1. Introduction
The Tiñu Formation in Oaxaca State, southern Mexico, provides the only record of fossiliferous lower Palaeozoic rocks south of the Laurentian successions in Sonora and Chihuahua states and Texas and north of the Gondwanan sequences in Andean Columbia and Venezuela. Pantoja-Alor (Reference Pantoja-Alor, Segura and Rodriguez-Torres1970) discovered the small inliers of the Tiñu Formation in 1964 (Fig. 1). Their first report by Pantoja-Alor & Robison (Reference Pantoja-Alor and Robison1967) was followed by descriptions of trilobites and molluscs from the inliers (Robison & Pantoja-Alor, Reference Robison and Pantoja-Alor1968; Yochelson, Reference Yochelson1968; Flower, Reference Flower1968). However, questions developed about the biostratigraphic and palaeogeographic significance of the Tiñu faunas, their depositional environments, and the early Palaeozoic location of southern Mexico.

Figure 1. Generalized locality maps. (a) Terminal Cambrian and lowest Ordovician outliers of the Tiñu Formation (tiny black areas) at the Tiñu (Tu) and Barranco de Santiago Ixtaltepec (SIx) inliers on the Oaxaca Complex, Oaxaca State. Mesozoic terranes include the Xolapa and mylonitic Juarez terranes. (b) Major tectonic subdivisions of Mexico, Belize and northern Guatemala and relationship of Oaxachia to Ouachita orogen in northwest Mexico and adjacent United States. Area of (a) outlined by box in southern Mexico (modified from Centeno-Garcia & Keppie, Reference Centeno-Garcia and Keppie1999, fig. 1).
A revision of the Tiñu trilobite fauna, based on new material, will be presented elsewhere, but preliminary assessments are included in this paper. Robison & Pantoja-Alor (Reference Robison and Pantoja-Alor1968) regarded the Tiñu trilobites as indicating correlation with lowest Ordovician (Tremadoc) faunas of the Avalon continent in England and Wales, as well as with the Saukia Zone of the classic Upper Cambrian succession of Laurentia. In particular, they suggested that the association of Saukia globosa Robison & Pantoja-Alor (now assigned to Mictosaukia Shergold, Reference Shergold1975) and Richardsonella variagranula Robison & Pantoja-Alor (variously assigned to Pseudokainella Harrington, Reference Harrington1938 or Elkanaspis Ludvigsen, Reference Ludvigsen1982; see Ludvigsen, Reference Ludvigsen1982; Jell, Reference Jell1985; Peng, Reference Peng1990b) in the lower Tiñu Formation corroborated Whittington's (Reference Whittington1966, table 1; see also Wolfart, Reference Wolfart1970, table 3) view that the Laurentian Saukia Zone was correlative with the Avalonian lower Tremadoc. Support for this latter correlation waned quickly (e.g. Ludvigsen, Reference Ludvigsen1982), but some workers regarded at least the lower part of the Tiñu as Upper Cambrian. Thus, similarities in Shumardia species led Rushton (Reference Rushton, Bassett and Dean1982) to propose that the lower Tiñu correlated with the uppermost Cambrian Acerocare Zone of North Wales. In addition, Shergold (Reference Shergold1988) emphasized Neoparabolina frequens (Barrande, Reference Barrande1868) (= Parabolina argentina (Kayser, Reference Kayser1876); see Nikolaisen & Henningsmoen, Reference Nikolaisen and Henningsmoen1985) as a key to correlation with British, Scandinavian and Argentinian successions, and implied an assignment to the Upper Cambrian, rather than the Tremadoc (Shergold, Reference Shergold1988, fig. 2). Shergold (Reference Shergold1988) also noted the significance of Mictosaukia, which indicates a correlation of the lower Tiñu Formation with pre-Tremadocian strata of South and North China (Peng, Reference Peng1984; Qian, Reference Qian and Chen1986).
However, these trilobite-based biostratigraphic re-evaluations ran counter to D. L. Clark's (in Robison & Pantoja-Alor, Reference Robison and Pantoja-Alor1968, figs 2B–4) unillustrated reports from the lower Tiñu of the characteristic Tremadocian conodont Cordylodus angulatus Pander, Reference Pander1856, and of Oneotodus simplex (Furnish, Reference Furnish1938), a species known from Laurentian Tremadoc-equivalent strata (Ethington & Brand, Reference Ethington and Brand1981). Recovery of nematophorous graptolites conclusively demonstrated that shales somewhat higher in the Tiñu at the Santiago Ixtaltepec locality (fig. 1a, locality SIx) are lower Tremadoc (Sour & Buitrón, Reference Sour and Buitrón1987; Sour Tovar, Reference Sour Tovar1990), but left open the precise correlation of the lower Tiñu. Finally, even the depositional environment of the Tiñu Formation remained problematical, with biotic content (abundant echinoderm debris and associated green algal fragments) suggesting shallow, apparently photic marine conditions (Pantoja-Alor, Reference Pantoja-Alor, Segura and Rodriguez-Torres1970; Cabaleri & Armella, Reference Cabaleri and Armella1990). However, lenticular debris flows and interpreted deeper-water environments have been claimed to occur at all localities (Centeno-Garcia et al. Reference Centeno-Garcia, Keppie, Sour-Tovar, Sanchez-Zavala, Ortega-Gutierrez and Keppie1998).
To resolve questions about the age, palaeobiogeographic significance and depositional environments of the Tiñu Formation, we undertook a restudy of the unit's conodont biostratigraphy and lithofacies. One likely consequence of this study was thought to be a refining of the early Palaeozoic location of southern Mexico, a region that has ping-ponged from proposed proximities with the northern Andes (Keppie, Reference Keppie1977), southern Laurentia (Scotese & McKerrow, Reference Scotese and McKerrow1990; Sedlock, Ortega-Gutiérrez & Speed, Reference Sedlock, Ortega-Gutiérrez and Speed1993), southern Andes (Dalziel, Dalla Salda & Gahagan, Reference Dalziel, Salda and Gahagan1994), and Amazon craton (Keppie & Ortega-Gutiérrez, Reference Keppie and Ortega-Gutiérrez1995; Keppie, Reference Keppie2004). Only much later in the Carboniferous did Oaxaquia, a continental fragment that comprises the middle Proterozoic basement and lower Palaeozoic cover sequence of central and southern Mexico (fig. 1b), collide and unite with southern Laurentia to uplift the Ouachita orogen of south-central North America (e.g. Keppie et al. Reference Keppie, Nance, Murphy and Dostal2003; Keppie, Reference Keppie2004).
2. Geological setting and collecting localities
The Tiñu Formation nonconformably overlies middle Proterozoic (c. 1.0 Ga) high-grade metamorphics and intrusives of the Oaxacan Complex in two small inliers located northwest of Oaxaca City (fig. 1a). Earlier overviews (Pantoja-Alor, Reference Pantoja-Alor, Segura and Rodriguez-Torres1970; Cabaleri & Armella, Reference Cabaleri and Armella1990; Centeno et al. Reference Centeno-Garcia, Keppie, Sour-Tovar, Sanchez-Zavala, Ortega-Gutierrez and Keppie1998) documented marine depositional environments through the thin Tiñu Formation (72+ m: this report). Initial epsilon neodymium (εNdi) and model ages (TDM) from the Tiñu Formation are consistent with a sediment source from the Oaxacan Complex (Centeno-Garcia et al. Reference Centeno-Garcia, Keppie, Sour-Tovar, Sanchez-Zavala, Ortega-Gutierrez and Keppie1998; Murphy et al. Reference Murphy, Keppie, Braid and Nance2005; Gillis et al. Reference Gillis, Gehrels, Ruiz and Flores de Dios-Gonzales2005) and with early Palaeozoic passive margin deposition.
2.a. Locality Tu
At its type locality, the Río Salinas section of Pantoja-Alor (Reference Pantoja-Alor, Segura and Rodriguez-Torres1970), the Tiñu Formation occurs in a NNE-trending and −plunging syncline that is unconformably overlain on its east flank either by Cretaceous limestone or Tertiary red beds (see Centeno-Garcia & Keppie, Reference Centeno-Garcia and Keppie1999, fig. 3, for a detailed map of the type locality). The locality is 59 km NW of Oaxaca City, and is reached by exiting Federal Route MEX135 at Nochixtlán, driving 10 km S on Route 190, and then driving 2 km W on the dirt road to Río Salinas village. We measured a N-dipping, structurally simple, 72 m thick section through the Tiñu Formation in a heavily grazed goat pasture just east of the synclinal axis at the formation's type locality (Figs 1–3, locality Tu). Our locality Tu corresponds to Robison & Pantoja-Alor's (Reference Robison and Pantoja-Alor1968, fig. 2A, B) locality west of Tiñu village. This section extends from a contact with middle Proterozoic gneiss south of the dirt road and up the grassy slope to the highest exposures of the Tiñu Formation on the north shoulder of the road.

Figure 2. Sections in the lower Tiñu Formation (Yudachica Member); conodont samples (SIx and Tu) indicated to right of columns; number after locality abbreviation is metres above Oaxaca Complex. Figure shows proposed depositional sequences of Yudachica Member.

Figure 3. Sections in the upper Tiñu Formation (Río Salinas Member); samples (SIx and Tu) indicated to right of columns. Lithologies as in Figure 2.
2.b. Locality SIx
Northern Tiñu Formation sections occur along the N–S-trending, W-dipping Barranco de Santiago Ixtaltepec inlier (fig. 1a). This inlier nonconformably overlies the middle Proterozoic Oaxaca Complex. The Tiñu Formation in the Barranco de Santiago Ixtaltepec inlier is not unconformably overlain by Carboniferous marine rocks (compare Robison & Pantoja-Alor, Reference Robison and Pantoja-Alor1968). Rather, the inlier is overlain to the west by a thrust slice of marine siliciclastics (Santiago Formation; Lower Carboniferous) and a higher slice of mixed marine siliciclastics and carbonates (Ixtaltepec Formation; Upper Carboniferous) (see Centeno-Garcia & Keppie, Reference Centeno-Garcia and Keppie1999, fig. 2, for a detailed map of the Barranco de Santiago Ixtaltepec inlier).
We did not re-investigate Robison & Pantoja-Alor's (Reference Robison and Pantoja-Alor1968, fig. 3) Arroyo Totoyac locality at the south end of the inlier, but measured and collected their northern section. This section is 66 km NW of Oaxaca City, and is reached by driving on a dirt road for 15.5 km NE of Nochixtlán village. The measured section lies 150 m past the last hairpin curve north of Santiago Ixtaltepec hamlet. It is reached by descending the steep path over the Carboniferous Ixtaltepec and Santiago formations 700 m north of Santiago Ixtaltepec (Figs 1–3; locality SIx). The lower 18 m of the Tiñu Formation at locality SIx is a monoclinal, W-dipping section, but higher shales and sheared fine-grained sandstones are increasingly faulted, folded, intruded with quartz veins and hydrothermally bleached toward the thrust contact with the coarse-grained, cliff-forming Santiago Formation.
3. Lithofacies and depositional environments
3.a. Members of the Tiñu Formation
As noted by Pantoja-Alor (Reference Pantoja-Alor, Segura and Rodriguez-Torres1970; also Robison & Pantoja-Alor, Reference Robison and Pantoja-Alor1968), the Tiñu Formation is divisible into a limestone-rich lower member and a shale- and sandstone-dominated upper member. We now distinguish these as the Yudachica Member (new) and Río Salinas Member (new), respectively (see Appendix 1). The base of the Río Salinas is a thin (to 15 cm), lenticular quartz arenite overlain by an equally thin, flat pebble conglomerate composed of limestone and silt-shale clasts derived from the Yudachica Member (Fig. 3).
3.b. Yudachica Member
The lower member of the Tiñu Formation consists of interbedded medium-grey limestones and light grey-brown, yellow-green weathering silt shales (Fig. 2). Up to 0.7 m of erosional relief on the Oaxacan Complex was observed at locality SIx, but abundant coarse sand derived from the Oaxacan Complex was observed only in the lower 0.85 m of locality Tu.
Diagenetic (nodular limestone and dolostone) carbonate horizons are rare in the Yudachica Member at locality Tu, but more common at SIx (Fig. 2). The dominant limestones at localities Tu and SIx range from minor calcisiltites, which are more abundant at SIx, to common fossil hash-intraclast wacke- to grainstones at both localities. The fossil allochems include disarticulated and fragmented trilobite debris, orthid brachiopods, and locally abundant gastropod conchs (particularly Tu-6.1, -6.2; SIx-6.5). Echinoderm ossicles are ubiquitous in the coarser limestones, and locally form grainstones (Tu-8.4–9.8). This abundance of fossil debris from a variety of benthic taxa, including rare calcified algae, in the coarser-grained limestones has been noted as evidence for lower Tiñu Formation deposition under relatively shallow, photic, unrestricted marine conditions (Cabaleri & Armella, Reference Cabaleri and Armella1990). The absence of carbonate ooids and aggregates, peloids, and any evidence of evaporates is consistent with unrestricted marine deposition, while the abundance of trilobite debris in almost all limestone beds comports with subtidal settings (e.g. Westrop & Adrain, Reference Westrop and Adrain1998; Adrain et al. Reference Adrain, Westrop, Chatterton and Ramsköld2000). The combination of an absence of carbonate ooids or evaporates in limestones dominated by ‘heterozoan’ (particularly trilobite) fragments, and which lack obvious precipitated lime mud or marine cement (Cabaleri & Armella, Reference Cabaleri and Armella1990; thin-sections and slabs made in this study) are consistent with cool-water/temperate depositional regimes (e.g. James, Reference James, James and Clarke1997).
A common association noted in the field and in sawn slabs is that thin planar-laminated calcisiltite or trilobite wackestone beds have erosive tops when they are directly overlain by 5–10 cm thick, trilobite–echinoderm–calcisiltite intraclast pack- and grainstone beds with very thin carbonate sand caps. These calcisiltite/wackestone–pack-/grainstone relationships are more common at locality Tu, and were observed only in the lower Yudachica Member (lower 6.25 m at Tu and several horizons below 8.8 m at SIx). These relationships are interpreted to indicate condensation during limestone accumulation, and depositional processes that included erosion, winnowing and sorting under episodic high-energy conditions produced by storm waves.
Despite this evidence for episodic high-energy, wave-dominated deposition, microfacies and faunal evidence suggest the occurrence of relatively low oxygenation of bottom sediments and, possibly, bottom waters during Yudachica Member deposition. Formic acid-breakdown of limestones for acid-resistant microfossils yielded small amounts of polished chlorite (probably originally glauconite) sand grains and large quantities of phosphatic debris (up to 10 % of original sample mass). The phosphatic residue consists of a variety of components: rounded and polished, black phosphatic sand grains; abundant phosphatized conchs and steinkerns of gastropods, orthid brachiopods, hyoliths and ostracodes, some of which show the abrasion and polishing of already phosphatized (‘pre-fossilized’) grains before final burial; common phosphate-replaced agnostids and ‘polymeroid’ trilobite debris; and very large quantities of spongy, partially phosphatized fragments of intraclasts and lime mud. This phosphatization suggests replacement of calcium carbonate allochems and sediment with intense denitrification under an oxygen minimum layer or within dysoxic sediment before final burial, while the glauconite grains suggest proximity to a mixing zone between oxygenated and dysoxic/anoxic water masses (e.g. Sageman, Wignall & Kauffman, Reference Sageman, Wignall, Kauffman, Einsele, Ricken and Seilacher1991). Evidence of low levels of bottom-water or sediment oxygenation is also suggested by the fact that intense burrowing activity is limited to burrow-churning in the wacke- and packstones, while the intervening grey-brown shales are either unburrowed or have only small Planolites-like horizontal burrows. Finally ‘Olenus’-like olenids and agnostids, trilobites characteristic of dark, organic-rich, low eH facies (e.g. Fortey & Cocks, Reference Fortey and Cocks2003), appear through the Yudachica Member (Robison & Pantoja-Alor, Reference Robison and Pantoja-Alor1968, figs 2–4; data from this study). Their presence through the Yudachica Member, but in association with abundant non-olenid trilobites (e.g. ‘Asaphellus’), echinoderms and brachiopods, suggests deposition of the Yudachica Member under water mass conditions that apparently ranged from weakly dysoxic to the lower end of ‘normal’ oxygenation (compare Schovsbo, Reference Schovsbo2001, fig. 3).
Primary sedimentary structures and depositional processes differ between localities Tu and SIx. Oscillatory wave activity is indicated at locality Tu by isolated sand ripples with bi-directional cross-beds (Tu-0.25–0.85), imbricated intraclast pebbles that form fans (Tu-7.1, -7.2), and a surface with small symmetrical ripples (Tu-11.3). Tidal action is also suggested at locality Tu by bi-directional troughs and climbing ripples in echinoderm grainstones (Tu-8.7–9.4 m) and a lensing echinoderm hash dune with west-dipping foresets (Tu-9.4 to Tu-9.8 locally).
Locality SIx differs by having a number of lenticular, intraclast conglomerates with particularly large (to 25 × 4 cm cross-sections), flat lime mudstone clasts in the lower 8.0 m of the Yudachica Member (Fig. 2; 1.7, 3.0, 5.1, 7.2, 7.9 m horizons). These conglomerates are debris flows, and the carbonate clasts generally ‘float’ in a muddy carbonate matrix with echinoderm fragments and sand- and granule-sized, lime mudstone fragments. In one bed (7.2 m), the flat limestone clasts are imbricated and dip to the south, and indicate transport to the north. Several normally graded, trilobite pack- to grainstone beds higher at locality SIx (8.0, 9.4 and 10.85 m) represent either turbidites or tempestites.
These observations agree with Centeno-Garcia et al.'s (Reference Centeno-Garcia, Keppie, Sour-Tovar, Sanchez-Zavala, Ortega-Gutierrez and Keppie1998) conclusion that debris flows occur in the lower Tiñu Formation. However, the debris flows occur to the north at locality SIx, while no evidence suggests that the locally intraclast-bearing pack- and grainstones at locality Tu reflect anything other than deposition on a storm wave-influenced shelf. The presence of debris flows, evidence for their northerly transport, and occurrence of turbidites/normally graded tempestite beds at SIx are all consistent with a northerly dipping ramp or slope during lower Tiñu Formation deposition.
3.c. Río Salinas Member
The sharp lithological contact of the Yudachica and Río Salinas members is marked by a thin, flat limestone and silt-shale clast conglomerate at localities Tu and SIx. The limestone clasts reach 30 × 20 × 7 cm at Tu, but are granule- and small pebble-sized at SIx, where the conglomerate is phosphatized and black in colour. The conglomerate and an underlying, lenticular green quartz arenite, which is present only at Tu, form the basal beds of the Río Salinas Member.
Limestones disappear at the Yudachica–Río Salinas contact, and the conglomerate is abruptly replaced by organic-rich, dark-grey, non-burrowed (laminated) shales with rare sideritic and pyrite nodules at Tu and SIx, respectively. This dysoxic facies comprises the lower 22.5 m of the Río Salinas Member at locality Tu. The dark shales have a low-diversity fauna that includes a few horizons with moldic olenid trilobites and nematophorous dendroid graptolites at localities Tu and SIx (Fig. 3).
A burrow-churned, nodular-bedded, trilobite wacke- to packstone with subangular phosphate pebbles overlain by a calcareous quartz arenite (Tu-35.7–36.0) marks the transition from dark-grey shale to medium-grey, structureless (burrow-churned), siliciclastic mudstone in the Río Salinas Member. (This limestone corresponds to Robison & Pantoja-Alor's (Reference Robison and Pantoja-Alor1968, fig. 2A) horizon 16000k in stratigraphic distance above the base of the Río Salinas Member, and is fault-duplicated in a flower structure on the west side of the syncline. Thus, we found that Robison & Pantoja-Alor's (Reference Robison and Pantoja-Alor1968, fig. 2A) trilobite-bearing horizons 16000i, 16000j are duplications of the 16000k bed.)
Above this lowest bedded limestone in the Río Salinas Member, the medium-grey siliciclastic mudstones have a number of limestones and calcareous, fine-grained sandstones, which are commonly burrow-mottled to -churned. Wave-produced sedimentary structures appear above this lowest bedded limestone, and include invaginated ellesmerceroid conchs (Tu-37.4; see Yochelson, Reference Yochelson1968), bi-directional (wave) ripples (Tu-39.75), surfaces with symmetrical ripples (Tu-46.5, -51), and N–S-trending gutter casts (Tu-48.1). This interbedded shale and sandstone facies is suggestive of a shallow subtidal, proximal tempestite facies (e.g. Myrow, Reference Myrow1992). Improved oxygenation is suggested by the transition from dark-grey shales with sparse olenids and nematophorous dendroids to medium-grey shales with limestones that yield asaphids and remopleurids (horizon Tu-43). Still higher in the succession, the impression of a shoaling-up succession in the Río Salinas Member is further suggested by the presence of 3.2 m of red (that is, well-oxygenated) shale and coarse-grained sandstone with fragmented orthoid brachiopod shells and bi-directional, apparently tidally generated, dunes at the top of the exposure at locality Tu (Fig. 3).
4. Conodonts and biostratigraphy of Yudachica Member
4.a. Conodonts and colour alteration index
Conodont elements were not recovered from samples from the fossiliferous (e.g. trilobite and echinoderm hash) lowest beds of the Yudachica Member above the Proterozoic–Tiñu Formation nonconformity, and their correlation is problematical (Fig. 4). Abundant conodont elements appear about 2 m above the nonconformity, and persist through the top of the Yudachica Member (Table 1). The colour alteration indices (CAI) of conodont elements (see Epstein, Epstein & Harris, Reference Epstein, Epstein and Harris1977) are low (CAI = 2) through most of the Yudachica Member at locality Tu, but abruptly increase to 3.5 in the upper 2 m, and this CAI is maintained in the Río Salinas Member at locality Tu. Colour alteration indices are much higher at locality SIx (CAI = 4), and this indicates deeper burial at Santiago Ixtaltepec.
Table 1. Upper Cambrian conodonts from productive samples from the Yudachica Member of the Tiñu Formation

Acid-disaggregated samples had a 6.0 kg mass. el. – element. Intense thermal blackening rendered some cordylodan elements from locality SIx- opaque; as the shape of their basal cavities cannot be determined, species-level identifications are not possible.

Figure 4. Conodont-based correlation of the Tiñu Formation into conodont and trilobite zonation of temperate successions of Baltica (left side of figure; see Szaniawski & Bengtson, Reference Szaniawski and Bengtson1998, and Löfgren, Repetski & Ethington, 1999) and into conodont succession of tropical Laurentia (e.g. Miller, Reference Miller1980). Figure shows proposed depositional sequences of Yudachica Member.

Figure 5. Upper Cambrian Cordylodus species from the Yudachica Member of the Tiñu Formation; hypotype specimens. (a–g) Cordylodus andresi Viira & Sergeeva in Kaljo et al. Reference Kaljo, Borovko, Heinsalu, Khasanovich, Mens, Popov, Sergeeva, Sobolevskaya and Viira1986, from Tu-4.95 unless otherwise indicated. (a) Rounded element, NYSM 17369, ×202; (b) rounded element, NYSM 17370, ×82; (c) rounded element, NYSM 17271, ×119; (d) rounded element, NYSM 17372, ×127, Tu-5.7 (see Fig. 10g); (e) rounded element, NYSM 17373, ×105; (f) compressed element, NYSM 17374, ×93 (see Fig. 10h); (g) compressed element, NYSM 17375, ×82. (h–l) Cordylodus proavus Müller, Reference Müller and Hinz1959. (h) Rounded element, NYSM 17376, ×98, Tu-9.8 (see Fig. 10i); (i) rounded element, NYSM 17377, ×87, Tu-13.0 (see Fig. 10j); (j) rounded element, NYSM 17378, ×69, Tu-8.5; (k) compressed element, NYSM 17379, ×69, Tu-9.8 (see Fig. 10k); (l) compressed element, NYSM 17380, ×84, Tu-9.8. (m–t) Cordylodus caseyi Druce & Jones, Reference Druce and Jones1971, emend. Landing, Reference Landing1993, from Tu-13.0 unless otherwise indicated. (m) Rounded element, NYSM 17381, ×79, Tu-9.8 (see Fig. 5m); (n) compressed element, NYSM 17382, ×62 (see Fig. 10p); (o) compressed element, NYSM 17383, ×65; (p) rounded (‘Cordylodus drucei’ sensu formo) element, NYSM 17384, ×57; (q) rounded element, NYSM 17385, ×78; (r) rounded element, NYSM 17386, ×69; (s) asymmetrical rounded element, NYSM 17387, ×78 (see Fig. 10n); (t) rounded element transitional into ‘Cordylodus drucei’ sensu formo, NYSM 17388, ×79. (u) Cordylodus prion Lindström, Reference Lindström1955, sensu Nicoll (Reference Nicoll1991), rounded element, NYSM 17389, ×116, Tu-13.0 (see Fig. 10q).
4.b. Cordylodus andresi Zone
The lower 7.2 m of the Yudachica Member at Tu and the lower 6.5 m at SIx has a distinctive conodont fauna characterized by the oldest known cordylodan, Cordylodus andresi Viira & Sergeeva in Kaljo et al. Reference Kaljo, Borovko, Heinsalu, Khasanovich, Mens, Popov, Sergeeva, Sobolevskaya and Viira1986 (fig. 5a–g). The definition of C. andresi followed herein is Szaniawski & Bengtson's (Reference Szaniawski and Bengtson1998), with the species’ elements having basal cavities that extend well above the zone of maximum cusp curvature, and sometimes almost reaching the tip of the element. As noted by Szaniawski & Bengtson (Reference Szaniawski and Bengtson1998, p. 16), C. primitivus Bagnoli, Barnes & Stevens, Reference Bagnoli, Barnes and Stevens1987, sensu Müller & Hinz, Reference Müller and Hinz1991, from the Upper Cambrian of Sweden is best assigned to C. andresi. We further note that the type specimens and illustrated elements of C. primitivus and C. andresi Barnes, Reference Barnes1988, have shallower basal cavities. As they fall within the range of morphology of C. proavus Müller, 1959, elements, the latter two ‘species’ should be assigned to C. proavus as junior synonyms.
Cordylodus andresi is reported only as low as the base of the Cordylodus proavus Zone on the Laurentian and North China carbonate platform (Miller et al. Reference Miller, Evans, Loch, Ethington, Stitt, Holmer and Popov2003; Dong, Repetski & Bergström, Reference Dong, Repetski and Bergström2004; see Fig. 4). However, its occurrence is much lower in the Upper Cambrian of Baltica, where it is the eponymous species of a sub-C. proavus Zone (Szaniawski & Bengtson, Reference Szaniawski and Bengtson1998). This ‘low’ occurrence of C. andresi is also shown in the Yudachica Member (Tu-2.05, −4.95). As in southern Sweden (Müller & Hinz, Reference Müller and Hinz1991, table 2; Szaniawski & Bengtson, Reference Szaniawski and Bengtson1998), C. andersi occurs with Proconodontus muelleri Miller, Reference Miller1969, and very weakly denticulated elements of P. serratus Miller, Reference Miller1969 (fig. 6n, o, t), species which have ranges that do not persist into the C. proavus Zone of Laurentia and North China. This lower part of the Yudachica Member is correlated with the Laurentian P. muelleri Zone (compare Szaniawski & Bengtson, Reference Szaniawski and Bengtson1998, p. 14).
Slightly higher in the Yudachica Member, the appearance of Eoconodontus notchpeakensis (Miller, Reference Miller1969) (fig. 6q, r) in Tu-5.7 and Tu-6.5 with all earlier-appearing Cordylodus andresi Zone taxa indicates a likely correlation with the Eoconodontus Zone of the Laurentian carbonate platform (e.g. Miller et al. Reference Miller, Evans, Loch, Ethington, Stitt, Holmer and Popov2003) (Fig. 4). Teridontus nakamurai (Nogami, Reference Nogami1967) (fig. 6i–l) is common in the lower Yudachica Member (Table 1), but provides no further resolution in biostratigraphic correlation. Although T. nakamurai appears in sub-Cordylodus proavus Zone strata in unrestricted marine facies marginal to Laurentia (Landing, Ludvigsen & von Bitter, Reference Landing, Ludvigsen and von Bitter1980; Landing, Reference Landing1983), its lowest occurrence is in the lower C. proavus Zone on the Laurentian platform (Miller, Reference Miller1980; Miller et al. Reference Miller, Evans, Loch, Ethington, Stitt, Holmer and Popov2003). Orminskia rexroadae Landing gen. et sp. nov. and species (fig. 6a–h) is newly recognized from the Yudachica Member, and does not yet assist in interregional correlation. Its seeming disappearance within the C. andresi Zone after the appearance of E. notchpeakensis (Table 1) may be of importance in subsequent studies of the C. andresi Zone. The superficial resemblance of O. rexroadae gen. et sp. nov. and a possible assignment of its elements to T. nakamurai may explain reports of exceptionally low, sporadic occurrences of Teridontus Miller, Reference Miller1980, on the Laurentian platform (Miller, Reference Miller1980; Miller et al. Reference Miller, Evans, Loch, Ethington, Stitt, Holmer and Popov2003, fig. 5).
4.c. Cordylodus proavus Zone
Several samples in the middle Yudachica Member (Tu-7.9, -8.5; SIx-6.9) are assigned to an undivided Cordylodus proavus Zone. With the exception of Eoconodontus nakamurai and Teridontus nakamurai, all Cordylodus andresi Zone taxa disappear in the Yudachica Member with the appearance of C. proavus Müller, 1959 (fig. 5h–l). None of the taxa used for subdivision of the C. proavus Zone into five subzones in Laurentian successions (Miller, Reference Miller1969, Reference Miller1980; Fig. 4) were recovered from this low-diversity (three euconodont species) interval. Thus, the C. proavus Zone of the middle Yudachica Member can only be considered as younger than the C. andresi Zone and older than the overlying terminal C. proavus Zone (Fig. 4).
4.d. Terminal Cordylodus proavus Zone–lower Fauna B interval
Horizons at least as low as Tu-9.0 and SIx-8.0 yield abundant elements of Cordylodus caseyi Druce & Jones, Reference Druce and Jones1971, emend. Landing, Reference Landing1993 (fig. 5m–t), with rare specimens of Semiacontiodus nogamii Miller, Reference Miller1969 (fig. 6m, p). Cordylodus caseyi emend. is the senior synonym for numerous reports of Cordylodus intermedius Furnish, Reference Furnish1938, and several other cordylodan ‘species’ from the terminal Upper Cambrian, upper C. proavus Zone on a number of Cambrian palaeocontinents (see synonymy and discussion in Landing, Reference Landing1993, pp. 13, 15, 16). An association of C. caseyi emend. and S. nogamii is present in the two upper subzones of the C. proavus Zone as originally defined by Miller (Reference Miller1969, Reference Miller1980) (see discussion in Section 6.a) in Laurentia and China (e.g. Ross et al. Reference Ross, Hintze, Ethington, Miller, Taylor, Repetski and Taylor1997; Miller et al. Reference Miller, Evans, Loch, Ethington, Stitt, Holmer and Popov2003; Dong, Repetski & Ethington, Reference Dong, Repetski and Bergström2004).
The low-diversity euconodont fauna from the upper Yudachica Member (five species) also includes a few elements of Cordylodus prion Lindström, Reference Lindström1955, sensu Nicoll, Reference Nicoll1991 (fig. 5u) recovered near the top of the Yudachica Member (Table 1; Tu-13.0 and SIx-11.5). Limited data exist on the stratigraphic range of C. prion in Laurentia and Australia (Nicoll, Reference Nicoll1991; Miller et al. Reference Miller, Evans, Loch, Ethington, Stitt, Holmer and Popov2003). Its lowest occurrence in these tropical successions is in post-Cordylodus proavus Zone and terminal Upper Cambrian strata variously referred to the lower Fauna B interval (Ethington & Clark, Reference Ethington, Clark, Sweet and Bergström1971; Fig. 4) or Cordylodus lindstromi Zone (see Miller et al. Reference Miller, Evans, Loch, Ethington, Stitt, Holmer and Popov2003). Significantly, although Cordylodus angulatus has been reported from the Yudachica Member (Robison & Pantoja-Alor, Reference Robison and Pantoja-Alor1968), this diagnostic lower Tremadoc species (e.g. Löfgren, Repetski & Ethington, Reference Löfgren, Repetski and Ethington1999) was not recovered in this study. In addition, Robison & Pantoja-Alor's (Reference Robison and Pantoja-Alor1968) report of Oneotodus simplex, a species described from Laurentian Tremadoc-equivalent strata (Ethington & Brand, Reference Ethington and Brand1981), was also not confirmed in this study. The report of O. simplex from the Yudachica Member may have been based on elements of Teridontus nakamurai. In short, all available data emphasize that no faunal evidence indicates that the uppermost Yudachica Member is Tremadoc and lowest Ordovician.
5. Biostratigraphy of Río Salinas Member
5.a. Lowest Río Salinas Member
We collected nematophorous dendroid graptolites as low as 5.0 m above the base of the Río Salinas Member at locality Tu, and duplicated Sour & Buitrón's (Reference Sour and Buitrón1987) collections somewhat higher in the member at locality SIx (Fig. 3; Tu-18.0, SIx-22.0, -22.5). Only one form, Rhabdinopora flabelliforme desmograptoides (Hahn, Reference Hahn1912), is present in our collections (Fig. 7). Sour & Buitrón (Reference Sour and Buitrón1987) and Centeno-García et al. (Reference Centeno-Garcia, Keppie, Sour-Tovar, Sanchez-Zavala, Ortega-Gutierrez and Keppie1998, fig. 11.2) reported a larger dendroid fauna (six taxa) in the tectonically contorted lower Río Salinas Member at SIx. However, all of their forms are synonymized with R. flabelliforme desmograptoides (see Systematic Palaeontology).
This subspecies is known from cool-water successions on the Avalon and Baltic palaeocontinents (Hahn, Reference Hahn1912; Bulman, Reference Bulman1954), where it is an element of the middle lower Tremadoc graptolite ‘Assemblage 2’ (Cooper, Reference Cooper1979). Conodont-based correlations indicate that the Assemblage 2 ‘Matane graptolites’ of southern Quebec (see Bulman, Reference Bulman1950) are all referable to the Rossodus manitouensis Zone in Laurentia (Landing, Barnes & Stevens, Reference Landing, Barnes and Stevens1986; Fig. 4). Consequently, the lower Río Salinas Member is also best correlated with the Rossodus manitouensis Zone. As the uppermost Yudachica Member is not younger than the lower conodont Fauna B interval, the dendroids from the lower Río Salinas Member provide evidence that the lensing sandstone and conglomerate at the Yudachica–Río Salinas contact marks a biostratigraphically significant depositional sequence boundary at the Cambrian–Ordovician boundary.
5.b. Middle Río Salinas Member
A moderately diverse euconodont fauna (seven species) was recovered from limestones and calcareous sandstones in the middle Río Salinas Member at locality Tu (35.7–43.0 m; Table 2). This interval is readily correlated by five euconodont species that occur in the upper lower Tremadoc Paltodus deltifer deltifer Subzone in Baltica (see Löfgren, Reference Löfgren1993, Reference Löfgren1997; Löfgren, Repetski & Ethington, Reference Löfgren, Repetski and Ethington1999) (Fig. 4). The association of the eponymous form P. deltifer deltifer (Lindström, Reference Lindström1955) (fig. 8a–q) with Paroistodus numarcuatus (Lindström, Reference Lindström1955) (fig. 8r–t), Variabiloconus variabilus (Lindström, Reference Lindström1955) (fig. 8u–cc), and early representatives of Drepanodus arcuatus Pander, Reference Pander1856 (fig. 9h, i) characterizes P. deltifer deltifer Subzone faunas in these cool-water Baltic assemblages (Löfgren, Reference Löfgren1993, Reference Löfgren1997). Variabiloconus transiapetus Löfgren, Repetski & Ethington, Reference Löfgren, Repetski and Ethington1999 (fig. 9f, g) also occurs in the Swedish P. deltifer deltifer Subzone, and its occurrence in unrestricted marine successions in the western Great Basin has been used in correlating the subzone into the Rossodus manitouensis Zone in Laurentia (Löfgren, Repetski & Reference Löfgren, Repetski and Ethington1999). This correlation of the middle Río Salinas Member and Baltic P. deltifer deltifer Subzone with the R. manitouensis Zone is compatible with recovery of a single hyaline element of Acanthodus uncinatus Furnish, Reference Furnish1938 (fig. 9v), a characteristic R. manitouensis Zone species in Laurentia (e.g. Landing, Westrop & Knox, Reference Landing, Westrop and Knox1996), but which is reported to range down into lowest Ordovician strata (Miller et al. Reference Miller, Evans, Loch, Ethington, Stitt, Holmer and Popov2003). The single element of Scolopodus filosus Ethington & Clark, Reference Ethington and Clark1964 (fig. 8dd), from the Río Salinas Member represents a low occurrence of an uncommon species previously reported only as low as strata just above the R. manitouensis Zone in Laurentia (Ethington & Clark, Reference Ethington and Clark1981; Repetski, Reference Repetski1982). Numerous elements of Cornuodus? clarkei Landing sp. nov. (Figs 9j–u, 10r, s) were recovered from the P. deltifer deltifer Subzone, but the biostratigraphic significance of the form is currently undetermined as it does not seem to have been recovered from other successions.
Table 2. Upper lower Tremadoc conodonts from productive samples from the middle Río Salinas Member of the Tiñu Formation

Acid-disaggregated samples had a 6.0 kg mass. el. – element.
6. Discussion
6.a. Conodont-based correlations
Despite the abundance of conodont elements in the Tiñu Formation, relatively few taxa are represented in each sample (Tables 1, 2). The conodonts and dendroid graptolites, however, show that the Tiñu Formation consists of an Upper Cambrian Yudachica Member and an unconformably overlying, upper lower Tremadoc Río Salinas Member.
One consequence of the low-diversity conodont faunas is that the finely subdivided, ‘standard’ conodont zonation and subzonation appropriate to the upper Upper Cambrian of tropical carbonate platform successions in western Laurentia (Miller, Reference Miller1969, Reference Miller1980; Miller et al. Reference Miller, Evans, Loch, Ethington, Stitt, Holmer and Popov2003) is not applicable to the Tiñu Formation. Thus, a ‘simplified’ conodont zonation with a coarser biostratigraphic resolution comparable to that appropriate in the cool-water Upper Cambrian of Baltica is appropriate to the Yudachica Member (Fig. 4).
A Cordylodus andresi Zone comparable to that recognized in the cool-water sequences of Baltica (Szaniawski & Bengtson, Reference Szaniawski and Bengtson1998) is appropriate for the conodont fauna of the lower Yudachica Member. As the lowest c. 2.0 m of the Yudachica Member did not yield biostratigraphically useful conodonts, Upper Cambrian marine onlap across the Oaxacan Complex is tentatively referred to the Cordylodus andresi Chron (Fig. 4).
A few samples from the middle Yudachica Member have low-diversity conodont assemblages with the lowest Cordylodus proavus elements, and are assigned to the C. proavus Zone. A finer biostratigraphic resolution is not possible. The five subzones of the terminal Upper Cambrian Cordylodus proavus Zone as originally defined by Miller (Reference Miller1969, Reference Miller1980) (Fig. 4) are based on species of Clavohamulus Furnish, Reference Furnish1938, Fryxellodontus Miller, Reference Miller1969, and Hirsutodontus Miller, Reference Miller1969, which were not recovered from the Tiñu Formation. These genera and species are uncommon in unrestricted marine settings, such as the continental slope successions marginal to Laurentia (Landing, Reference Landing1983; Landing, Barnes & Stevens, Reference Landing, Barnes and Stevens1986) and unknown in the cool-water successions of Avalon and Baltica (Landing, Taylor & Erdtmann, Reference Landing, Taylor and Erdtmann1978; Bruton, Koch & Repetski, Reference Bruton, Koch and Repetski1988; Szaniawski & Bengtson, Reference Szaniawski and Bengtson1998). The presence of low-diversity conodont faunas that lack characteristic tropical/low-latitude uppermost Cambrian conodont genera and species is consistent with the apparent cool-water depositional setting of the Yudachica Member, and not with the low-latitude setting of Laurentia in the terminal Upper Cambrian.
The uppermost Yudachica Member is referred to the terminal Cordylodus proavus–lower Fauna B interval. The association of Cordylodus caseyi emend. and Semiacontiodus nogamii appears in the upper two subzones of the C. proavus Zone (Fig. 4), an interval which has been designated the Cordylodus intermedius Zone in many reports on the Laurentian and Chinese platforms (Bagnoli, Barnes & Stevens, Reference Bagnoli, Barnes and Stevens1987; Miller, Reference Miller and Clark1984, Reference Miller1988; Miller et al. Reference Miller, Evans, Loch, Ethington, Stitt, Holmer and Popov2003; Dong, Repetski & Bergström, Reference Dong, Repetski and Bergström2004).
The upper Yudachica Member correlates into this so-called ‘C. intermedius Zone’, but we reject this biostratigraphic designation, and refer the lower part of the upper Yudachica to the upper C. proavus Zone for two important reasons (Fig. 4). The first reason is that C. intermedius sensu formo is apparently an element and junior synonym of the younger Tremadoc species Cordylodus angulatus (G. S. Nolan, unpub. Ph.D. thesis, Univ. Waterloo, Ontario, 1976; Nicoll, Reference Nicoll1990; Landing, Reference Landing1993). Thus, C. intermedius is inappropriate as an eponymous species for a terminal Cambrian interval. The second reason to suppress the so-called ‘C. intermedius Zone’ derives from the fundamental rules of stratigraphic nomenclature. Indeed, separating off the top two subzones of the C. proavus Zone as a ‘C. intermedius Zone’, while maintaining the name C. proavus Zone for the lower three subzones (Ethington & Clark, Reference Ethington and Clark1981; Bagnoli, Barnes & Stevens, Reference Bagnoli, Barnes and Stevens1987; Miller, Reference Miller1988; Miller et al. Reference Miller, Evans, Loch, Ethington, Stitt, Holmer and Popov2003; Dong, Repetski & Bergström, Reference Dong, Repetski and Bergström2004), creates a homonym, and is not acceptable (North American Stratigraphic Commission, 1983, article 19g). In short, the Tremadoc species C. intermedius sensu formo is not appropriate for a biostratigraphic zone that comprises the terminal Cambrian, while separating off the upper two subzones of the original C. proavus Zone (Miller, Reference Miller1969, Reference Miller1980) as a separate zone and retaining ‘C. proavus’ zone for the lower three subzones creates a homonym with the original definition of the C. proavus Zone.
6.b. Depositional history of Yudachica Member
Conodont biostratigraphy and lithofacies analysis of the Tiñu Formation help in a synthesis of the development of an Early Palaeozoic, passive margin succession on the Oaxacan Complex (Keppie et al. Reference Keppie, Nance, Murphy, Dostal and Miller2006). The Yudachica Member marks latest Cambrian marine onlap in Oaxaca. It is significant that this thin member (12 and 13.3 m at SIx- and Tu) spans the lower–upper C. andersi Zone, a very thin, undifferentiated Cordylodus proavus Zone, and a terminal C. proavus Zone–lower Fauna B interval. The Yudachica is very condensed when correlated into coeval successions (upper Proconodontus Zone–lower Fauna B interval) in marginal Laurentian platform settings such as that of the western Great Basin (c. 150 m: Miller et al. Reference Miller, Evans, Loch, Ethington, Stitt, Holmer and Popov2003). Indeed, the Yudachica Member does not differ greatly in thickness from coeval sections in Baltica, and it is only 3 to 4 times as thick as the very condensed upper Peltura Zones–Acerocare Zone in the Oslo Region (e.g. Henningsmoen, Reference Henningsmoen1957). U–Pb zircon geochronology shows that 4 Ma separate the middle Peltura Zones from the Cambrian–Ordovician boundary (Landing et al. Reference Landing, Bowring, Davidek, Rushton, Fortey and Wimbledon2000). Simple division of this figure into the thickness of the Yudachica Member yields an exceptionally low, average rate of accumulation (c. 0.3–0.4 cm ka−1) of the compacted rock of the Yudachica Member.
The slow rate of development of accommodation space for Yudachica Member deposition may reflect either slow subsidence early in the cooling history of a rifted margin on the southern margin of the Rheic Ocean (e.g. Keppie & Ortega-Gutiérrez, Reference Keppie, Ortega-Gutiérrez, Ramos and Keppie1999; Keppie et al. Reference Keppie, Nance, Murphy, Dostal and Miller2006) or deposition in the interior of a large, slowly subsiding cratonic block, perhaps following a strong eustatic rise. The resultant aggradation rate of the Yudachica Member seems to have been so low that relatively monofacial sequences persisted through the latest Cambrian at the southern and northern exposures of the Yudachica Member (storm wave-dominated to upper tidally influenced shelf at Tu and slope/ramp facies at SIx).
The low accumulation rate of the Yudachica Member and its apparent condensation may reflect unconformities and the presence of two or three depositional sequences in the member. An evaluation of possible depositional sequences within the Yudachica Member (Figs 3, 4) would require a more detailed, bed-by-bed analysis of the faunas and microfacies of the unit than was undertaken in this study. However, the presence of exceptionally thin conodont zones by comparison with those in other regions and the seeming coincidence of abrupt biostratigraphic changes (that is, appearance of new taxa and disappearance of taxa present in lower parts of the member) with apparent lithological breaks suggest a sequence stratigraphic architecture of the Yudachica Member.
Possible sequence boundaries may be marked at locality Tu by the appearance of a relatively thick, shale-dominated interval with the only large diagenetic (methanogenic?) nodules in the section (7.2–8.6 m) above the highest known Cordylodus andresi Zone fauna (Tu-7.2). This shale-dominated interval has limestones with an undifferentiated (lower?) C. proavus Zone fauna (sample Tu-8.5) near its top. Similarly, the appearance of a terminal Cordylodus proavus Zone fauna (sample Tu-9.0) is associated with the abrupt change into echinoderm tidalites in the upper Yudachica Member, and a sequence boundary may lie at the base of this facies.
Laterally equivalent horizons at locality SIx may include evidence for possible offlap and a sequence boundary marked by the thick, particularly coarse-grained debris flow (locally 7.1–7.35 m). This debris flow has an undifferentiated (lower?) Cordylodus proavus Zone fauna and lies above the highest known C. andresi Zone fauna (SIx-6.5). The proposed upper sequence boundary at Tu may be marked by a lenticular trilobite grainstone at SIx-8.0 with a terminal C. proavus Zone fauna. This latter possible sequence boundary lies at the base of a succession through the upper Yudachica Member that lacks the debris flows present in the lower part of the member.
These suggested depositional sequences in the Yudachica Member may correspond to several depositional sequences defined in the Ibex area on the western Laurentian margin (Miller et al. Reference Miller, Evans, Loch, Ethington, Stitt, Holmer and Popov2003). Interregional conodont-based correlations suggest correspondences between the Tiñu Formation C. andresi Zone and Ibex-area sequences 4 and 5, the undifferentiated (lower?) C. proavus Zone with sequences 7 and 8, and the terminal C. proavus–lower Fauna B interval with sequences 10 and lower 11.
Persistently low average aggradation rates of the Yudachica Member may help explain the faunal and lithological indications for low bottom-water oxygenation through most of the unit. Indeed, evidence exists for persistent dysoxia/anoxia of upper continental slope waters through the Late Cambrian and earliest Ordovician, and for the movement of these low-oxygen slope waters across platforms during times of particularly high eustatic sea levels (Landing, Benus & Whitney, Reference Landing, Benus and Whitney1992; Landing & Bartowski, Reference Landing and Bartowski1996; Landing, Geyer & Bartowski, Reference Landing, Geyer and Bartowski2002).
In the late Late Cambrian (that is, Saukia Chron sensu lato in Laurentian successions), strong eustatic rise brought shorelines into the upper Mississippi River valley (e.g. Landing, Benus & Whitney, Reference Landing, Benus and Whitney1992). Thus, the simplest interpretation of the Yudachica Member is that it apparently reflects three particularly high sea-level intervals within the late Late Cambrian. Just as the highly condensed, organic-rich, black ‘Alum Shale’ facies records dysoxic/anoxic bottom environments in the interior of Baltica during Late Cambrian–earliest Ordovician high eustatic sea levels, most facies of the roughly coeval Yudachica Member, with exception of the echinoderm tidalites in the upper part of locality Tu, record bottom waters that were most likely mildly dysoxic by comparison with the highly dysoxic facies that appeared in coeval parts of the Alum Shale in Baltica (Schovsbo, Reference Schovsbo2001).
6.c. Depositional history of Río Salinas Member
A biostratigraphically resolvable depositional sequence boundary (lower Fauna B interval–lower Rossodus manitouensis) spans the Cambrian–Ordovician boundary at the Yudachica Member–Río Salinas Member contact. A thin conglomerate and abrupt facies change between the members mark this contact. With exception of minor (2–3 cm deep) erosional bevelling of the top limestone bed of the Yudachica Member, little evidence for stratigraphic cut-out at this sequence boundary is indicated at localities SIx and Tu. Robison & Pantoja-Alor (Reference Robison and Pantoja-Alor1968, fig. 3) measured a thicker section of the Yudachica Member at Arroyo Totoyac (16 m) than at localities SIx and Tu (11.95 m and 13.3 m, respectively: this report). These lateral changes in thickness may reflect differential erosion of the Yudachica Member before deposition of the Río Salinas Member. However, they did not report any change in trilobite faunas in the upper Yudachica member at Arroyo Totoyac, and it is possible that the locality only preserves a somewhat greater thickness of the lower conodont Fauna B interval.
Marine onlap and abrupt deepening is recorded by the olenid- and nematophorous dendroid-bearing, unburrowed, lower (but not lowest) Tremadocian dark-grey mudstones of the lower Río Salinas Member above its basal conglomerate. Investigation of this basal conglomerate was not undertaken, and it may prove to represent a deposit of a low-stand systems tract or a condensed transgressive systems tract.
This sea-level rise is apparently eustatic, as it correlates with sea-level rise within the Rossodus manitouensis Chron that drove shorelines into the upper Mississippi River valley region in Laurentia (Landing, Benus & Whitney, Reference Landing, Benus and Whitney1992; Ross & Ross, Reference Ross, Ross, Cooper, Droser and Finney1995; Landing, Westrop & van Aller Hernick, Reference Landing, Westrop and van Aller Hernick2003; Landing & Westrop, Reference Landing and Westrop2006) and across Baltica during the ‘early to middle Tremadoc highstand interval’ (Nielsen, Reference Nielsen, Webby, Paris, Droser and Percival2004). Development of a strongly dysoxic mid-water mass on continental slopes is recorded during this interval (middle–late part of the early Tremadoc) of high eustatic levels (Landing, Benus & Whitney, Reference Landing, Benus and Whitney1992; Landing, Westrop & van Aller Hernick, Reference Landing, Westrop and van Aller Hernick2003), and an onlap of this low-oxygen water is apparently recorded by the lower Río Salinas Member.
A shoaling-up succession is recorded through the overlying siliciclastic mudstone-dominated, middle part of the Río Salinas Member. Improved bottom-water oxygenation is suggested by the colour change from dark-grey to grey-green mudstone, and is indicated by the appearance of intense burrow churning of the mudstone at about the level of the first limestone (Tu-35.7), and by the appearance of trilobite assemblages with asaphids and remopleurids in slightly higher limestone beds (Tu-43).
This facies change and the appearance of wave-generated fabrics are within the upper lower Tremadocian Paltodus deltifer deltifer Subzone. The near identity of conodont assemblages from the middle Tiñu Formation with P. deltifer deltifer Subzone assemblages from Poland and Sweden (Dzik, Reference Dzik1976; Löfgren, Reference Löfgren1993, Reference Löfgren1997; Löfgren, Repetski & Ethington, Reference Löfgren, Repetski and Ethington1999) and their dissimilarity with coeval Laurentian faunas reflects both the degree of faunal exchange between these regions and their environmental similarities in the Early Ordovician. In lieu of any particularly useful palaeoclimatic evidence, the conodont faunas from the middle Río Salinas Member are most suggestive of temperate, unrestricted marine environments.
Shallow siliclastic shelf deposition is recorded through the higher strata of the Río Salinas Member, with interbedded mudstones and sandstones of a proximal tempestite facies capped by well-oxygenated, reddish purple shale and tidalite sandstones at the top of the section. However, we did not recover any biostratigraphically useful fossils above the P. deltifer deltifer Subzone limestones and sandstones. If no temporally significant stratigraphic discontinuities exist above this fossiliferous upper lower Tremadocian interval, the upper Tiñu Formation may provide a record of the highstand facies of the early–middle Tremadoc eustatic highstand and, possibly, the late Tremadoc–early Arenig eustatic lowstand (see Nielsen, Reference Nielsen, Webby, Paris, Droser and Percival2004). In short, just as the Yudachica Member is interpreted to reflect a submergence of the Oaxaca Complex coeval with particularly high eustatic levels that brought shorelines into the upper Mississippi River valley, the Río Salinas Member reflects resubmergence of parts of Oaxaquia during the middle–late early Tremadoc eustatic high.
Unlike the Yudachica Member, no lithological or biostratigraphic evidence for exceptionally slow accumulation rates exists in the Río Salinas Member. A very rough comparative figure for the accumulation rate of the Río Salinas can be calculated. If the exposed, c. 60 m thick Río Salinas Member at locality Tu represents most of the 6 Ma long Tremadoc Epoch (Landing et al. Reference Landing, Bowring, Davidek, Rushton, Fortey and Wimbledon2000), its rate of accumulation of compacted rock is almost three times as fast as that of the Yudachica. In addition, the dominant facies (condensed interbedded limestones and siliciclastic mudstones of the Yudachica v. siliciclastic mudstones and sandstones of the Río Salinas) strongly differ between the two members, and there is an abrupt lithofacies contact between the members. These contrasts are interesting because both members were deposited during separate intervals of particularly high sea levels in the Late Cambrian and early Early Ordovician when Laurentian shorelines reached the upper Mississippi River valley. The difference between the two members in the lithofacies deposited and rate of production of accommodation space during times of comparable sea-level rise probably reflect epeirogenic changes on the Oaxacan passive margin that included increased availability of siliciclastic sediment and increased subsidence rates.
6.d. Palaeogeographic implications
Restudy of the Tiñu Formation, the lowest cover unit on the middle Proterozoic Oaxaca Complex, emphasizes that its Upper Cambrian–lowest Ordovician biotas and lithofacies do not support a palaeogeographic proximity to such tropical palaeocontinents as Laurentia (see Scotese & McKerrow, Reference Scotese and McKerrow1990; Sedlock, Ortega-Gutiérrez & Speed, Reference Sedlock, Ortega-Gutiérrez and Speed1993).
As noted by Shergold (Reference Shergold1988), olenids are a significant proportion of the trilobites of the Tiñu Formation. Almost 40 % of the 21 genera reported by Robison & Pantoja-Alor (Reference Robison and Pantoja-Alor1968) are olenids, and this has influenced some biogeographic assessments of the fauna. For example, Shergold (Reference Shergold1988) used the diversity of olenids to assign Oaxaca to the Baltic Province, which he interpreted as a set of cool-water biofacies. We agree that the trilobite faunas of Baltica, Avalonia and Gondwanan South America and Oaxaca likely lived under temperate conditions. However, Shergold's approach to trilobite biogeography focused on the biostratigraphic significance of faunas and does not lead to an improved understanding of global palaeogeography. Associated non-olenid taxa need to be considered, as well as the fact that olenid distribution reflects the wide occurrence of dysoxic water (e.g. Schovsbo, Reference Schovsbo2001) both at shallow depths at high latitudes and in deeper settings at mid- to low latitudes. Taylor (Reference Taylor, Kauffman and Hazel1977) attributed this wide latitudinal distribution of olenids to their preference for cold water. However, his approach assumed a ‘modern ocean’ with a source of cold polar water, a situation that likely did not exist in the Cambrian–Early Ordovician, an interval with no evidence for polar ice caps (Landing, Benus & Whitney, Reference Landing, Benus and Whitney1992). As he recognized, Shergold's (Reference Shergold1988, p. 364) assessment actually reflected similarities in environmental conditions, and his palaeogeographic maps (Shergold, Reference Shergold1988, fig. 1) and those of Fortey & Cocks (Reference Fortey and Cocks2003, fig. 6) place the Mexican terranes in Gondwanan or peri-Gondwanan positions.
In their original study, Robison & Pantoja-Alor (Reference Robison and Pantoja-Alor1968) commented upon the close relationship with faunas from Argentina (e.g. Harrington & Leanza, Reference Harrington and Leanza1957), which was considered to be part of a ‘core area’ of Gondwana by Fortey & Cocks (Reference Fortey and Cocks2003). The dikelocephalid Mictosaukia globosa (Robison & Pantoja-Alor) is a genus that supports a Gondwana or peri-Gondwana of Oaxaquia, as the genus occurs in Australia (Shergold, Reference Shergold1975, Reference Shergold1991), Afghanistan (Wolfart, Reference Wolfart1970), south China (Peng, Reference Peng1984), north China (Endo & Resser, Reference Endo and Resser1937; Qian, Reference Qian and Chen1986) and, possibly, Thailand (Shergold, Reference Shergold1975).
The distributions of Onychopyge Harrington, Reference Harrington1938 (represented in the Tiñu by O. sculptura Robison & Pantoja-Alor) and Koldinioidia Kobayashi, Reference Kobayashi1931 (represented in the Tiñu by K. sulcata Robison & Pantoja-Alor, Reference Robison and Pantoja-Alor1968) are broadly congruent with that of Mictosaukia, with both occurring in Australia (Shergold, Reference Shergold1972, Reference Shergold1975, Reference Shergold1991; Jell, Reference Jell1985), South China (Lu et al. Reference Lu, Lin, Han, Li and Ju1984; Peng, Reference Peng1984, Reference Peng1990b) and North China (Endo & Resser, Reference Endo and Resser1937). Onychopyge has also been recorded from Argentina (Harrington & Leanza, Reference Harrington and Leanza1957; Zeballo & Tortello, Reference Zeballo and Tortello2005).
The asaphid Asaphellus Callaway, Reference Callaway1877, has been reported from several palaeocontinents and terrains, although there is considerable variation in such features as the size and position of the palpebral lobes in species assigned to the genus (e.g. Robison & Pantoja-Alor, Reference Robison and Pantoja-Alor1968, pl. 98; Peng, Reference Peng1990a, pl. 6, figs 3–7, 9, 10, 15; Shergold Reference Shergold1991, pl. 9, figs 9–14; Fortey & Owens, Reference Fortey and Owens2001, fig. 7a, b), and monophyly seems doubtful. As recognized by Shergold (Reference Shergold1975, p. 33), the pre-Tremadocian asaphid Golasaphus Shergold, Reference Shergold1972, is closely comparable to species attributed to Asaphellus by Robison & Pantoja-Alor, and this may be where the affinities of the Mexican species lie (e.g. compare Shergold, Reference Shergold1972, pl. 10, fig. 1, with Robison & Pantoja-Alor, Reference Robison and Pantoja-Alor1968, pl. 98, figs 12, 14). If correct, this provides additional support for a Gondwanan to peri-Gondwanan position for the Oaxaca.
This trilobite-based Gondwanan affinity agrees with middle Proterozoic lead isotopes (Ruiz et al. Reference Ruiz, Tosdal, Restrepo, Murillo-Muñetón, Ramos and Keppie1999; Cameron et al. Reference Cameron, Lopez, Ortega-Gutiérrez, Solari, Keppie, Schulze, Tollo, Corriveau, McLelland and Bartholomew2004), thermal histories (Keppie & Ortega-Gutiérrez, Reference Keppie, Ortega-Gutiérrez, Ramos and Keppie1999; Keppie et al. Reference Keppie, Solari, Orgega-Gutiérrez, Elias-Herrera, Nance and Keppie2004), and a late Proterozoic–Early Cambrian rifting history (Keppie et al. Reference Keppie, Nance, Murphy, Dostal and Miller2006) that indicate a similarity of the Oaxacan Complex to the Santa Marta and Garzón massifs in the Columbian Andes (Keppie, Reference Keppie2004).
Upper Cambrian and lowest Ordovician conodont faunas from the Tiñu Formation are most similar to coeval faunas from Baltica and allow relatively precise biostratigraphic correlations into that palaeocontinent. However, as the olenid trilobites, this similarity reflects the ease of biotic exchange between temperate, unrestricted marine successions, and not palaeogeographic proximity of palaeocontinents in the Late Cambrian–Early Ordovician. It is important to note that conodonts from this time interval remain very poorly known from the temperate, unrestricted marine successions of Avalon (Landing, Taylor & Erdtmann, Reference Landing, Taylor and Erdtmann1978). The recovery of a more complete Avalonian conodont record through this interval would likely document a succession of low-diversity conodont faunas with the same taxa as those known in the Tiñu Formation.
Lithofacies and faunas indicate movement of the West African margin of Gondwana from equatorial latitudes in the early Cambrian to the South Pole in the latest Ordovician (e.g. Landing, Reference Landing, Nance and Thompson1996, Reference Landing2005). In the course of this southerly movement of West Gondwana, bedded carbonates became a limited component of platform successions as early Cambrian carbonate platforms disappeared, and bedded limestones were reduced to minor shell-hash beds. The Tiñu Formation provides the best-known record of these temperate, ‘heterozoan’ limestones in the Upper Cambrian and Lower Ordovician of West Gondwana. Similar Tremadoc limestones occur in a thick Cambrian–Lower Ordovician succession on the Garzón massif in Andean Columbia (Harrington & Kay, Reference Harrington and Kay1951; C. S. Bridger, unpub. Ph.D. thesis, Univ. National de Columbia, Bogota, 1982). As lithological and faunal data on this ‘mainland’ Gondwanan succession are limited, those from the Tiñu Formation provide the best palaeogeographic information on the Early Palaeozoic location of the Mexican–northern South American margin of Gondwana (Fig. 11).
7. Systematic palaeontology
Many taxa have been well discussed elsewhere or are represented by a limited number of specimens, and are not discussed below. Illustrated specimens are reposited in the New York State Museum (NYSM) Palaeontology Collection.
Class CONODONTA Eichenberg, Reference Eichenberg1930 Order PROTOCONODONTIDA Landing, Reference Landing1995
Genus Phakelodus Miller, Reference Miller and Clark1984
Type species
Oneotodus tenuis Müller, 1959, from the Upper Cambrian of Wyoming (? = Proconodontus elongatus Zhang in An et al. Reference An, Zhang, Xiang, Zhang, Xu, Zhang, Jiang, Yang, Lin, Cui and Yang1983, from the Upper Cambrian of north China).
Discussion
Two species of Phakelodus with similar, very elongate elements have been recognized in a number of reports. They are distinguished by the transverse cross-sections of their elements. Phakelodus tenuis (see synonymy in Müller & Hinz, Reference Müller and Hinz1991) elements have a rounded-oval cross-section. By comparison, P. elongatus (Zhang in An et al. Reference An, Zhang, Xiang, Zhang, Xu, Zhang, Jiang, Yang, Lin, Cui and Yang1983) (see synonymy in Müller & Hinz, Reference Müller and Hinz1991) elements have a teardrop-shaped cross-section with a sharply rounded posterior margin. However, these taxa may be synonymous as elements of both species regularly co-occur in samples and have comparable stratigraphic ranges (e.g. An et al. Reference An, Zhang, Xiang, Zhang, Xu, Zhang, Jiang, Yang, Lin, Cui and Yang1983; Müller & Hinz, Reference Müller and Hinz1991). Both Phakelodus ‘species’ also co-occur in the Tiñu Formation (Table 1). Because P. elongatus may prove to be a junior synonym of P. tenuis, its elements are distinguished as ‘P. elongatus’ in this report (fig. 6u, Table 1).
Order CONODONTOPHORIDA Eichenberg, Reference Eichenberg1930
Genus Cornuodus Fåhraeus, Reference Fåhraeus1966, emend. Dzik, Reference Dzik1994
Type species
Cornuodus erectus Fåhraeus, Reference Fåhraeus1966, from the Middle Ordovician of southeastern Sweden (= Drepanodus longibasis Lindström, Reference Lindström1955, from the Lower Ordovician of southeastern Sweden).
Cornuodus ? clarkei Landing sp. nov
Figures 9j–u, 10r, s
Holotype
NYSM 17447 from sample Tu-43.0, upper lower Tremadoc Paltodus deltifer deltifer Subzone, middle Río Salinas Member of the Tiñu Formation, Oaxaca State, Mexico (fig. 9j, k).
Paratypes
NYSM 17448–17452 and NYSM 17454–17456 from Tu-43.0 and NYSM 17453 from Tu-37.4.
Diagnosis
Probable Cornuodus species known from elongate, proclined to slightly reclined, anteriorly and posteriorly keeled, laterally compressed, coniform elements that form a weakly differentiated symmetry transition series (symmetrical drepanodiforms; weakly differentiated scandodiforms; asymmetrical paltodiforms with short, low lateral costa in zone of maximum curvature; and acodiform-like elements); cusps of many elements wider antero-posteriorly above zone of maximum curvature and lack posterolateral costae.
Etymology
For David L. Clark, who first examined conodonts from the Tiñu Formation (in Robison & Pantoja-Alor, Reference Robison and Pantoja-Alor1968) and who was an important influence on EL.
Description
Coniform elements opaquely albid above basal cavity that extends into zone of maximum curvature (fig. 10r, s). Cusp of some elements conspicuously broadens above zone of maximum curvature (fig. 9j, p); bases narrow to broad in lateral view (fig. 9j, u). Elements elongate; proclined to gently reclined (fig. 9j, o); cusp lanceolate with sharp anterior and posterior keels; aboral margin circular (Fig. 9j) to strongly laterally compressed ellipse (Fig. 10s) to strongly flattened triangle with rounded outer-lateral costa (Fig. 9r, s). Development of fine longitudinal striae highly variable, may cover all surfaces of element (Fig. 9k) or be present only on posterolateral flanks in zone of maximum element curvature; microstriae become obsolescent in broad band anterior to aboral margin (Fig. 9j). Sharp anterior and posterior keels appear anterior to aboral margin and extend to tip; fine costae never present immediately lateral to posterior keel; drepanodiforms symmetrical (Fig. 9j, n) and transitional into scandodiforms with weakly laterally deflected base (Fig. 9m); posterior costa of scandodiforms may continue onto posterolateral surface of base as fine ridge (Fig. 9u). Paltodiform-like elements with low, short, anterolateral to posterolateral costa in zone of maximum curvature (Fig. 9p, q); in a few elements, a short, low anterolateral costa originates near the anterior keel in the zone of maximum element curvature, and curves slightly posteriorly and onto middle of cusp before becoming obsolescent. Acodiform-like elements asymmetrical with laterally deflected anterior keel (Fig. 9s) and broadly rounded medial costa on opposite side (Fig. 9r).
Discussion
The available specimens are morphologically simple elements of a weakly differentiated apparatus that is most similar to that of the stratigraphically long-ranging (Tremadoc–Ashgill) form Cornuodus longibasis (see Löfgren, Reference Löfgren1999). Similarities between C. longibasis and C.? clarkei sp. nov. include element coloration and shape (albid, elongate, coniforms with short to long bases, lanceolate cusps, narrow to broad bases that are not strongly deflected laterally, and basal cavity extending to zone of maximum element curvature). The elements of both species have longitudinal microstriae that tend to be restricted to the posterior cusp margin (see Löfgren, Reference Löfgren1999, p. 182), although those of C.? clarkei sp. nov. may extend along all surfaces of the elements. Löfgren's (Reference Löfgren, Repetski and Ethington1999) illustrations of C. longibasis elements shows that many of its elements have the posterolateral keel flanked by a low longitudinal costa immediately to the right and/or left. These costae do not occur in C.? clarkei sp. nov. elements. The apical widening of C.? clarkei sp. nov. elements is apparently not present in C. longibasis. Most elements of C.? clarkei sp. nov. recovered from the Tiñu Formation collections are drepanodiform-like, and correspond to Löfgren's (Reference Löfgren, Repetski and Ethington1999) S-series elements. The laterally costate acodiform-like elements (Fig. 9r, s) may be homologous to the asymmetrical P-elements in C. longibasis.
Cornuodus? clarkei sp. nov. is referable neither to Acanthodus Furnish emend. Landing, Westrop & Knox (Reference Landing, Westrop and Knox1996), which has hyaline or weakly albid elements, nor to Laurentoscandodus Landing in Landing, Westrop & Knox (Reference Landing, Westrop and Knox1996), a genus with nongeniculate oistodiforms. Scalpellodus Dzik, Reference Dzik1976, emend. Landing, Westrop & Knox (Reference Landing, Westrop and Knox1996) is an uppermost Cambrian–lower Middle Ordovician genus also known from longitudinally microstriated, simple, albid, short- to long-based coniform elements arranged in a weakly to strongly differentiated symmetry transition series (see Löfgren, Reference Löfgren1978; Landing, Westrop & Knox, Reference Landing, Westrop and Knox1996). However, Scalpellodus elements typically have lateral keels. The oldest known Scalpellodus species, the uppermost Cambrian–lowest Ordovician S. utahensis (Miller, Reference Miller1969), is known from less elongate, proclined scandodiforms with antero- and posterolateral keels commonly bordered by a longitudinal sulcus (see Paltodus utahensis in Miller, Reference Miller1969, pl. 63, figs 33–40). Scalpellodus utahensis has rare symmetrical, laterally compressed, ‘unicostate elements’ (Miller, Reference Miller1980, p. 35) similar to the drepanodiforms of the new species.
Scalpellodus longipinnatus (Ji & Barnes, Reference Ji and Barnes1994), which ranges from the terminal Cambrian into upper lower Tremadoc-equivalent strata coeval with those yielding C.? clarkei sp. nov. (that is, the Rossodus manitouensis Zone), also has very elongate elements. However, S. longipinnatus had laterally compressed, anteriorly and posteriorly keeled scandodiforms comparable to those in C.? clarkei sp. nov., but also had S. utahensis-like scandodiforms in which the costae are bordered by a deep sulcus (Landing, Westrop & Knox, Reference Landing, Westrop and Knox1996). At present, S. longipinnatus is known from tropical carbonate platform successions in Laurentia, North China, South Korea and, probably, Australia (Landing, Westrop & Knox, Reference Landing, Westrop and Knox1996, pp. 675–6). Upper Lower–lower Middle Ordovician species of Scalpellodus include three Baltoscandian taxa: S. viruensis Löfgren, Reference Löfgren1978, with similar but less elongate elements than C.? clarkei sp. nov.; S. gracilis (Sergeeva, Reference Sergeeva1964), in which the scandodiforms have very elongate bases and a strongly differentiated anterolateral costa; and S. latus (van Wamel, Reference Van Wamel1974), with drepanodiforms that are acontiodiform, not laterally compressed and lanceolate in cross-section as in C.? clarkei sp. nov. (see Löfgren, Reference Löfgren1978).
Genus Orminskia Landing gen. nov
Type species
Orminskia rexroadae Landing gen. et sp. nov. from the Upper Cambrian (lower Cordylodus andersi Zone), lower Yudachica Member of the Tiñu Formation, Oaxaca State, Mexico.
Diagnosis
Euconodont genus with apparatus consisting of a weakly differentiated symmetry transition in hyaline, elongate, coniform elements with rounded cross-sections and deep basal cavities; elements lack surface micro-ornament in genotype species.
Etymology
For Edward A. Orminski (1924–2003), Anne Kaletta (nee Orminski) (1920–2005), Anna V. Orminski (1900–1992), and Helen B. Landing (nee Orminski) (1922–2006); maternal uncle, aunt, grandmother, and mother of EL, who encouraged an early interest in palaeontology.
Description
Apparatus consists of simple, elongate, proclined to reclined coniform elements (Fig. 6a–g) with circular to weakly laterally compressed cusps and bases, weakly differentiated symmetry transition series embraced by bilaterally symmetrical elements to elements with weakly laterally deflected bases (Fig. 6c); basal cavity deep and extends to zone of maximum cusp curvature (Fig. 10a, b); cusp completely lacks white matter and is transparent above tip of basal cavity; no surface micro-ornament present in elements of genotype species (Fig. 6d).

Figure 6. Upper Cambrian conodonts with coniform apparatuses from the Yudachica Member of the Tiñu Formation; hypotype specimens unless otherwise indicated. (a–h) Orminskia rexroadae Landing gen. et sp. nov. (a) Holoytpe NYSM 17390, ×108, locality Tu-2.05 (see Fig. 10a); (b) paratype NYSM 17391, ×178, Tu-2.05; (c, d) paratype NYSM 17392, lateral view ×126, and magnified view ×347 of smooth surface, Tu-4.3; (e) paratype NYSM 17393, ×160, Tu-4.95 (see Fig. 10b); (f) paratype NYSM 17394, ×198, Tu-4.95; (g) paratype NYSM 17395, ×212, Tu-4.95; (h) paratype NYSM 17396, ×174, Tu-5.7. (i–l) Teridontus nakamurai (Nogami, Reference Nogami1967). (i) NYSM 17397, ×85, Tu-5.7; (j, k) NYSM 17398, lateral view ×81 and detail of longitudinal microstriae ×775, Tu-9.8; (l) NYSM 17399, ×150, Tu-13.0. (m, p) Semiacontiodus nogamii Miller, Reference Miller1969, from Tu-13.0. (m) Asymmetrical (anterolaterally weakly costate) element, NYSM 17400, ×154; (p) symmetrical (posteriorly costate) element, NYSM 17401, ×156. (n, o) Proconodontus muelleri Miller, Reference Miller1969, from Tu-4.95. (n) Element with long oral edge (tip broken off during scanning microscopy), NYSM 15239, ×157; (o) NYSM 17402, ×127 (see Fig. 10d). (q, r) Eoconodontus notchpeakensis (Miller, Reference Miller1969) from Tu-5.7. (q) Symmetrical (rounded) element, NYSM 17403, ×118 (see Fig. 10e); (r) asymmetrical (laterally deflected) element, NYSM 17404, ×144 (see Fig. 10f). (s) Phakelodus tenuis (Müller, 1959) from Tu-13.0; element with rounded cross-section, NYSM 17405, ×62. (t) Proconodontus serratus Miller, Reference Miller1969; element with low serrations on oral margin, NYSM 17407, ×104, Tu-6.25 (see Fig. 10c). (u) ‘Phakelodus elongatus’ (Zhang in An et al. Reference An, Zhang, Xiang, Zhang, Xu, Zhang, Jiang, Yang, Lin, Cui and Yang1983), element with sharp posterior margin, NYSM 17406, ×86.

Figure 7. Rhabdinopora flabelliforme desmograptoides (Hahn, Reference Hahn1912) from the lower Río Salinas Member of the Tiñu Formation; hypotype specimens. (a, e) Part and counterpart, ×4.0 and ×3.8, of specimen showing branching in and shape of apical region of rhabdosome, NYSM 17408A and 17408B, SIx-22.0; (b) rhabdosome fragment in inverted view to emphasize stipes and dissepiments, NYSM 17409, ×7.1, SIx-22.0; (c) rhabdosome fragments with dissepiments that are thin (upper left) and ontogenetically thickened with thick bases (right), ×2.8, Tu-18.4; NYSM 17410; (d) rhabdosome fragment with gently convex left margin, elongate interstipe spaces, and stipe branching limited to apical region at top of figure, ×2.5, NYSM 17411, SIx-22.0.
Comparison
Orminskia gen. nov. is known from exceptionally simple, coniform, euconodont elements. The form and basal cavities of its elements closely resemble those in Teridontus nakamurai (Nogami, Reference Nogami1967) (Fig. 6i, j, l). However, Orminskia gen. nov. elements have hyaline (glassy, transparent) cusps, and can be immediately distinguished from T. nakamurai elements with their opaquely white (‘albid’: see Müller, Reference Müller and Robison1981, pp. W31–41) cusps in the same sample (Table 1). Scanning microscopy shows that the surfaces of Orminskia gen. nov. elements are smooth, while those of T. nakamurai have longitudinal microstriae on all external surfaces (Fig. 6k). Although a shallow longitudinal groove or subtle costa occurs on some T. nakamurai elements (e.g. Ji & Barnes, Reference Ji and Barnes1994, pl. 24, figs 4, 8, 9), these have not been noted on Orminskia gen. nov. elements. Thermal alteration and blackening of Orminskia new genus elements with deep burial or igneous intrusions (see Epstein, Epstein & Harris, Reference Epstein, Epstein and Harris1977) would obscure their differences with T. nakamurai elements. It is likely that Orminskia elements have been recovered in earlier studies of Upper Cambrian rocks from orogenic belts, but have not been recognized.
Discussion
Orminskia gen. nov. occurs in the Upper Cambrian, and is the oldest known euconodont with hyaline elements. The absence or weak development of highly reflective albid matter in cusps and denticles is generally regarded as an important histological feature with significance in conodont taxonomy (e.g. Müller, Reference Müller and Robison1981). Conodont genera with coniform to ramiform elements with hyaline or diffusely albid cusps are best known in the Ordovician from tropical carbonate successions (e.g. Acanthodus Furnish, Reference Furnish1938, emend. Landing, Westrop & Knox, Reference Landing, Westrop and Knox1996; Eoneoprioniodus Mound, Reference Mound1965; Multioistodus Cullison, Reference Cullison1938; Tropodus Kennedy, Reference Kennedy1980, sensu Bagnoli, Stouge & Tongiorgi, Reference Bagnoli, Stouge and Tongiorgi1988; Ulrichodina Furnish, Reference Furnish1938, emend. Landing, Westrop & van Aller Hernick, Reference Landing, Westrop and van Aller Hernick2003), but are also present in cool-water successions in the Baltic (e.g. Scandodus Lindström, Reference Lindström1955; Scolopodus Pander, Reference Pander1856). These hyaline genera are presently assigned to a number of families seemingly arbitrarily assigned to superfamilies that include albid and hyaline taxa (see Clark et al. Reference Clark, Sweet, Bergström, Klapper, Austin, Rhodes, Müller, Ziegler, Lindström, Miller, Harris and Robison1981, pp. W111–48). However, a shared histology of densely laminated apatite without internal spaces and pores may better reflect a phyletic and taxonomic relationship. If so, Orminskia new genus from the Upper Cambrian is potentially ancestral to Ordovician hyaline conodont genera.
Orminskia rexroadae Landing gen. et sp. nov
Figures 6a–h, 10a, b
Holotype
NYSM 17390 from sample Tu-2.05, Upper Cambrian (lower Cordylodus andersi Zone), lower Yudachica Member of the Tiñu Formation, Oaxaca State, Mexico (Fig. 6a).
Paratypes
NYSM 17391 (sample Tu-2.05), NYSM 17392 (Tu-4.3), NYSM 17393–17395 (Tu-4.95), and NYSM 17396, Upper Cambrian (lower Cordylodus andresi Zone), lower Yudachica Member of the Tiñu Formation, Oaxaca State, Mexico.
Diagnosis and description
As Orminskia gen. nov. is currently monospecific, the diagnosis of the type species and description of its elements correspond to that of the genus.
Etymology
For Carl B. Rexroad, who introduced EL to conodont studies during a National Science Foundation High School Summer Science Program in 1966.
Genus Paltodus Pander, Reference Pander1856, emend.
Lindström, Reference Lindström, Sweet and Bergström1971
Type species
Paltodus subaequalis Pander, Reference Pander1856, from the Lower Ordovician of Estonia.
Paltodus deltifer deltifer (Lindström, Reference Lindström1955) Figure 8a–q
1955 Drepanodus deltifer Lindström, p. 563, pl. 2, figs 42, 43.
1997 Paltodus deltifer deltifer (Lindström). Löfgren, pp. 264–5, figs 5Z–AG, 6H–N (source includes synonymy).
Material and occurrence
Hypotypes NYSM 17412–17426 from sample Tu-43.0, upper lower Tremadoc (Paltodus deltifer deltifer Subzone), middle Río Salinas Member of the Tiñu Formation, Oaxaca State, Mexico.
Discussion
The apparatuses of Paltodus deltifer deltifer and P. deltifer pristinus (Viira, Reference Viira1970) have been illustrated and discussed by Szaniawski (Reference Szaniawski1980) and Löfgren (Reference Löfgren1997) from Baltic successions. The abundant Paltodus elements from the middle Río Salinas Member have oistodiform elements with relatively elongate bases (Fig. 8a–e), suberectiforms with fairly prominent anterolateral costae (Fig. 8j, k), and acodiform-like elements with a lateral costa (Fig. 8q). These are features reported in P. deltifer deltifer (see Löfgren, Reference Löfgren1997, fig. 6N, H, M, respectively). In addition, the distinctive scandodiform elements with broad, short cusps and antero-posteriorly elongated bases of P. deltifer pristinus (the ‘varanguensiform’ elements of Szaniawski (Reference Szaniawski1980); see Löfgren (Reference Löfgren1997, fig. 5S)) were not recovered from the Tiñu.

Figure 8. Lower Ordovician (upper lower Tremadocian) conodonts from the middle Río Salinas Member of the Tiñu Formation; hypotype specimens. (a–q) Paltodus deltifer deltifer (Lindström, Reference Lindström1955) from Tu-43.0. (a–e) oistodiform elements. (a) NYSM 17412, ×83; (b) NYSM 17413, ×78; (c) NYSM 17414, ×111; (d) NYSM 17415, ×82; (e) NYSM 17416, ×108. (f–i) Scandodiform elements. (f) NYSM 17417, ×63; (g) NYSM 17418, ×118; (h) NYSM 17419, ×75; (i) NYSM 17420, ×98. (j, k) Suberectiform elements, NYSM 17421 and NYSM 17422, ×108 and ×140; (l) symmetrical drepanodiform, NYSM 17423, ×109; (m) subsymmetrical drepanodiform with inner lateral sulcus on oral and aboral margins, NYSM 17424, ×159; (n) subsymmetrical drepanodiform with laterally deflected aboral keel elongated into anticusp, NYSM 15240, ×95; (o, p) subsymmetrical drepanodiform with weakly laterally deflected anterior keel, lateral view ×88 and detail of longitudinal microstriae ×330, NYSM 17425; (q) anterolaterally costate (‘acodiform’) element, NYSM 17426, ×122. (r–t) Paroistodus numarcuatus (Lindström, Reference Lindström1955) from Tu-35.9. (r) Oistodiform (M) element, NYSM 17427, ×118; (s, t) drepanodiform element, lateral view ×125 and enlarged view ×465 of longitudinal microstriae, NYSM 17428. (u–cc) Variabiloconus variabilis (Lindström, Reference Lindström1955) from Tu-35.9 unless otherwise indicated. (u–w) Symmetrical drepanodiforms; (u) NYSM 17429, ×86; (v, w) lateral view ×112 and enlarged view ×815 of longitudinal microstriae, NYSM 17430. (x) Weakly asymmetrical drepanodiform element with gentle anterolateral flare of aboral margin, NYSM 17431, ×89; (y) asymmetrical drepanodiform element with laterally displaced sulcus on oral margin, NYSM 17432, ×95; (z–bb) acontiodiform elements from Tu-43.0 with weakly developed anterolateral costae, NYSM 17433–17435, ×103, ×74 and ×93, respectively; (cc) asymmetrical element with anterolateral costa, NYSM 17436, ×80; (dd) Scolopodus filosus Ethington & Clark, Reference Ethington and Clark1964, from Tu-37.4, NYSM 17437, ×155.
Genus Variabiloconus Landing, Barnes & Stevens, Reference Landing, Barnes and Stevens1986
Type species
Paltodus bassleri Furnish, Reference Furnish1938, from the Lower Ordovician Oneota Dolostone of Minnesota.
Variabiloconus transiapeticus
Löfgren, Repetski & Ethington, Reference Löfgren, Repetski and Ethington1999 Figure 9f, g
1999 Variabiloconus transiapeticus Löfgren, Repetski & Ethington, pp. 166, 168, 170–1, pl. 3, figs 1–12, 14, 16–20, 22; text-fig. 3A–H.
Material and occurrence
Hypotypes NYSM 17443 and 17444 from Tu-35.9, upper lower Tremadoc (Paltodus deltifer deltifer Subzone), middle Río Salinas Member of the Tiñu Formation, Oaxaca State, Mexico.
Discussion
Single specimens of the proclined long- and reclined short-based scandodiform elements were recovered from the middle Río Salinas Member. Recovery of the species from the Tiñu Formation extends its range from the unrestricted marine successions of western Laurentia and Baltica (Löfgren et al. Reference Löfgren, Repetski and Ethington1999) to the Mexican margin of Gondwana.
Variabiloconus variabilis (Lindström, Reference Lindström1955) Figure 8u–cc
1955 Oneotodus variabilis Lindström, p. 582, pl. 2, figs 14–18, pl. 5, figs 4, 5; text-fig. 6.
1974 Oneotodus variabilis Lindström. Viira, Reference Viira1974, p. 97, pl. 1, figs 14, 15; text-fig. 118.
1994 Teridontus obesus Ji & Barnes, p. 65, 66, pl. 24, figs 10–17; text-fig. 37B.
1999 Variabiloconus variabilis Lindström. Löfgren, Repetski & Ethington, p. 162, 164, 166, pl. 1, figs 1–20, pl. 2, figs 1–17; text-fig. 2A–S (source includes partial synonymy).
Material and occurrence
Hypotypes NYSM 17429–17432 and 17436 from Tu-37.4 and NYSM 17433–17435 from Tu-43.0, upper lower Tremadoc (Paltodus deltifer deltifer Subzone), middle Río Salinas Member of the Tiñu Formation, Oaxaca State, Mexico.
Discussion
A thorough description and illustration of the elements that compose the species’ apparatus is in Löfgren et al. (Reference Löfgren, Repetski and Ethington1999). The elements from the Tiñu Formation are opaquely albid above the tip of a deep basal cavity that extends into the zone of maximum curvature. They are weakly to strongly reclined (Fig. 9u, x) and covered by longitudinal microstriae (Fig. 9w). A relatively rapid rate of tapering means the elements generally have a small cusp and large base (Fig. 9v–aa); however, a few elements are elongate (Fig. 9u). The drepanodiforms are symmetrical (Fig. 9u, v) or show slight a asymmetry with lateral deflection of the aboral margin (Fig. 9bb). The symmetrical acontiodiforms are not strongly antero-posteriorly compressed, and have weakly defined anterolateral costae (Fig. 9z, aa). Asymmetrical variants of the latter are laterally compressed, and have an anterolateral costa (Fig. 9cc) or a shallow posterior sulcus that is laterally displaced in the zone of maximum element curvature (Fig. 9y). The cross-section of most elements is circular to a laterally compressed oval, but sharply compressed cusps with narrowly rounded anterior and posterior margins of the distal end of cusp were recovered.

Figure 9. Lower Ordovician (upper lower Tremadocian) conodonts from the middle Río Salinas Member of the Tiñu Formation; hypotype specimens unless otherwise indicated. (a, b) Coelocerodontus bicostatus van Wamel, Reference Van Wamel1974. (a) Symmetrical tetracostate element, NYSM 17438, ×119, Tu-35.9; (b) asymmetrical (laterally unicostate) element, NYSM 17439, Tu-43.0, ×67. (c) Furnishina furnishi Müller, 1959, posterolateral view, NYSM 17440, Tu-43.0, ×88. (d, e) Prooneotodus gallatini (Müller, 1959) from Tu-35.9, NYSM 17441 and NYSM 17442, ×71 and ×90. (f, g) Variabiloconus transiapetus Löfgren et al. Reference Löfgren, Repetski and Ethington1999, from Tu-35.9, proclined long- and reclined short-based scandodiform elements, NYSM 17443, ×91, and NYSM 17444, ×77. (h, i) Drepanodus arcuatus Pander, Reference Pander1856, from Tu-43.0. (h) Oistodiform (‘pipaform’) element, NYSM 17445, ×68; (i) elongate drepanodiform (‘arcuatiform’) element, NYSM 17446, ×75. (j–u) Cornuodus? clarkei Landing sp. nov. from Tu-43.0 unless otherwise indicated. (j–l, n, u) Symmetrical drepanodiform elements. (j, k) Holotype NYSM 17447, lateral view, × 120 (see Fig. 10s), and enlarged view of longitudinal microstriae, ×280; (l) paratype NYSM 17448, ×99; (n, u) paratypes NYSM 17449 and NYSM 17465, ×100. (m, p, q, t) Subsymmetrical drepanodiform elements. (m) Paratype NYSM 17450 with weak posterolateral sulcus extending above zone of maximum curvature, ×120; (p) paratype NYSM 17551 with weak anterolateral sulcus and costa, ×98; (q) paratype NYSM 17452 with deep posterolateral sulcus, ×125; (t) paratype NYSM 17453 with weak posterolateral sulcus and very low anterolateral costa, ×111, Tu-37.4. (o) Scandodiform paratype NYSM 17454 with weakly laterally deflected base, ×95. (r, s) Acodiform-like elements. (r) Outer-lateral view showing rounded, medial costa, paratype NYSM 17455, ×79 (see Fig. 10r); (s) inner-lateral view showing anterolateral costa, paratype NYSM 17456, ×117. (v) Acanthodus uncinatus Furnish, Reference Furnish1938, subsymmetrical element with weak lateral deflection of anterior keel at aboral margin, NYSM 17457, ×88, Tu-43.0.

Figure 10. Conodont elements from the Tiñu Formation; outline of basal cavities dashed; hypotype specimens unless otherwise indicated. (a, b) Orminskia rexroadae Landing gen. et sp. nov. (a) Holotype NYSM 17390, ×54 (see Fig. 6a); (b) paratype NYSM 17393, ×80 (see Fig. 6e). (c) Proconodontus serratus Miller, Reference Miller1969, NYSM 17407, ×52 (see Fig. 6t). (d) Proconodontus muelleri Miller, Reference Miller1969, NYSM 17403, ×64 (see Fig. 6o). (e, f) Eoconodontus notchpeakensis (Miller, Reference Miller1969); symmetrical (rounded) and asymmetrical (laterally deflected) elements, NYSM 17403 and 17404, ×60 and ×72, respectively (see Fig. 6q, r). (g, h) Cordylodus andersi Viira & Sergeeva in Kaljo et al. Reference Kaljo, Borovko, Heinsalu, Khasanovich, Mens, Popov, Sergeeva, Sobolevskaya and Viira1986; (g) rounded element, NYSM 17372, ×64 (see Fig. 5d); (h) compressed element, NYSM 17374, ×47, (see Fig. 5f). (i–l) Cordylodus proavus Müller, 1959. (i) Rounded element, NYSM 17376, ×49 (see Fig. 5h); (j) rounded element with secondary (‘Cordylodus lindstromi’) basal cavity tip, NYSM 17377, ×44 (see Fig. 5i); (k) compressed element with secondary (‘Cordylodus lindstromi’) basal cavity tip, NYSM 17379, ×35 (see Fig. 5k); (l) compressed element, NYSM 17380, ×42 (see Fig. 5l). (m–p) Cordylodus caseyi Druce & Jones, Reference Druce and Jones1971. (m) Rounded element, NYSM 17381, ×40 (see Fig. 5m); (n) subsymmetrical rounded element, NYSM 17387, ×39 (see Fig. 5s); (o) rounded (‘Cordylodus drucei’) element, NYSM 17384, ×29 (see Fig. 5p); (p) compressed (cyrtoniodiform) element, NYSM 17382, ×31 (see Fig. 5n). (q) Cordylodus prion Lindström, Reference Lindström1955, sensu Nicoll (Reference Nicoll1991), rounded element, NYSM 17389, ×59 (see Fig. 5U). (r, s) Cornuodus? clarkei Landing sp. nov. (r) Paratype acodiform-like element, NYSM 17455, ×40 (see Fig. 5r); (s) holotype, NYSM 17447, ×60 (see Fig. 5j, k).
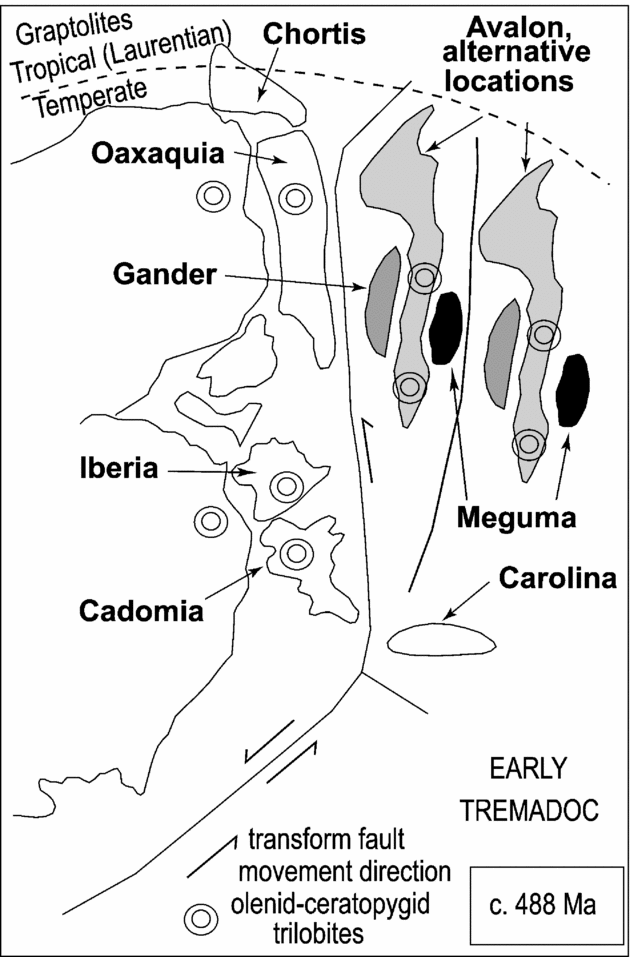
Figure 11. Generalized Cambrian–Ordovician boundary interval palaeogeography of southern hemisphere. Figure shows proximity of Oaxaquia to South American margin of West Gondwana. Iberia and Cadomia are contiguous with Moroccan margin of West Gondwana (Landing, Reference Landing, Nance and Thompson1996; Landing, Geyer & Heldmaier, Reference Landing, Geyer and Heldmaier2006). After Avalon–Gondwana separation in latest Proterozoic times, increasing similarity of faunas and lithofacies of West Gondwana and Avalonia begins in the early–middle Cambrian boundary interval (c. 510 Ma) and is heightened into Early Ordovician times (e.g. Landing, Reference Landing, Nance and Thompson1996, Reference Landing2005); this suggests that Avalon and West Gondwana came to lie at comparable palaeolatitudes, although the distance of their separation is undetermined.
The elements of Teridontus nakamurai and T. gracillimus Nowlan, Reference Nowlan1985, are more elongate, and include proclined variants (e.g. see Ji & Barnes, Reference Ji and Barnes1994). Lindström (Reference Lindström1955) emphasized a rounded cross-section in his small collection of Oneotodus variabilis elements (40 specimens), but the larger collection from the Tiñu Formation includes forms with laterally compressed cusps. The rapidly tapering, reclined elements of the species, some of which have sharp anterior and posterior cusp margins, seem indistinguishable from those of T. obesus, and T. obesus is regarded as a junior synonym. ‘Teridontus obesus’ occurs in somewhat older strata (Cordylodus angulatus Zone) in western Newfoundland (Ji & Barnes, Reference Ji and Barnes1994) than the middle Tiñu Formation. However, Löfgren (Reference Löfgren1997) and Löfgren, Repetski & Ethington (1999) recorded the species’ lowest occurrence in the C. angulatus Zone in Sweden.
Class GRAPTOLITHINA Bronn, Reference Bronn1846
Order DENDROIDEA Nicholson, Reference Nicholson1872
Family anisograptidae Bulman, Reference Bulman1950
Genus Rhabdinopora Eichwald, Reference Eichwald1855
Type species
Gorgonia flabelliformis Eichwald, Reference Eichwald1840, from the Tremadocian of Estonia.
Rhabdinopora flabelliforme desmograptoides
(Hahn, Reference Hahn1912)
Figure 7
1912 Dictyonema flabelliforme var. desmograptoides Hahn, p. 139, pl. 20.
1954 Dictyonema flabelliforme desmograptoides Hahn. Bulman, pl. 5, figs 1–4; text-fig. 6a–c.
1987 Callograptus cf. C. elegans Hall. Sour & Buitrón, pp. 387–8, figs 1, 2.
1987 Dictyonema flabelliforme var. oaxacensis Sour & Buitrón, pp. 388–9, figs 3, 4.
1987 Dictyonema flabelliforme forma typica Brögger. Sour & Buitrón, pp. 389–90, fig. 5.
1987 Dictyonema scitulum Harris & Keble. Sour & Buitrón, p. 390, fig. 6.
1987 Anisograptus delicatus Cooper & Stewart. Sour & Buitrón, p. 391, fig. 7.
1987 Bryograptus kjerulfi Lapworth. Sour & Buitrón, pp. 391–2, fig. 8.
1987 Staurograptus dichotomus Emmons. Sour & Buitrón, p. 392, fig. 9.
Material and occurrence
Hypotypes NYSM 17408A, 17408B 17409, and 17411 from SIx-22.0 and NYSM 17410 from Tu-18.4, middle lower Tremadoc, lower Río Salinas Member of the Tiñu Formation, Oaxaca State, Mexico.
Description
Rhabdosomes relatively large, broken fragments reach 45 mm wide and 40 mm high, broadly conical with very broadly convex lateral margins (Fig. 7d, left side) and apparently parabolic apical end (Fig. 7a), length/breadth ratio about 1/1 in nearly complete specimens (Fig. 7a); 9–10 stipes/10 mm, with interstipe spaces wider than stipes (Fig. 7a, d); stipe branching primarily near apical end of rhabdosome (Fig. 7a, d); dissepiment spacing variable (6.5–7.5/10 mm but reaches 11.5/10 mm), regularly to irregularly spaced (Fig. 7b, c), wide spacing creates elongate interstipe spaces (Fig. 7a, d); locally ovoid mesh produced by thickening of dissepiments at junction with stipes (Fig. 7c, right side); autotheca number c. 15/10 mm (Fig. 7e, right three stipes).
Discussion
The dendroid specimens recovered from the lower Río Salinas Member at the Tu and SIx localities (Fig. 3) are referable to one morphologically defined subspecies of Rhabdinopora. Overall stipe and dissepiment spacing, relatively large rhabdosome size, the shape and variability of shape of interstipe spaces, and the lateral broadening of dissepiments match Bulman's (Reference Bulman1954) definition of a subspecies known from Avalon (southern New Brunswick) and Baltica. All of these morphological features are evident in the six dendroid taxa that Sour & Buitrón (Reference Sour and Buitrón1987) recognized in a collection from the upper part of the exposed Río Salinas Member at locality SIx. Our dendroid collections from their locality (SIx-20, −20.5) and from a newly discovered horizon somewhat lower in the Río Salinas Member (Tu-18.4) suggest that Sour & Buitrón's (Reference Sour and Buitrón1987) taxa are best regarded as fragmentary taphonomic variants of one Rhabdinipora subspecies.
Acknowledgements
EL gratefully acknowledges a travel grant from the Rockland County Mineral Society and access to scanning microscopy facilities via W. Samsonoff at the Wadsworth Center for Laboratories and Research, New York State Department of Health (under National Science Foundation grant 0116551). R. Barber and F. Mannolini prepared the Figure 7 illustrations. A. Löfgren provided comments on the Paltodus and Paroistodus specimens. B. Murphy and O. Lehnert reviewed the manuscript for Geological Magazine.
Appendix 1. Members of the Tiñu Formation
Yudachica Member (new)
The Yudachica Member (new) comprises the lower Tiñu Formation. The Yudachica is a lithologically distinctive succession of thin- to medium-bedded limestone (rare mudstone and dominant fossiliferous wacke- to grainstones with intraclast granules and pebbles, with carbonate clast debris flows at Santiago Ixtaltepec) and light brownish green silt-shale with minor calcareous concretions. Thin calcareous quartz arenites are limited to the base of the member at its type section (Figs 1, 2). The Yudachica nonconformably overlies middle Proterozoic (c. 1 Ga) gneisses and granite at all localities; its top is an unconformity marked by a thin lensing sandstone and overlying limestone clast-dominated conglomerate bed or a phosphatized limestone clast conglomerate that forms the base of the overlying Río Salinas Member. The thickness of the Yudachica Member ranges from 12 m at Santiago Ixtaltepec (Fig. 1) to 16.4 m at Arroyo Totoyac (Pantoja-Alor, Reference Pantoja-Alor, Segura and Rodriguez-Torres1970, fig. 4). The Yudachica Member is named for the hamlet of Yudachica, approximately 0.5 km north of the type section of the Yudachica Member and Tiñu Formation at locality Tu (Figs 1, 2). ‘Yudachica’ has not been used previously for any Mexican stratigraphic unit. Fossils show that the Yudachica Member (new) is a condensed, upper Upper Cambrian succession interpreted to consist of three depositional sequences (Figs 2, 4).
Río Salinas Member (new)
The Río Salinas Member comprises the upper Tiñu Formation. This lithologically distinctive unit includes a thick, lower interval dominated by dark-grey and higher green-grey shale (55.4 m thick at the type section at locality Tu; Figs 1, 3). Isolated beds of medium- to thick-bedded, medium-grained sandstone and several trilobite-wacke- to packstone beds appear in the green-grey shale of the middle Río Salinas. The uppermost Río Salinas Member at locality Tu is composed of red silt-shale and trough cross-bedded quartz arenite with abundant orthoid brachiopods. The base of the member is an unconformity with the underlying Yudachica Member that is marked by a lensing, thin green quartz arenite (up to 10 cm thick) and an overlying conglomerate (up to 12 cm thick) composed of large, flat limestone and silt-shale clasts (locality Tu). This conglomerate is thin (7 cm) and phosphatized at locality SIx. The top of the Río Salinas is an unconformity locally overlain by Cretaceous limestone or Tertiary red beds at locality Tu and a thrust fault with lower Carboniferous sandstone at locality SIx. The thickest section (58.6 m) of the member is at the structurally undeformed type section of the Tiñu Formation. The top of the Río Salinas Member overlooks the deep Río Salinas valley to the north, and the member is named for this valley. ‘Río Salinas’ has not been used previously for any stratigraphic unit in Mexico. Fossils show that the lower–middle Río Salinas Member (new) is lower Lower Ordovician (middle–upper lower Tremadocian). Biostratigraphically useful fossils have not been recovered from the upper Río Salinas Member.















