INTRODUCTION
Ascaridia galli (Schrank, 1788), the large roundworm of chickens has a direct life cycle during which orally ingested infective eggs hatch in the duodenum and larvae (either second stage larvae (L2) or third stage larvae (L3)) are released in the intestine (Ackert, Reference Ackert1923, Reference Ackert1931; Herd and McNaught, Reference Herd and McNaught1975; Araujo and Bressan, Reference Araujo and Bressan1977). The larvae penetrate the intestinal mucosa and most of them then initiate a so-called histotropic or mucosal phase beginning at day 1 post infection (Tugwell and Ackert, Reference Tugwell and Ackert1952). On day 3 post infection they have been observed dominantly in the crypts of the jejunoileum (Luna-Olivares et al. Reference Luna-Olivares, Ferdushy, Kyvsgaard, Nejsum, Thamsborg, Roepstorff and Iburg2012). The histotropic phase can persist from 2 to 7 weeks depending on the dose (Herd and McNaught, Reference Herd and McNaught1975). After maturation to the adult stage (L5) the parasites reside in the lumen of the intestine where they excrete eggs after a prepatent period usually of 5–8 weeks (Ackert, Reference Ackert1931; Kerr, Reference Kerr1955).
In recent years, due to increased consumer demand for organic food products and improved animal welfare, traditional battery cage systems for poultry have been replaced with enriched cage or floor systems with or without outdoor access, as stipulated in the European legislation for the protection and welfare of laying hen (1999/74/EC, Anonymous, 1999). The characteristics of these alternative housing systems (close contact with faecal matter, use of bedding material) and the nature of this parasite (direct life cycle and highly resistant eggs) all favour the persistence of A. galli in the chicken populations (Permin and Hansen, Reference Permin and Hansen1998; Permin et al. Reference Permin, Bisgaard, Frandsen, Pearman, Kold and Nansen1999). High prevalence of this parasite in free range systems have been reported in many countries including Denmark (63·8%) (Permin et al. Reference Permin, Bisgaard, Frandsen, Pearman, Kold and Nansen1999), Sweden (77·1%) (Jansson et al. Reference Jansson, Vågsholm, Nyman, Christensson, Göransson, Fossum and Höglund2010) and Germany (88%) (Kaufmann et al. Reference Kaufmann, Das, Sohnrey and Gauly2011). This worldwide parasite may cause substantial economic losses due to impaired body weight gain, decreased feed conversion rates and increased mortality as well as its potential role as a vector for Salmonella enterica (Ackert and Herrick, Reference Ackert and Herrick1928; Chadfield et al. Reference Chadfield, Permin, Nansen and Bisgaard2001; Phiri et al. Reference Phiri, Phiri, Ziela, Chota, Masuku and Monrad2007).
Most of the studies on the population dynamics of A. galli have focused on the effects of host genetics, age and nutrition on worm burden (Ackert et al. Reference Ackert, Eisenbrandt, Wilmoth, Glading and Pratt1935; Permin and Ranvig, Reference Permin and Ranvig2001; Schou et al. Reference Schou, Permin, Roepstorff, Sørensen and Kjær2003; Idi et al. Reference Idi, Permin and Murrell2004, Reference Idi, Permin, Jensen and Murrell2007; Gauly et al. Reference Gauly, Homann and Erhardt2005; Das et al. Reference Das, Kaufmann, Abel and Gauly2010). However, information on the possible existence of acquired immunity and its effect on parasite population dynamics is not well documented. Recently, it has been found that birds infected with A. galli develop both cellular (Th2 type cytokine-selectively IL-4 and IL-13) and humoral (IgY antibodies, referred as IgG) immune responses against A. galli antigen (Degen et al. Reference Degen, van Daal, Rothwell, Kaiser and Schijns2005; Marcos-Atxutegi et al. Reference Marcos-Atxutegi, Gandolfi, Arangüena, Sepúlveda, Arévalo and Simón2009; Schwarz et al. Reference Schwarz, Gauly, Abel, Das, Humburg, Rohn, Breves and Rautenschlein2011; Norup et al. Reference Norup, Dalgaard, Pleidrup, Permin, Schou, Jungersen, Flink and Juul-Madsen2013). Based on these findings of specific immunological reactions against the parasite, we hypothesized that acquired immunity takes place after long-term infection. Therefore, the present study was carried out to estimate the establishment of worms following trickle-infection and to measure the effect of previous exposure to infection on a subsequent challenge infection in quantitative terms of worm establishment, growth and localization.
MATERIALS AND METHODS
Experimental animals
Seventy-two 18-week-old white Leghorn inbred chickens from the Department of Animal Science, Aarhus University (ANIS-AU), 8830 Tjele, Denmark were used in this experiment. The chickens belonged to line 2 which carry the Major Histocompatibility Complex (MHC) haplotype B12 and were randomly allocated into four groups consisting of 15 chickens (G1, G2, G4 and G5) and two groups of six chickens (G3 and G6). Each group of chickens was housed separately without outdoor access. They were offered feed formulated at the ANIS-AU and water ad libitum.
The experimental animals were treated according to Danish ethical guidelines. A licence to conduct the animal experiment was obtained from the Danish Ministry of Justice, Animal Experimentation Inspectorate by Helle R. Juul-Madsen (2011/561–98).
Collection and preparation of infection dose
Fresh chicken faeces collected from an organic farm with a high prevalence of A. galli were used for isolation of eggs by the wet sieving method according to Ferdushy et al. (Reference Ferdushy, Nejsum, Roepstorff, Thamsborg and Kyvsgaard2012). Isolated eggs were embryonated in 0·05 m H2SO4 (pH = 1) at 22 °C for 6 weeks, and then stored at 5 °C until use (within 9 weeks).
Experimental design
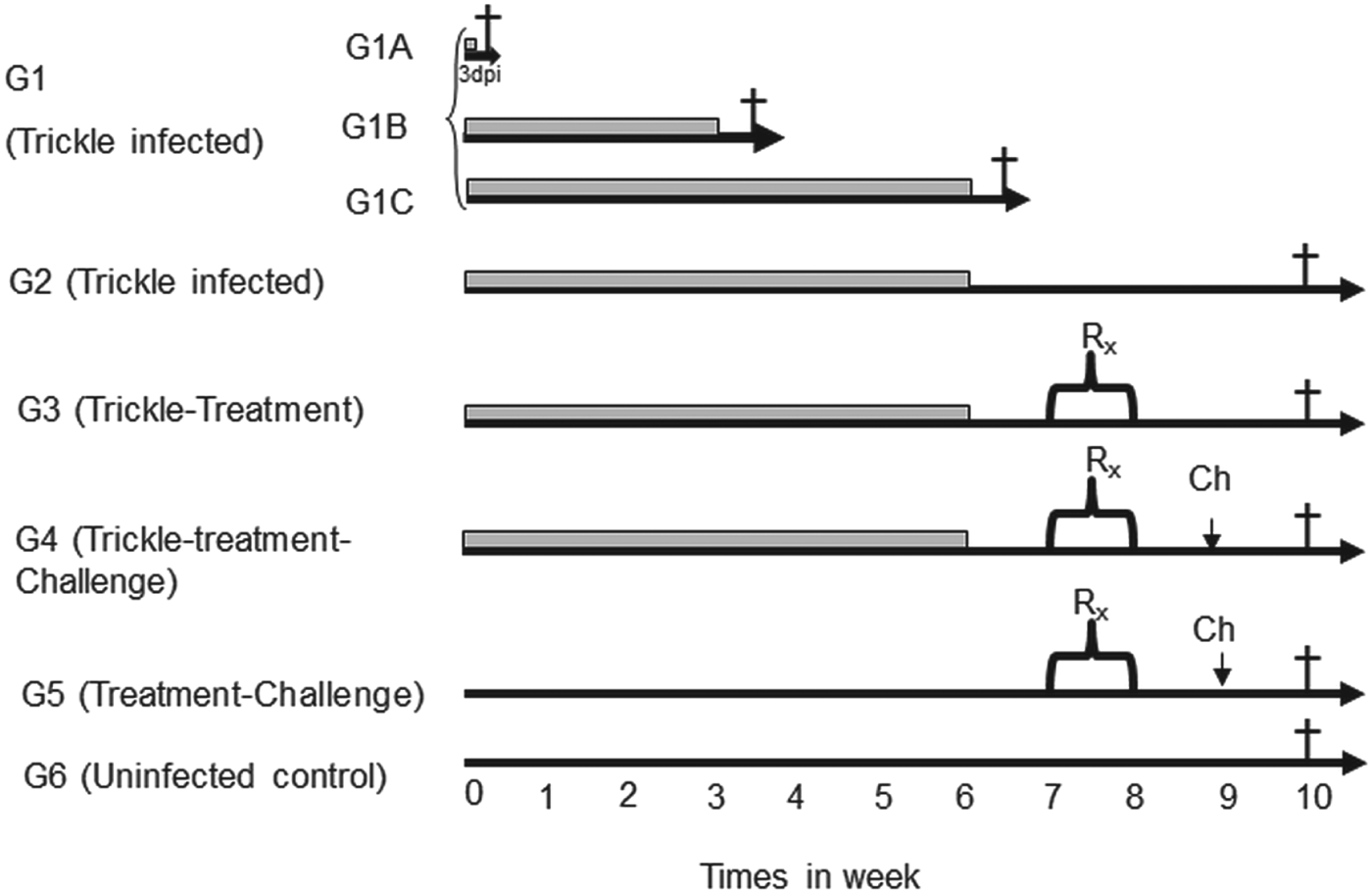
The six experimental groups were treated according to Fig. 1. Chickens in G1 were divided into three sub-groups i.e. G1A, G1B and G1C (5 birds per group). Each of the birds in G1A were orally infected with 100 embryonated A. galli eggs and necropsied at 3 days post infection (dpi). Birds of G1B and G1C were each trickle-infected with 100 A. galli eggs twice weekly for 3 weeks and 6 weeks, respectively, and necropsied 3 days after the last inoculation. All birds in G2, G3 and G4 were trickle-infected with 100 eggs twice weekly for 6 weeks. In addition, birds of G3, G4 and G5 were treated with flubendazole (Flubenol® 1·43 mg flubendazol kg−1 body weight, daily in the feed for 7 days) in week 7 pi. Furthermore, birds of G4 and G5 received a challenge inoculation with 500 embryonated A. galli eggs at week 9 while birds of G6 were left as uninfected controls. All the birds from G2–G6 were necropsied in week 10 after the first inoculation (Fig. 1). Moreover, weekly blood samples were collected from each chicken of G2, G4, G5 and G6 until the end of experiment.

Fig. 1. Experimental design of population dynamics study of Ascaridia galli infection, showing anthelmintic treatments and inoculations in six groups of chickens (G1–G6). Trickle-infection periods are indicated with grey lines (2 times 100 eggs/week). G1A, G1B, G1C and G2 (only trickle-infected), G3 (trickle-infected-treated), G4 (trickle-infected-treated-challenged), G5 (non-trickle-infected-treated-challenged), G6 (uninfected control).
The rationale behind inclusion of the different groups were: to test the infectivity of the egg batch (G1A); to describe the dynamics of establishment of worms during the 6-week period (comparing G1A, G1B, G1C and G2); to estimate the total worm burden following trickle-infection (G2); to evaluate the efficacy of treatment (G3 compared with G2); to assess the establishment of a challenge infection in previously trickle-infected-treated birds (G4) and in non-trickle-infected birds (G5).
Necropsy and larval recovery from intestinal content and wall
At each time point of intervention respective numbers of infected and control chickens were euthanized by decapitation. The gastrointestinal tract was removed from the proventriculus to the cloaca, divided into two main sections; (i) duodenum (defined by the duodenal loop and referred to as section D) (Schummer et al. Reference Schummer, Vollmerhaus, Sinowatz, Frewein, Waibl, Nickel, Schummer and Seiferle1992), and (ii) jejunoileum (from entry of the bile duct to the origin of caeca as defined by Schummer et al. Reference Schummer, Vollmerhaus, Sinowatz, Frewein, Waibl, Nickel, Schummer and Seiferle1992) divided into four equally sized subsections (J1, J2, J3, J4). Each intestinal section was opened separately in a longitudinal direction and washed by dipping the intestinal wall 10 times in 150 mL 0·9% NaCl solution (38 °C). The washing water together with the intestinal contents was embedded in agar and incubated as described by Ferdushy et al. (Reference Ferdushy, Nejsum, Roepstorff, Thamsborg and Kyvsgaard2012). In short, the samples containing the intestinal content in 150 mL 0·9% NaCl solution were mixed with 150 mL of 2% agar solution, and immediately poured onto a humid agar cloth (45104S, Johnson's Universalduk Talousliina, Johnson and Johnson AB Sweden), placed on a tray and allowed to solidify for a few minutes at room temperature (RT). These agar gels were then incubated in warm physiological saline overnight at 38 °C. The following day larvae were collected on a 15 μm sieve and stored in 70% alcohol until counting. The sections of intestinal wall were processed by artificial pepsin-HCl digestion (12 mL HCl (30%), 30 mL liquid Pepsin (660 U mL−1, Orthana Biofac A/S, Denmark) in 1 L 42 °C tap water) (Ferdushy et al. Reference Ferdushy, Nejsum, Roepstorff, Thamsborg and Kyvsgaard2012). Briefly, the small intestinal wall was cut into small pieces of 0·5 cm and digested in 200 mL of digestion fluid under constant magnetic stirring of 250 rpm at 38 °C for 90 min or until full digestion of the tissue. Then the larvae were collected on a 15 μm sieve and stored in 70% alcohol until counting.
A. galli-specific IgY ELISA
The A. galli-specific IgY ELISA was performed essentially as in Norup et al. (Reference Norup, Dalgaard, Pleidrup, Permin, Schou, Jungersen, Flink and Juul-Madsen2013). Briefly, microtitre plates (Maxisorp, Nunc, Roskilde, Denmark) were coated with 100 mL of 5 mg mL−1A. galli crude extract in a carbonate buffer (50 mm –CO3; pH 9·6), incubated overnight at 4 °C and then washed in PBS-BSA (PBS with 0·1% BSA, pH 7·4). Plates were then blocked using 200 mL blocking solution (PBS with 0·5% BSA, pH 7·4) for 30 min at RT and washed. 100 mL serum, standards and controls (all diluted in the PBS-BSA washing buffer) were added and incubated for another 2 h at RT. Plates were then washed and 100 mL horseradish peroxidase (HRP) conjugated goat anti chicken IgY (referred as IgG) (AAI29P, AbD Serotec, Oxford, UK) diluted 1:20 000 in blocking solution was added, and plates were incubated for 1 h at RT. Finally plates were washed with PBS-BSA and 100 mL of substrate solution (<0·05% w/w 3,3′,5,5″ tetramethylbenzidine) was added. After 15 min of incubation at RT and in the dark, colour development was stopped with a 1 m H2SO4 solution and determined by absorbance at 450 nm with absorbance at 650 nm as a reference.
Data analysis and statistical methods
Comparison of worm counts between groups was carried out by non-parametric statistics (Mann–Whitney test). The mean localization index for each bird was calculated by multiplying the number of larvae in sections D, J1, J2, J3 and J4 with 1, 2, 3, 4 and 5, respectively and dividing this number with the total number of parasites. The mean length index was calculated by multiplying the number of larvae belonging to different sizes <1, 1–2, 2–3, 3–4, 4–5, 5–6 and >6 mm with 0·5, 1·5, 2·5, 3·5, 4·5, 5·5 and 10, respectively and divided as above. The differences in mean worm localization and length indices between the groups were compared by one-way ANOVA. Statistical analysis and graphical presentations were made in GraphPad Prism (version 5) and Microsoft Excel 2007. The level of significance was considered as P < 0·05.
RESULTS
Clinical observations
Two birds from G2 and G4 each and one bird from G5 died due to cannibalism. No clinical signs of infection were observed in any birds.
Larval counts during establishment of infection, after anthelminthic treatment and after challenge infection
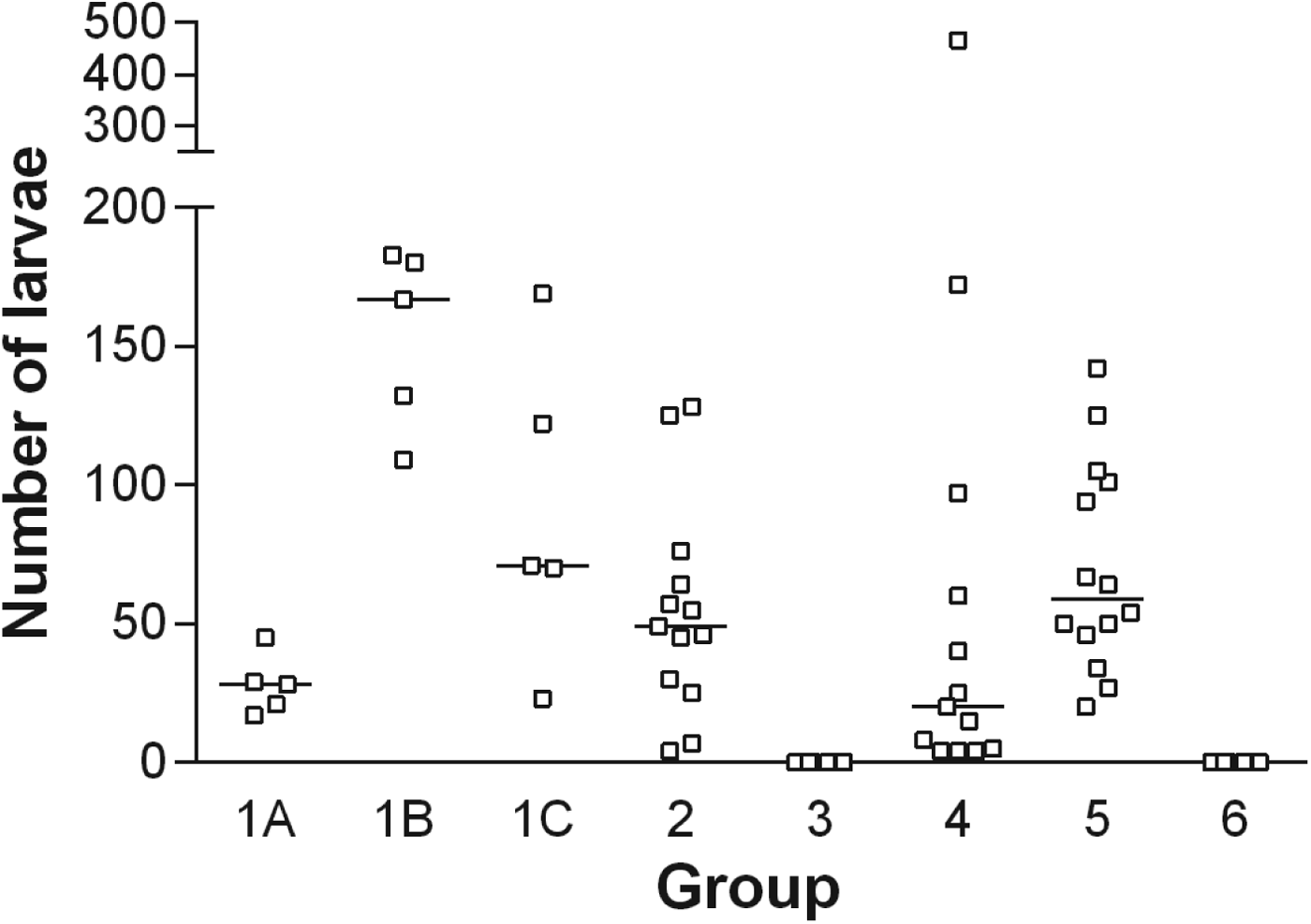
During the infection period each of the birds in the G1 family (G1A, G1B and G1C) and G2 had received a total of 100, 600, 1200, 1200 A. galli eggs, respectively. The median larvae numbers (min–max) recovered were 28 (range 17–45), 167 (109–183), 71 (23–167), 49 (4–128) for groups G1A, G1B, G1C and G2, respectively (Fig. 2). It is evident that the number of established larvae increased initially (between G1A and G1B, P < 0·01) and thereafter decreased with time (from G1B over G1C although not significantly different) despite a continuous re-infection regime. Four weeks after the termination of the trickle-infection, a significant reduction in larval burden had taken place as compared with peak level (G2 vs. G1B, P < 0·05) whereas no major reduction in larval burden had taken place between G1C (slaughtered 3 days after last inoculation) and G2 (slaughtered 4 weeks after the last inoculation) (P > 0·05).

Fig. 2. Individual (□) and median (–) number of larvae recovered from different groups of chickens infected with the respective number of embryonated A. galli eggs (for trickle-infection 100 eggs per chicken twice weekly and for challenge 500 eggs per chicken were used). G1A, G1B, G1C and G2 (only trickle-infected), G3 (trickle-infected-treated), G4 (trickle-infected-treated-challenged), G5 (non-trickle-infected-treated-challenged), G6 (uninfected control).
In G4 (trickle-infected-treated-challenged) and G5 (non-trickle-infected-treated-challenged) the median numbers of larvae recovered were 20 (4–466) and 59 (20–142), respectively (P < 0·05). It was furthermore noticed that the variation in the number of larvae was much higher in G4 than in G5. No A. galli were recovered from the uninfected controls (G6) or flubendazole-treated birds (G3) (Fig. 2).
Distribution of larvae in the intestine
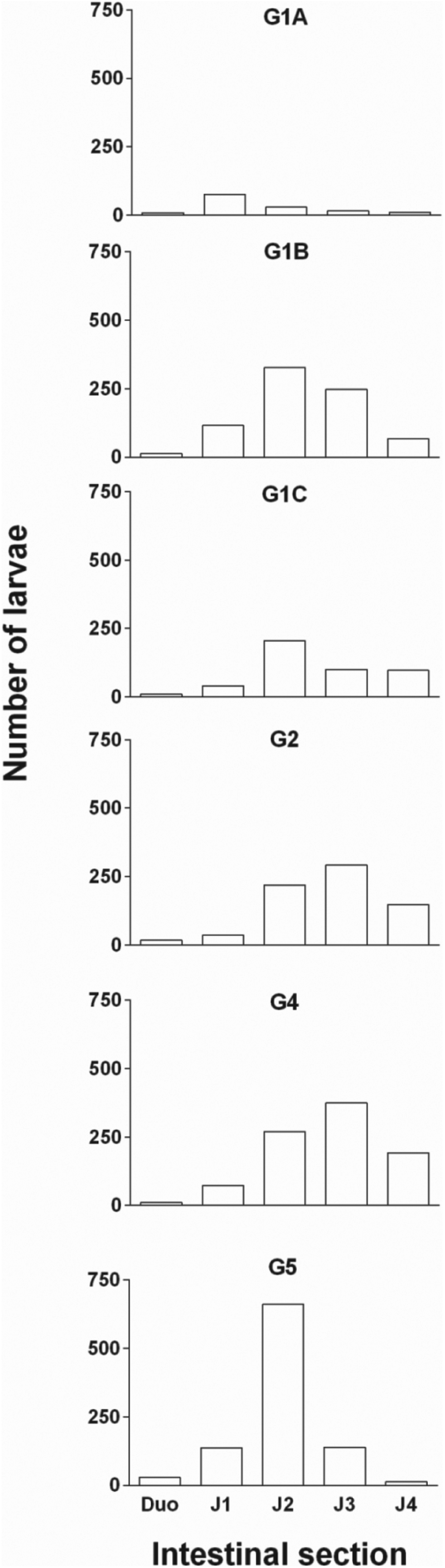
The localization of the larvae in the different sections of the intestine differed significantly between the groups (mean localization index, P < 0·0001). During the initial establishment period (3 dpi for G1A) most of the larvae were located in section J1. However, at 3 and 6 weeks pi (G1B and G1C) they were most abundant in sections J2 and J3 (Fig. 3). In the trickle-infected group (G4), the larvae from the challenge infection were located more posteriorly (found with the highest density in J3) compared with the non-trickle-infected group (G5) where they were mainly found in section J2.

Fig. 3. Total number of larvae recovered from the intestinal sections of different groups of chickens infected with the respective number of embryonated A. galli eggs (for trickle-infection 100 eggs per chicken twice weekly and for challenge 500 eggs per chicken were used). G1A, G1B, G1C and G2 (only trickle-infected), G4 (trickle-infected-treated-challenged), G5 (non-trickle-infected-treated-challenged). No larvae were recovered from G3 and G6 and thus not included in the figure.
The proportion of larvae in the intestinal wall was higher than in the intestinal content. More than 70% of the larvae were found in the intestinal wall except for G1C where the distribution between wall and content was almost equal.
Distribution of larvae according to size
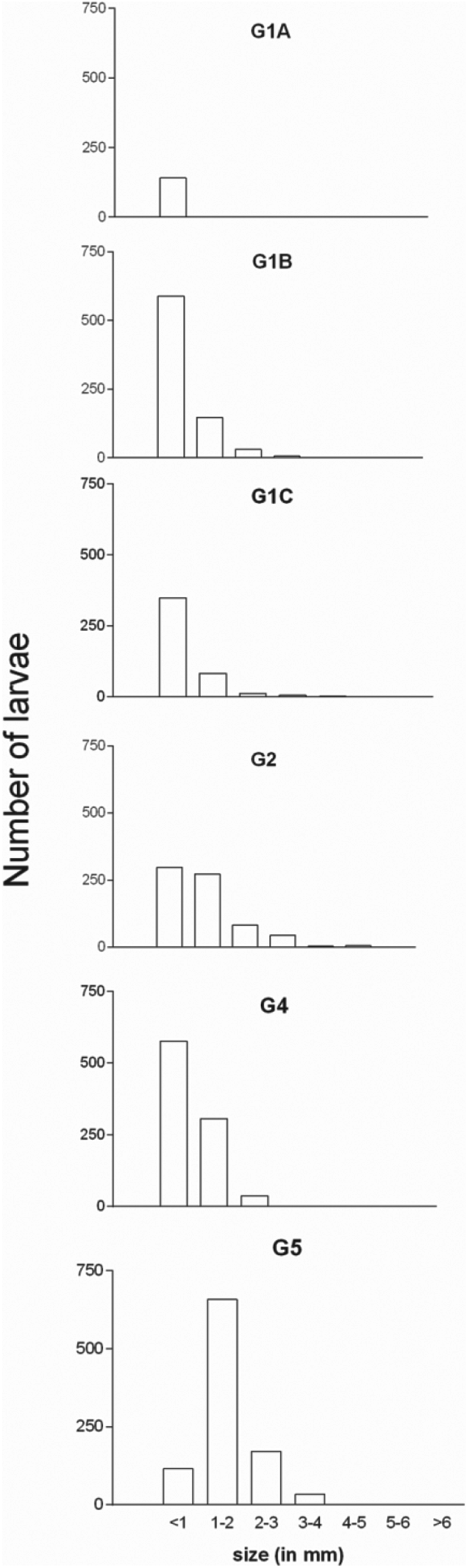
The size of the larvae also varied greatly according to the group (mean length index, P < 0·0001). All the larvae recovered at 3 dpi from G1A were <1 mm. A subpopulation of larvae was growing in size as the time progressed which can be seen from the time-line formed by G1B over G1C to G2. A significantly (P < 0·05) larger proportion of the larvae in the trickle-infected-challenged (G4) remained below the length of 1 mm compared with the non-trickle-infected-challenged (G5) although they received the same challenge inoculation (Fig. 4).

Fig. 4. Total number of larvae recovered according to size from the intestine of different groups of chickens infected with respective number of embryonated A. galli eggs (for trickle-infection 100 eggs per chicken twice weekly and for challenge 500 eggs per chicken were used). G1A, G1B, G1C and G2 (only trickle-infected), G4 (trickle-infected-treated-challenged), G5 (non-trickle infected-treated-challenged). No larvae were recovered from G3 and G6 thus these are not included in the figure.
Antigen-specific IgY in serum
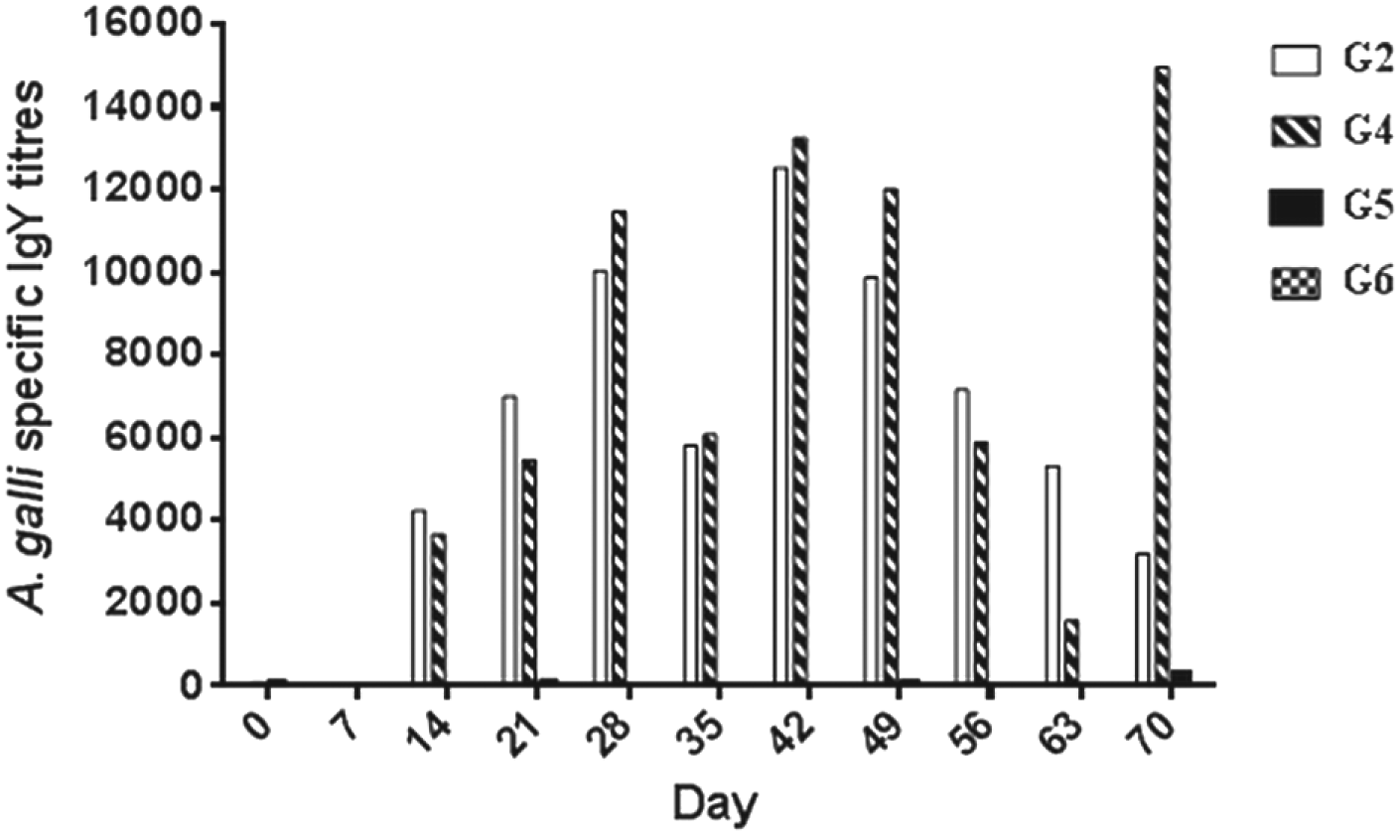
Individual titre from G2 and G4 chickens showed that the antibody titres reached a noticeable level at 14 dpi in both groups. The levels of antibody in G2 and G4 waned by 56–63 dpi but in G4 the challenge infection boosted antibody markedly at 70 dpi. In both groups large variation was observed among the birds. In the birds from G5 and G6 no serum antibodies were detected by the assay (Fig. 5).

Fig. 5. Mean IgY titres in different groups of chickens infected with A. galli eggs. G2 (only trickle- infected), G4 (trickle- infected-treated-challenged), G5 (non-trickle-infected-treated-challenged), G6 (uninfected control). For trickle-infection each chicken was inoculated with 100 eggs twice weekly for 6 weeks and for challenge infection (at day 63) 500 eggs per chicken were used.
DISCUSSION
This study provides clear evidence that resistance to A. galli acquired during trickle-infection followed by anthelminthic treatment will lead to a significantly lower establishment rate of a subsequent challenge infection as seen when comparing the trickle-infected group (G4) with the parasite-naïve group (G5). Also, the established larvae of the challenge infection in the trickle-infected-treated group (G4) were shorter and located more posteriorly than those of the previously unexposed group (G5). The resistance against re-infection did not confer absolute protection as some larvae from the challenge infection established in all trickle-infected-treated birds with a high between-bird variation in larval number. It is interesting that a few of the trickle-infected-treated birds did not seem to have any protection against re-infection. This could be an accidental finding but supports the evidence that protection against incoming A. galli infections is incomplete or may even be absent in some chickens despite heavy previous exposure. This result supports the findings of Norup et al. (Reference Norup, Dalgaard, Pleidrup, Permin, Schou, Jungersen, Flink and Juul-Madsen2013) who observed that the high antibody titres were not associated with protection against re-infection or continued infection.
In the initial phase of the trickle-infection the establishment rate was constant and an accumulation of larvae was observed when comparing the group receiving a single dose (G1A) with the group infected 6 times over a 3-week period (G1B). However, after this initial period, the total number of established larvae declined as seen when observing the time-line represented by G1B over G1C to G2. This may be an indication of the onset of acquired resistance leading to both a lower establishment rate of the incoming infections and an expulsion of an already established infection. Similarly, Permin and Ranvig (Reference Permin and Ranvig2001) suggested the expulsion of primary infection and demonstrated a lower establishment rate of larvae after challenge infection without any intervening anthelminthic treatment.
We found that the proportion of smaller-sized larvae (less than 1 mm) after challenge were higher in the trickle-infected group compared with the non-trickle-infected group which is in accordance with the findings of Permin and Ranvig (Reference Permin and Ranvig2001).
Although the larvae recovered from the serial killing of the trickle-infected birds were increasing in size from 3 weeks pi (G1B) and onwards, a population of smaller-sized larvae always remained and we did not find any worms even close to the size of the adult worms (51–76 and 72–116 mm for male and female, respectively (Ackert, Reference Ackert1931) at the end of experiment i.e. 10 weeks after the first infection dose. This is in contrast with the findings of Permin et al. (Reference Permin, Bojsen, Nansen, Bisgaard, Frandsen and Pearman1997) who recovered adult worms at 8 weeks pi with establishment rates between 0·5 and 14·2% depending on the infection dose. Arrest or inhibition in larval development could happen because of the prior exposure of the infective stages of the parasite to a hostile environment (e.g. shorter photoperiod, decreasing temperature or humidity), crowding effect/density dependency or because of the acquisition of resistance (Michel, Reference Michel1974; Eysker, Reference Eysker1997). Environmentally induced inhibition is a very common phenomenon for trichostrongylid nematodes of ruminants; in our study it was not possible to determine whether the storage of embryonated eggs under refrigeration could be responsible for lack of development to patency. However, it has been a standard procedure in our laboratory also yielding patent infections previously (e.g. Permin et al. Reference Permin, Bojsen, Nansen, Bisgaard, Frandsen and Pearman1997). Crowding or density dependency could also be the reason as this was observed by Ikeme (Reference Ikeme1970) who trickle-infected the chickens with either 1000 or 10 A. galli embryonated eggs daily for 6 weeks and recovered small-sized larvae until the end of the experiment (19 weeks) in the high-dose group and up to 7 weeks in the low-dose group. In our study the trickle-infection in G4 was removed before challenge and thus the presence of smaller-sized larvae is most likely caused by an immune reaction. The role of host resistance on A. galli growth and development has also been well documented by Herd and McNaught (Reference Herd and McNaught1975). They found that in the birds which were treated with immunosuppressive drugs the proportion of larger larvae were considerably higher than the non-treated group.
After challenge the larvae were located more caudally in the trickle-infected-treated group (G4) than the non-trickle-infected group (G5). Moreover, we have also seen that the larvae of the trickle-infected group move posteriorly with time. Our findings of G1 (G1A, G1B, G1C) correspond well with our single A. galli infection experiment where we recovered more larvae in section J3 from 10 dpi up to 28 dpi, and at 42 dpi larvae were mainly located in section J2. This posterior localization of larvae might be related with the expulsion of comparatively larger worms (Ferdushy et al. Reference Ferdushy, Luna-Olivares, Nejsum, Roepstorff, Thamsborg and Kyvsgaard2013). Roepstorff et al. (Reference Roepstorff, Eriksen, Slotved and Nansen1997) also found that in Ascaris suum infection in pig the larvae are located more posteriorly during the initial expulsion phase.
In this experiment the inbred line was chosen to minimize variation due to differences in MHC type/genotype. We have no reason to expect major qualitative differences to commercial hybrids, although some quantitative differences between breeds are common. Regarding the age of the birds, Gauly et al. (Reference Gauly, Homann and Erhardt2005) infected birds at 6, 12, 18 and 24 weeks of age with 250 embryonated A. galli eggs, and at 10 weeks pi highest worm burden and faecal egg counts was observed in the group infected at the age of 18 weeks; they assume that the hormonal changes around the time of laying make the birds more prone to infection. Thus we selected the birds with this age limit (18 weeks at the time of infection).
We chose 100 embryonated A. galli eggs as the inoculation dose for trickle-infection on the basis of the findings of Permin et al. (Reference Permin, Bojsen, Nansen, Bisgaard, Frandsen and Pearman1997) who infected three groups of chickens either with 100, 500 or 2500 embryonated A. galli eggs and documented the reverse dose dependency on establishment of A. galli infection (14·2, 2·9 and 0·5% for 100, 500 and 2500 infection dose, respectively).
With regard to the efficacy of flubendazole little information is available in the public domain about the efficacy against larval stages. The present study showed a very high efficacy (100%) against larval stages of 0–7 weeks of age when the 7-day treatment schedule found in the Summary of Product Characteristics (SPC) was followed. This is in contrast with the findings of Höglund and Jansson (Reference Höglund and Jansson2011) who reported a re-infection within 2–4 weeks post treatment with flubendazole (SID Po verminator®, Boehringer Ingelheim Vetmedica, Malmö, Sweden) in drinking water at a concentration of 1·43 mg kg−1 for 5–7 days. The authors claim the possibility of maturation of larvae in a histotropic phase that were not affected by treatment. It should however be noted that the deworming in the field trial was performed exclusively by the farmers and ours was performed on a research facility where the treatment protocol is followed strictly.
Serum antibody levels in trickle-infected chickens (G2 and G4) reached a noticeable level 14 days after the first infection. Schwarz et al. (Reference Schwarz, Gauly, Abel, Das, Humburg, Rohn, Breves and Rautenschlein2011) also observed the presence of IgY antibody in chicken serum 2 weeks after the infection with A. galli eggs. A drop in antibody titre was observed in those groups at 35 dpi. The reason for this drop in antibody level is not well understood but it could be associated with immune-directed migration of the larvae from their initial predilection site to the posterior part of the jejunoileum, thereby facilitating the expulsion of some of the already established larvae. In a single infection experiment performed by our group we have seen that the larvae move more posteriorly and migrate towards the intestinal content in the course of the infection (during 2–4 weeks pi) (Ferdushy et al. Reference Ferdushy, Luna-Olivares, Nejsum, Roepstorff, Thamsborg and Kyvsgaard2013) and we can speculate a similar migration strategy for G2 and G4 chickens during the trickle-infection period. Secondly, the birds entered into the laying phase during the experimental period and, as we mentioned before, the hormonal changes during the period of lay make the bird immunocompromised which may lower the antibody production (Gauly et al. Reference Gauly, Homann and Erhardt2005). The drop in specific antibody levels from day 49 may be a reflection of the end of the trickle-infection (birds were trickle-infected for 6 weeks) and previous elimination of the larvae. The elevated level of the specific antibody in G4 chickens after challenge infection is definitely evidence of immunological memory and also a reflection of the relative protection against re-infection.
In conclusion, the present study demonstrated that repeated low-level exposure as mimicked by trickle-infection with A. galli in chickens leads to a lower rate of establishment and impaired larval growth rather than a complete protection against re-infection. Whether lack of patent infections is a feature of acquired resistance cannot be decided as none of the infection regimes led to egg-laying infections within study period. Thus, it can be speculated that the effect is rather in the form of a modulation of the establishment and development of the larvae rather than elimination. This is also in line with the non-invasive nature of the infection observed by Luna-Olivares et al. (Reference Luna-Olivares, Ferdushy, Kyvsgaard, Nejsum, Thamsborg, Roepstorff and Iburg2012) and the moderate degree of cellular response observed by Luna-Olivares et al. (Reference Luna-Olivares, Kyvsgaard, Ferdushy, Nejsum, Thamsborg, Roepstorff and Iburg2014). Thus, acquired resistance may not be able to eliminate the parasite but may make the environment more hostile at the predilection site between the villi of the interior half of the jejunoileum and force the larvae to a more superficial as well as a more posterior localization.
ACKNOWLEDGEMENTS
We deeply acknowledge the contribution of Dr Allan Roepstorff, and are grateful to Luz Adilia Luna-Olivares, Lise-Lotte Christiansen and Lene R. Dal for their skilful assistance.