another measure derived from this task is the number of low-frequency but high-magnitude punishment choices (decks B and D) and the number of high-frequency but low-magnitude punishment choices (decks A and C).
DLPFC tasks
To obtain measures of cognitive functions associated with DLPFC, such as working memory and executive function, we selected three tasks, the WAIS-III Backward Digits, a fluency test (FAS) and the Trail Making Test (TMT), that have been extensively studied in relation to working memory and executive function (Lezak, Reference Lezak1995). In the Backward Digits task the subject has to repeat in inverse order digit lists presented verbally. In the FAS the individual has to generate as many words as possible beginning with letters F, A and S, in a given time. The TMT is a set-shifting task with two parts. In part A the subject has to link spots in a sheet of paper with a continuous line. All spots contain numbers, and they have to be linked in numerical order. In part B spots contain either numbers or letters. The subject has to link them, alternating numerical and alphabetical order. As it is part B that taps DLPFC function whereas part A measures psychomotor speed and visual scanning, the final score is obtained by calculating a quotient between parts B and A (Arbuthnott & Frank, Reference Arbuthnott and Frank2000).
Statistical analysis
Independent-sample t tests were used for between-group comparisons on the GT (total score and periods scores), DLPFC functions tasks, VC index, and age. χ2 tests were used to compare sex distribution. Repeated measures analyses of variance (ANOVAs) were used for within-group comparisons among the five periods of the GT, and also to estimate whether within-group differences existed in the preference for choices: advantageous versus disadvantageous, low frequency–high magnitude versus high frequency–low magnitude, specific decks.
Results
The sociodemographic characteristics of each of the two groups are shown in Table 1. According to our criteria, the group of cannabis abusers was composed of 61 patients (46.2%), and the group of non-abusers of 71 patients (53.8%). We found that a larger proportion of males than females abused cannabis before their illness began. Moreover, patients who had abused cannabis were significantly younger at illness onset, even when analysis was adjusted for gender (F=29.18, df=1, p<0.001), and at the time of clinical and neuropsychological evaluation, and had a lower estimated IQ as measured by the WAIS-III VC index, even when the analysis was adjusted for age and sex (F=4.66, df=1, p=0.03). Mean SAPS and SANS scores for patients who had or had not abused cannabis are also presented in Table 1. No significant differences were found at the time when the neuropsychological assessment took place. Antipsychotic treatment, which was randomly assigned, is shown in Table 1. An equal distribution in both groups can be observed. Regarding use of other illicit drugs, we found that although none of the cannabis non-abusers had abused other illicit drugs, 10 (16.4%) of the patients who had abused cannabis had also used other illicit drugs (three had used amphetamines, six cocaine, one heroin, and two hallucinogens). However, none of the analyses carried out in this study changed significantly when adjusted for past use of other drugs.
Table 1. Sociodemographic and clinical data
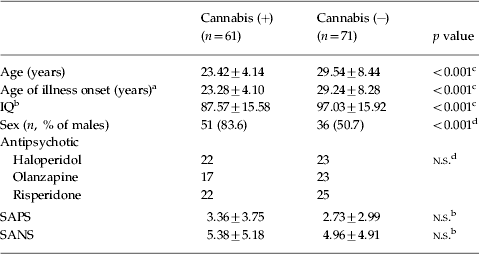
SAPS, Scale for the Assessment of Positive Symptoms; SANS, Scale for the Assessment of Negative Symptoms; n.s., non-significant at a p value <0.05.
IQ estimated by the Weschler Adult Intelligence Scale – III (WAIS-III) Verbal Comprehension (VC) index.
a When the analysis was adjusted for sex: F=29.18, df=1, p<0.001.
b When the analysis was adjusted for age and sex: F=4.66, df=1, p=0.03.
c Student t test.
d χ2 test.
Data on the performance on the cognitive tasks are shown in Table 2. In a first step, the influence of sex and age on these cognitive tasks was examined. We found that females had higher scores than males in the verbal fluency task (p=0.009), with no significant differences in any other task. Younger age was correlated with worse GT total performance (p=0.001), worse performance on the verbal fluency task (p<0.001), and lower estimated IQ (p=0.005). However, only the correlation with verbal fluency achieved statistical significance in both groups of patients: those who had abused (p=0.04) and those who had not abused cannabis (p=0.002).
Table 2. Cognitive tasks

The possible correlation between pre-morbid IQ, as measured by the WAIS-III VC index, and the other cognitive measures was examined. In the whole sample, higher estimated pre-morbid IQ was correlated with better performance on the GT (r=0.25, p=0.006), verbal fluency (r=0.40, p<0.001), TMT (r=0.25, p=0.006) and Backward Digits (r=0.28, p=0.002). These results remained significant after adjusting for age and sex. When these correlations were analysed separately in both groups of patients, we found that, in the group of non-abusers, all the correlations were still significant, whereas in the group of abusers the only one that remained significant was the one with verbal fluency (r=0.30, p=0.02).
Univariate t tests revealed significant differences between groups in the performance on the GT, patients who had abused cannabis performing worse than patients who had not abused in the total task (t=3.60, df=130, p<0.001). Patients who had abused cannabis made significantly more disadvantageous than advantageous choices (F=9.74, df=1, p=0.003), while the opposite was seen among patients who had not abused (F=4.36, df=1, p=0.04). Both groups significantly preferred those choices with low frequency–high magnitude of punishment (B+D) over those with high frequency–low magnitude (A+C) (F=65.26, df=1, p<0.001), although no differences were observed between the two groups.
Separate analyses were carried out to examine whether these differences were due to the effect of younger age or lower VC index of patients who had abused compared with those who had not abused cannabis (as patients who had abused cannabis had an earlier age of onset and lower VC index than patients who had not abused). ANOVA, adjusting for patients' age and VC index, also revealed that patients who had abused cannabis performed significantly worse than patients who had not abused in the GT total task (F=5.57, df=1, p=0.02).
Both groups of patients performed increasingly better along the five periods of the task (see Fig. 1) (overall F=13.24, df=4, p<0.001). This improvement was significant in both the group of patients who had abused cannabis (F=3.49, df=4, p=0.009) and in those who had not abused (F=10.42, df=4, p<0.001), although the improvement was significantly greater in those who had not abused (F=12.16, df=1, p=0.001). Between-group differences in the GT total task performance began to reach statistical significance from period 3 onwards (period 3: t=2.72, df=129, p=0.007; period 4: t=2.97, df=129, p=0.004; period 5: t=3.10, df=129, p=0.002).

Fig. 1. Evolution of performance along the five periods of the Iowa Gambling Task (GT) for the patients who had (—◆—) or had not (- -■- -) abused cannabis before their illness began.
Regarding the performance on DLPFC-sensitive tasks, no significant differences were found in any of the tasks (FAS, TMT and Backward Digits). When the analyses were carried out adjusting for the effect of age and pre-morbid IQ (VC), between-group differences remained non-significant.
Discussion
This study has demonstrated, in a sufficiently large sample of subjects with a first episode of schizophrenia, that cannabis abuse prior to the onset of psychosis is associated with greater impairment in a decision-making task linked to orbitofrontal function, but not with more severe deficits on the performance of working memory and executive function tasks, which are sensitive to dorsolateral prefrontal function. Patients who abused cannabis also had lower estimated IQ, as measured by the WAIS-III VC index, than those who had not abused. When the analyses of the prefrontal tasks were adjusted for the effect of estimated IQ, differences remained significant in the same direction. Although not an objective of this study, in our sample of first-episode psychosis patients cannabis abuse was not associated with clinical symptoms, as assessed by the SANS and SAPS scales after 6 weeks of programme entry.
Regarding decision making in schizophrenia, three studies have examined the performance of early-psychosis patients on the GT, one finding differences from controls (Kester et al. Reference Kester, Sevy, Yechiam, Burdick, Cervellione and Kumra2006) and the other two failing to find any difference (Hutton et al. Reference Hutton, Murphy, Joyce, Rogers, Cuthbert, Barnes, McKenna, Sahakian and Robbins2002; Rodriguez-Sanchez et al. Reference Rodriguez-Sanchez, Crespo-Facorro, Perez-Iglesias, Gonzalez-Blanch, Alvarez, Llorca and Vazquez-Barquero2005). In a preliminary report on part of the same sample as the present study, Rodriguez-Sanchez et al. (Reference Rodriguez-Sanchez, Crespo-Facorro, Perez-Iglesias, Gonzalez-Blanch, Alvarez, Llorca and Vazquez-Barquero2005) found that, compared to a control sample of age- and sex-matched healthy volunteers (n=22), patients with first-episode schizophrenia (n=80) were unimpaired on the global scores of the decision-making task (GT). We reanalysed these data, finding no differences between patients who had not abused cannabis and healthy controls (t=0.75, df=91, p=0.45), and a trend towards significant differences between patients who had abused cannabis and controls (t=1.81, df=81, p=0.07). Results from studies on chronic schizophrenia patients are also inconclusive, with a similar number of studies finding and not finding differences between patients and controls. Thus, it cannot be concluded from previous research whether schizophrenia patients show impairment in OFC function as assessed by the GT.
Findings from neuroimaging studies have implicated the OFC in several behavioural aspects of substance abuse, including anticipation of drug reward, craving for the drug, and impairments in judgement that could influence the decision to abstain or take the drug (London et al. Reference London, Ernst, Grant, Bonson and Weinstein2000). Cannabis users have been found to perform significantly worse than non-users on the GT, even after a 25-day abstinence period (Bolla et al. Reference Bolla, Eldreth, Matochik and Cadet2005). Impairments on the performance in decision-making tasks have also been reported in abusers of other drugs such as alcohol (Mazas et al. Reference Mazas, Finn and Steinmetz2000; Bechara et al. Reference Bechara, Dolan, Denburg, Hindes, Anderson and Nathan2001), cocaine (Grant et al. Reference Grant, Contoreggi and London2000; Bechara et al. Reference Bechara, Dolan, Denburg, Hindes, Anderson and Nathan2001), opiates (Rogers et al. Reference Rogers, Everitt, Baldacchino, Blackshaw, Swainson, Wynne, Baker, Hunter, Carthy, Booker, London, Deakin, Sahakian and Robbins1999b; Mintzer & Stitzer, Reference Mintzer and Stitzer2002) and 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’; Quednow et al. Reference Quednow, Kuhn, Hoppe, Westheide, Maier, Daum and Wagner2006); and lesion (Bechara et al. Reference Bechara, Tranel and Damasio2000) and neuroimaging (London et al. Reference London, Ernst, Grant, Bonson and Weinstein2000) studies have provided evidence that performance on the GT is correlated with OFC function. Thus, there is strong evidence linking drug abuse, including cannabis abuse, and OFC function impairment among non-psychotic individuals. Our findings provide preliminary support for this association among subjects experiencing their first episode of schizophrenia.
There is some evidence that repeated exposure to Δ9-tetrahydrocannabinol (THC) reduces dopamine transmission in the medial PFC of the rat (Jentsch et al. Reference Jentsch, Verrico, Le and Roth1998), and that neuropsychological prefrontal abnormalities shown by THC-exposed rats can be reversed with an acute amphetamine challenge (Verrico et al. Reference Verrico, Jentsch, Roth and Taylor2004), supporting the hypothesis that cannabis abuse can alter dopaminergic modulation in the PFC, leading to changes in decision making. However, this could not explain why DLPFC-related functions were not affected in our cannabis users compared to non-users. Another possible explanation is that decision-making differences between cannabis abusers and non-abusers reflect a pre-existing condition that led abusers to make costly decisions in the GT. Previous studies using the GT have found impairments in patients with borderline personality disorder (Haaland & Landrø, Reference Haaland2007) or bulimia nervosa (Boeka & Lokken, Reference Boeka and Lokken2006), and in suicide attempters (Jollant et al. Reference Jollant, Bellivier, Leboyer, Astruc, Torres, Verdier, Castelnau, Malafosse and Courtet2005), raising the possibility that individuals with affective dysregulation and/or impulsivity make costly decisions (as shown by the performance in the GT) and tend to take risks and seek sensations, this substance misuse being one of these costly decisions and sensation-seeking behaviours. Unfortunately, the question about which is the direction of causality between cannabis abuse and GT impairment cannot be answered from our results.
Both groups of patients improved their performance on the GT, although this improvement was significantly greater in the group of patients who had not abused cannabis. The patients who had not abused cannabis kept improving along the five periods into which the task was divided, whereas those patients who had abused improved from period 1 to period 2, but failed to improve from then on. Reanalysing the data of Rodriguez-Sanchez et al. (Reference Rodriguez-Sanchez, Crespo-Facorro, Perez-Iglesias, Gonzalez-Blanch, Alvarez, Llorca and Vazquez-Barquero2005), we found that the pattern of improvement in controls and cannabis non-abusers was similar, and significantly different from that of cannabis abusers. Thus, it appears that cannabis abusers compared to non-abusers, regardless of having or not having schizophrenia, have an impaired capacity to improve their decision-making skills. This is concordant with previous studies of cannabis users (Whitlow et al. Reference Whitlow, Liguori, Livengood, Hart, Mussat-Whitlow, Lamborn, Laurienti and Porrino2004; Bolla et al. Reference Bolla, Eldreth, Matochik and Cadet2005), and in some sense with the results of Grant et al. (Reference Grant, Gonzalez, Carey and Natarajan2001), who found in a meta-analysis of the long-term effects of cannabis on cognition, an association with lower capacity in the area of learning new information.
Regarding cognitive functions related to the DLPFC, such as working memory and executive function, it is widely accepted that patients with first-episode schizophrenia show deficits on the performance of neuropsychological tasks sensitive to this area (Hutton et al. Reference Hutton, Puri, Duncan, Robbins, Barnes and Joyce1998). Neuroimaging studies have also demonstrated the involvement of abnormal DLPFC functioning on the deficits that patients with schizophrenia show on working memory and executive function (Van Snellenberg et al. Reference Van Snellenberg, Torres and Thornton2006). In the preliminary report on part of this sample, Rodriguez-Sanchez et al. (Reference Rodriguez-Sanchez, Crespo-Facorro, Perez-Iglesias, Gonzalez-Blanch, Alvarez, Llorca and Vazquez-Barquero2005) showed that patients with a first episode of schizophrenia, compared to healthy controls, were impaired in the performance on tasks sensitive to DLPFC. When the analyses were repeated on the extended sample (132 patients and 22 controls), the differences were highly significant: FAS (t=3.71, df=152, p=0.0003); TMT (t=3.14, df=152, p=0.002); Backward Digits (t=3.93, df=152, p=0.0001).
However, we did not find any significant difference between patients who had or had not abused cannabis on any of these tasks. The effect of cannabis use on the performance in DLPFC-related tasks among non-psychotic subjects remains controversial, but some preliminary evidence indicates that executive function may only become impaired after long-term heavy cannabis use (Bolla et al. Reference Bolla, Brown, Eldreth, Tate and Cadet2002), and not after light or moderate use (Grant et al. Reference Grant, Gonzalez, Carey and Natarajan2001; Pope et al. Reference Pope, Gruber, Hudson, Huestis and Yurgelun-Todd2001; Fried et al. Reference Fried, Watkinson and Gray2005). Among patients with schizophrenia, Joyal et al. (Reference Joyal, Halle, Lapierre and Hodgins2003) found that those with co-morbid drug abuse/dependence, as compared to those with no history of drug abuse, performed better on prefrontal cognitive tasks. Furthermore, Stirling et al. (Reference Stirling, Lewis, Hopkins and White2005) found that, after a 10-year follow-up period, schizophrenic patients with pre-psychotic or current cannabis abuse performed significantly better than non-abusers on a range of neuropsychological, including prefrontal, tasks. These studies suggest two possible explanations: it could be that planning and organizational skills, reflected by fewer prefrontal abnormalities, are needed to initiate and maintain drug use (Joyal et al. Reference Joyal, Halle, Lapierre and Hodgins2003); or it could be that those schizophrenia cases that develop with no substance abuse are more linked to genetic and neurodevelopmental factors that give rise to the characteristic cognitive abnormalities of schizophrenia (Stirling et al. Reference Stirling, Lewis, Hopkins and White2005).
Although not a primary objective of this study, we found that first-episode psychosis patients who had abused cannabis had a lower estimated pre-morbid IQ, as measured by the WAIS-III VC index, than those who had not abused cannabis. This finding is coherent with the results of the meta-analysis by Grant et al. (Reference Grant, Gonzalez, Carey and Natarajan2001) that only found slight decrements in the area of learning new information among long-term non-psychotic cannabis users.
Taken together, and considering previous research in this field, the results of our comparisons between first-episode schizophrenia patients who had and had not abused cannabis before their illness onset suggest that pre-psychotic cannabis abuse is associated with impairment in orbitofrontal but not dorsolateral prefrontal cortex functions among first-episode schizophrenia patients. However, further studies are needed to examine the direction of causality between cannabis abuse and OFC impairments, and the long-term consequences of pre-psychotic cannabis abuse on DLPFC and OFC functions.
Acknowledgements
We thank the PAFIP researchers who helped with data collection, and G. Pardo for assistance during the investigations. We also thank the study participants and their families for enrolling in this study. The present study received the following financial support: Instituto de Salud Carlos III PI050427, Plan Nacional de Drogas Research Grant 2005-Orden sco/3246/2004 and SENY Fundació Research Grant CI 2005-0308007.
Declaration of Interest
None.





