Introduction
We document the occurrences and properties of two minerals of nickel, i.e. ognitite and Co-rich maucherite, discovered in the Ognit ultramafic complex, in Eastern Sayans, Russia. Ognitite has been approved as a valid species (IMA2018-006a, Barkov et al. Reference Barkov, Bindi, Winkler, Morgenroth, Shvedov, Martin, Zaccarini, Stan, Tamura and Stanley2019) by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA). The name ognitite (Cyrillic: огнитит) is after the Ognit complex, in which the new mineral occurs. The catalogue number of the ognitite single crystal is 3292/I at the Museo di Storia Naturale of the University of Firenze, Italy.
Ognitite (Ni1.1Bi0.9Te) has a distinctly Bi-rich composition; in addition, it differs structurally from melonite. With an ideal composition NiTe2, melonite generally contains no more than 0.25 atoms of Bi per formula unit (apfu), and exceptionally up to 0.4 apfu in complex solid solutions that also involve Pt and Pd (Barkov et al., Reference Barkov, Shvedov, Flemming, Vymazalová and Martin2017a, and references therein). Such an enrichment reflects the incorporation of substantial levels of the merenskyite (PdTe2) and moncheite (PtTe2) components (e.g. Garuti and Rinaldi, Reference Garuti and Rinaldi1986). However, ognitite shows a striking Bi enrichment without those two components.
The Co-rich maucherite represents a novel variety, unusual and hitherto unreported. Maucherite, ideally Ni11As8, is one of the principal carriers of Pd in various Ni–Cu–PGE (platinum-group element) deposits. We report a major enrichment of cobalt in the core of a zoned grain. At more than 9 wt.% Co, or roughly 2 apfu Co, this composition far surpasses what is normally encountered: ≤1.2 wt.% Co (e.g. Fleet, Reference Fleet1973a; Wagner and Lorenz, Reference Wagner and Lorenz2002; Makovicky and Merlino, Reference Makovicky and Merlino2009) and, occasionally, up to 3.1–3.9 wt.% Co (Petruk et al., Reference Petruk, Harris and Stewart1971; Gritsenko and Spiridonov, Reference Gritsenko and Spiridonov2008; Raič et al., Reference Raič, Mogessie, Benkó, Molnár, Hauck and Severson2015).
We seek to establish the circumstances that could have led to the mineralogical anomalies observed. In addition, we describe a characteristic zonation, hitherto unreported, in Ni–(Co) arsenides at Ognit. Our findings extend the knowledge of the pattern of behaviour and extent of solid solution, and provide a useful insight into the ore-forming environments leading to Cu–Ni–PGE mineralisation in ultramafic complexes.
Background information
As noted, melonite and its synthetic analogue normally contain <0.4 apfu Bi (Barkov et al., Reference Barkov, Shvedov, Flemming, Vymazalová and Martin2017a, and references therein). Maucherite generally contains between 18 and 265 ppm Pd (Gervilla et al., Reference Gervilla, Cabri, Kojonen, Sie, Papunen and Hach-Alí2000). In experiments, the extent of Pd-enrichment increases with decreasing temperature, to attain 5.5 at.% Pd at 450°C (Gervilla et al., Reference Gervilla, Makovicky, Makovicky and Rose-Hansen1994). Elevated contents, up to 1.5–1.8 wt.% Pd, are known in grains of maucherite from the Sudbury, Canada and Noril'sk, Russia complexes (Cabri and Laflamme, Reference Cabri and Laflamme1976; Gritsenko and Spiridonov, Reference Gritsenko and Spiridonov2008). In addition, maucherite is one of the principal arsenides deposited from melts rich in As under experimental and natural conditions (Prichard et al., Reference Prichard, Fisher, McDonald, Knight, Sharp and Williams2013). Arsenic-rich melts segregated experimentally via immiscibility from sulfide melts are capable of efficiently scavenging the initially dissolved PGE, especially Pd (Fleet et al., Reference Fleet, Chryssoulis, Stone and Weisener1993; Makovicky et al., Reference Makovicky, Makovicky and Rose-Hansen1992). Elevated contents of Cu (and S), up to ~5 wt.% each, were documented in grains of maucherite from metamorphosed Cu–Ni ores of the Noril'sk complex, Russia (Gritsenko and Spiridonov, Reference Gritsenko and Spiridonov2008).
Geological setting and ore-mineral associations
Both ognitite and Co-rich maucherite occur in dunite in zones of disseminated Cu–Ni–PGE sulfide mineralisation in the Ognit (or Medek) dunite–wehrlite complex of Neoproterozoic age, located at the southern margin of the Siberian Craton, Irkutskaya (Irkutsk) oblast, Russia (Figs 1a, b). The Ognit complex belongs to a suite of dunite–peridotite–pyroxenite complexes, namely Shumikha, Kingash, Golumbei, Tartai, Ognit, Zhelos, Tokty-Oi and Malyi Zadoi, associated with the Yoko–Dovyren complex in the Baikal–Patom zone. These complexes formed in a continental margin setting in the East Siberian metallogenic province (e.g. Mekhonoshin et al., Reference Mekhonoshin, Tolstykh, Podlipsky, Kolotilina, Vishnevsky and Benedyuk2013; Tolstykh et al., Reference Tolstykh, Polyakova, Izokh, Podlipsky, Mekhonoshin, Orsoev and Kolotilina2014). They give a radiometric age in the range 731–710 Ma, which is close to the range reported for the Franklin Province in northwestern Canada, 725–710 Ma, and is thus consistent with the inferred breakup of Rodinia in Neoproterozoic time (Gladkochub et al., Reference Gladkochub, Wingate, Pisarevsky, Donskaya, Mazukabzov, Ponomarchuk and Stanevich2006; Ernst et al., Reference Ernst, Hamilton and Soderlung2012; Tolstykh et al., Reference Tolstykh, Polyakova, Izokh, Podlipsky, Mekhonoshin, Orsoev and Kolotilina2014).

Fig. 1. (a) The location of the Ognit complex in the Russian Federation and (b) geological map of the Ognit complex, Eastern Sayans, Russia, based on various sources (Mekhonoshin et al., Reference Mekhonoshin, Tolstykh, Podlipsky, Kolotilina, Vishnevsky and Benedyuk2013, Reference Mekhonoshin, Kolotilina and Doroshkov2018, and references therein; Oleshkevich et al., pers. comm.). The sample location is shown for ognitite (labelled Ogn) and Co-rich maucherite (Mch).
The Ognit complex is a relatively small ultramafic body ≤1.5 km long at the contact of granodiorite and gneiss. It is mostly composed of dunite, wehrlite, and olivine pyroxenite. The core-like zone is essentially dunitic; elements of large-scale layering are recognised in the complex (Fig. 1b). A notable feature is the development of a pronounced zone of metasomatic alteration, especially at the southeastern contact.
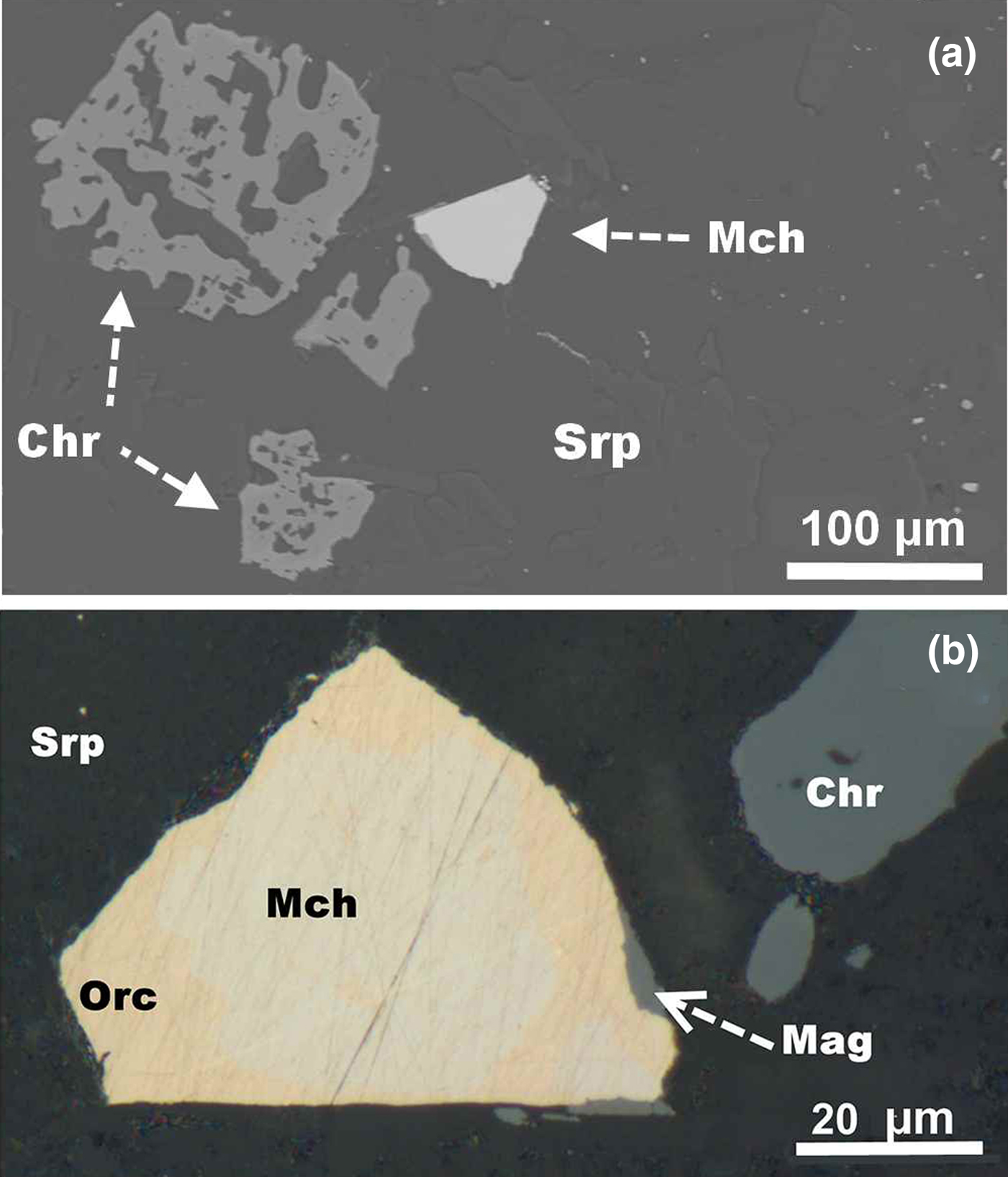
The ultramafic rocks of the Ognit complex are variably serpentinised; they are composed of a serpentine-group mineral, clinochlore and calcic amphiboles (actinolite and tschermakite). Chromite, including skeletal grains of low-Al compositions (Figs 2a, 3, Table 1), and magnetite are common accessories. The ore zones consist of sulfides (≤3 modal%), mainly pentlandite and chalcopyrite, subordinate troilite and cubanite, rare heazlewoodite, pyrite, bornite, mackinawite, secondary chalcocite, covellite, violarite, valleriite and, occasionally, sphalerite and molybdenite. Less common/rare species of ore minerals include orcelite, maucherite, cobaltite–gersdorffite, altaite, hessite, Se-bearing galena, shandite, parkerite, native silver and Ag–Au alloy (Ag-dominant), auricupride, awaruite, native copper, graphite and native bismuth (Shvedov and Barkov, Reference Shvedov and Barkov2017).

Fig. 2. A back-scattered electron (BSE) image (a) and reflected light microphotograph (b) showing the zoned grain of maucherite (Mch)–orcelite (Orc), associated with skeletal grains of chromite (Chr), hosted by fine intergrowths of serpentine (Srp) and clinochlore; Mag is secondary magnetite.

Fig. 3. Compositions of skeletal grains of chromite and associated magnetite zones (shown by blue and brown symbols, respectively) from the Ognit complex in terms of the Cr–Fe3+–Al plot (atomic %). The positions of Fe–Ti and Cr–Al trends and the miscibility gap are from Barnes and Roeder (Reference Barnes and Roeder2001).
Table 1. Compositions of skeletal grains of chromite from the Ognit complex, Eastern Sayans.

Notes: 10(R) refers to a rim zone of skeletal grain of chromite; *FeO(tot): all Fe is expressed as FeO. FeO and Fe2O3 are calculated on the basis of stoichiometry and charge balance.
Mg# is 100Mg/(Mg + Fe2++Mn); Cr# is 100Cr/(Cr + Al), and Fe3+# is 100Fe3+/(Fe3+ +Cr + Al).
‘–’ = below the detection limit
Two associations of platinum-group minerals (PGM) exist in the Ni–Cu–PGE zones at Ognit. The first includes species rich in Ir–Ru–Os: Ru–Os disulfides (laurite–erlichmanite series), Ru–Os diarsenides (anduoite–omeiite) and sulfarsenides (platarsite–osarsite–ruarsite). These PGM, crystallising relatively early, exhibit a close affinity to primary grains of base-metal sulfides. The second association is late, more diverse, and mainly consists of Pd-rich PGM: palladium bismuthotellurides and antimonides, such as phases of the type Pd(Bi,Te,Sb)1+x, merenskyite, michenerite, mertieite-II and stibiopalladinite, naldrettite, polarite, urvantsevite, froodite, palladogermanide (Pd2Ge: cf. IMA2016-086) and unnamed Pd2+xCu1–x(Sb,Sn) (Shvedov and Barkov, Reference Shvedov and Barkov2017).
Analytical methods
Compositions of ognitite were established via wavelength-dispersive spectrometry (WDS) using a JEOL JXA-8900L electron microprobe, at 20 kV, 50 nA, and with a 5-μm beam size at McGill University, Montreal, Canada. The following lines and standards were used: Kα and CoNiAs (for Ni), Kα and metallic iron (Fe), Kα and CuFeS2 (Cu), Kα and CoNiAs (Co), Lα and Pd3HgTe3 or metallic Pd (Pd), Mβ and metallic Pt (Pt), Lα and PbTe (Te), Mα and Bi2Te3 (Bi), Mα and PbTe (Pb), Kα and Bi2S3 (S), Lβ and CoNiAs (As), and Lβ and pure Sb (Sb). The PRZ correction was applied. The counting times were 150 s for As, 60 s for Pd, Pt, Co, Sb and Pb, and 20 s for Ni, Fe, Cu, Te, Bi and S. Minimum detection limits (wt.%) are: 0.01 (S and Co), 0.02 (Fe), 0.03 (Ni), 0.04 (Cu, Pd, Pt, Pb and As), 0.05 (Sb), 0.06 (Te) and 0.08 (Bi).
The Co-rich maucherite and associated minerals (chromite, clinochlore and serpentine) were analysed with a JEOL JXA-8200 microprobe at the E.F. Stumpfl laboratory, University of Leoben, Austria. The analytical conditions (WDS with a ≤2 µm beam) used for the zoned maucherite grain were: 20 kV, 10 nA, and 20 s counting times for the peak and 10 s for the backgrounds. The following lines and standards were used: NiKα (pentlandite), FeKα, SKα (pyrite), AsLα, CoKα (skutterudite), BiMα, TeLα (synthetic Bi2Te3), and SbLα and SbLβ (Sb2Te3). The detection limits expressed in ppm are: 100 for As and S, 150 for Ni, Fe and 600 for Co, Sb, Bi and Te. Levels of Sb, Bi and Te were below the detection limit.
Scanning-electron microscopy (SEM) and energy-dispersive analysis (EDS) were also employed, using a MIRA 3 LMU (Tescan Ltd.) system combined with INCA Energy 450+ XMax 80 (Oxford Instruments Ltd), at the Institute of Geology and Mineralogy, Novosibirsk, Russia. The reflectance measurements on ognitite were carried out using a WTiC standard at the Natural History Museum, London, UK.
Single-crystal X-ray diffraction data were collected on the ognitite specimen using an Oxford Diffraction Xcalibur diffractometer equipped with an Oxford Diffraction CCD detector, with graphite-monochromatised MoKα radiation (λ = 0.71073 Å).
The powder X-ray diffraction data were obtained on the same fragment of the ognitite grain used for the single-crystal study with an Oxford Diffraction Xcalibur PX Ultra diffractometer fitted with a 165 mm diagonal Onyx CCD detector and using copper radiation [CuKα (λ = 1.54184 Å)]. The working conditions were 40 kV and 40 nA with 3 hr of exposure; the detector-to-sample distance was 7 cm. The program CrysAlis RED (Oxford Diffraction, 2006) was used to convert the observed diffraction rings to a conventional powder-diffraction pattern. The least-squares refinement gave the unit-cell values listed below.
We also provide results of synchrotron X-ray Laue microdiffraction measurements at beam line 12.3.2 of the Advanced Light Source (ALS), Berkeley, California, USA. A polychromatic X-ray beam (5–24 keV) was focused to approximately 2 µm × 2 µm (full width at half maximum) with Kirkpatrick–Baez mirrors. Laue diffraction patterns were collected with a PILATUS 1M area detector in reflection geometry, with the sample inclined 45° to the primary beam, and the area detector was set to 90°. The Laue diffraction patterns were collected with raster scanning across the entire sample area. The patterns were indexed and analysed using XMAS v.6 (Tamura, Reference Tamura, Barabash and Ice2014). An energy scan (10–20 keV, 10 eV step size) was performed on single reflections to determine the lattice parameters.
Occurrence, physical and optical properties, and composition of ognitite
Ognitite forms a single homogeneous grain (80 µm × 30–40 µm) hosted by chalcopyrite; it is anhedral, irregularly shaped, and occurs next to a small grain of native bismuth (Fig. 4). The new species is metallic and opaque; its colour and streak are metallic black (powder). The value of its density (calculated) is 8.75 g/cm3.

Fig. 4. Reflected light microphotograph (a) showing the ognitite grain (Ogn), which is hosted by chalcopyrite (Ccp), and is associated with native bismuth, hessite (Hs), altaite (Alt), and also with late veinlets of magnetite (Mag). (b) BSE image showing ognitite (Ogn) and associated grains, complementary to those shown in the optical photograph in (a). Type specimen (#3292/I).
Under reflected light, ognitite is creamy white. It is slightly to distinctly bireflectant, with a pleochroism from creamy white to slightly lighter creamy white. Ognitite is slightly to distinctly anisotropic. Internal reflections were not observed. Reflectance values of ognitite (WTiC standard, in air) are listed in Table 2.
Table 2. Reflectance values for ognitite.

Notes: These values were measured in air; the standard used is WTiC.
The values required by the Commission on Ore Mineralogy are given in bold.
Representative compositions of ognitite are given in Table 3. Other elements (Co, Pt, Sb, Pb, As and S) were also sought, but not detected. The mean composition (and ranges for seven point analyses, n = 7) are: Ni 17.05 (16.91–17.15), Fe 0.07 (0.02–0.16), Cu 0.14 (0.08–0.26), Pd 0.14 (0.09–0.16), Te 32.53 (31.79–33.67), Bi 49.64 (48.27–50.31), total 99.57 (99.01–100.07) wt.%. Minor Pd is thus present. Results of two independent methods of microprobe analyses (WDS and SEM/EDS) are in excellent agreement; they yield essentially the same empirical formula: (Ni1.11Cu0.008Fe0.005Pd0.005)Σ1.13Bi0.90Te0.97, calculated on the basis of a total of 3 apfu. A minor extent of Ni-for-Bi substitution is thus implied. Examples of Ni–Bi substitution are known in some compounds: LaNi4.7−xBixAl0.3 with x = 0.1, 0.2 or 0.3 (Yilmaz et al., Reference Yilmaz, Kilicaslan, Atanur, Hong and Uzun2012) and Bi2−xNixSr2CaCu2Oy with x = 0, 0.05, 0.1 or 0.2 (Özkurt, Reference Özkurt2012). In addition, a mean electron number <83 at the Bi site of ognitite is consistent with the inferred presence of a lighter element (presumably Ni) at the 1b position. The ideal formula of ognitite is NiBiTe, which requires Ni 14.85, Bi 52.87 and Te 32.28, for a total of 100 wt.%.
Table 3. Compositions of ognitite from the Ognit complex.

Note: Analyses #1–7 are results of WDS analyses listed in weight %. Composition #8 is mean result of the seven point analyses (WDS). Analysis #9 pertains to results of an SEM/EDS analysis. Other elements (Co, Pt, Sb, Pb, As and S) were sought (WDS), but not detected. The bottom part of the table shows the compositions recalculated on the basis of three atoms per formula unit.
An initial study of the ognitite grain was undertaken using synchrotron micro-Laue diffraction followed by a monochromator energy scan, results of which indicate that is a single phase, apparently untwinned, with a = 3.925(5) Å and c = 5.381(7) Å. These patterns were indexed and analysed using XMAS v.6 (Tamura, Reference Tamura, Barabash and Ice2014).
Ognitite is trigonal; its space group is P3m1. The unit-cell parameters derived from the single-crystal X-ray diffraction data are: a = 3.928(1) Å, c = 5.385(1) Å and V = 71.95(4) Å3, with Z = 1. The c:a ratio calculated from the unit-cell parameters is 1.37.
The powder X-ray diffraction data (Table 4), obtained on the same fragment as used for the single-crystal study, are: a = 3.9332(4) Å, c = 5.3920(6) Å and V = 72.24(1) Å3.
Table 4. Measured and calculated X-ray powder diffraction data (d in Å) for ognitite*.

*Calculated diffraction pattern obtained with the atom coordinates reported in Table 6 (only reflections with I rel ≥ 1 are listed). The strongest diffraction lines are given in bold.
Crystal structure of ognitite
A small fragment (0.040 mm × 0.050 mm × 0.060 mm) was handpicked from the ognitite grain (Fig. 4) and examined with an Oxford Diffraction Xcalibur single-crystal diffractometer equipped with an Oxford Diffraction CCD detector, with graphite-monochromatised MoKα radiation (λ = 0.71073 Å). The collected data were integrated and corrected for standard Lorentz and polarization factors with the CrysAlis RED package (Oxford Diffraction, 2006). The program ABSPACK in CrysAlis RED (Oxford Diffraction, 2006) was used for the absorption correction. Details of the selected crystal, data collection, and refinement are given in Table 5. The calculated value of the R-factor is R 1 = 0.0276 for 81 reflections with F o > 4σ(F o).
Table 5. Data and experimental details for the selected ognitite crystal.

Although the statistical tests (|E 2–1| = 0.756) suggested an acentric space-group, the crystal structure was initially refined starting from the atomic coordinates of melonite, NiTe2 (Peacock and Thompson, Reference Peacock and Thompson1946) and using the program SHELXL-97 (Sheldrick, Reference Sheldrick2008). Given the observed larger unit-cell volume of ognitite (i.e. 71.95 Å3) compared to melonite (i.e. 66.93 Å3; Peacock and Thompson, Reference Peacock and Thompson1946), the site-occupancy factor at the crystallographic sites was allowed to vary (Ni vs. structural vacancy at the 1a site, and Bi vs. Te at the 2d site) using scattering curves for neutral atoms taken from the International Tables for Crystallography (Wilson, Reference Wilson1992). After several cycles of anisotropic refinement, a final R 1 = 0.0795 was achieved, but the atomic displacement parameter at the 2d position was abnormally high and asymmetric, thus suggesting a split into two positions. The introduction of two partially occupied (50%) positions (one partially occupied by Bi and one partially occupied by Te) led the R 1 index to 0.0608. At this stage, given the indication coming from the normalised structure-factors, we decided to try to solve the structure in the non-centrosymmetric space-group P3m1, which allows the separation of the 2d position into two positions, 1b and 1c. The new refinement (R 1 = 0.0276) showed that Bi and Te are completely ordered at 1b and 1c, respectively, with Bi probably being replaced by 0.03 Ni (according to the refined mean number of electrons at the site). The Te–Ni and Bi–Ni bond distances vary accordingly: the Te–Ni distance (2.575 Å) is identical to that observed in melonite (2.575 Å; Peacock and Thompson, Reference Peacock and Thompson1946), whereas the Bi–Ni distance (2.696 Å) is in agreement with those observed in parkerite, Ni3Bi2S2 (2.64–2.86 Å; Fleet, Reference Fleet1973b). Ognitite exhibits a brucite-type structure (Fig. 5). The edge-sharing NiTe3Bi3 octahedra form sheets parallel to (0001); the sheets are linked by van der Walls interactions.

Fig. 5. The crystal structure of ognitite. (a) Ball and stick representation: red, white and grey spheres refer to Ni, Te and Bi, respectively; (b) polyhedral representation. The unit-cell and the orientation of the figures are outlined.
Atom coordinates, site occupancies, and equivalent isotropic displacement parameters are given in Table 6. Selected bond-distances are given in Table 7. A crystallographic information file has been deposited as supplementary material (see below)
Table 6. Atoms, site occupancy factors (s.o.f.), fractional atomic coordinates and anisotropic displacement parameters (Å2) for the ognitite crystal extracted.

Table 7. Bond distances (in Å) in the structure of ognitite.

Ognitite is thus related to melonite; however, it differs compositionally (i.e. the marked Bi-enrichment) and structurally, because atoms of Bi and Te are ordered at two specific sites, leading to the loss of the centre of symmetry in the crystal structure, corresponding to a variant of the CdI2-2H structure-type. An additional characterisation of kitkaite (NiTeSe; Häkli et al., Reference Häkli, Vuorelainen and Sahama1965) is probably required to check whether it also belongs to that structure-type.
Co-rich maucherite and zoning in Ni–(Co) arsenides
As noted, the anomalously Co-rich variant of maucherite occurs in the core of a zoned grain (Figs 2b, 6a,b). The rim, composed of orcelite, is essentially devoid of Co (<0.1 wt.%), in contrast to the core zone, which contains ~8 to 10 wt.% Co (or 1.7–2.0 Co apfu: Table 8, #1–6). At least in some cases, the quantitative SEM/EDS method is preferable in analysing narrow zones or tiny phases. Indeed, the SEM/EDS composition (#6, Table 8) is somewhat closer to the ideal stoichiometry; nevertheless, the WDS results obtained (Table 8, #1–5) are consistent.

Fig. 6. BSE image (a) of the zoned grain of Co-rich maucherite (core: Mch) and orcelite (rim: Orc) hosted by serpentine. (b) False-colour X-ray map showing the distribution of Co in the zoned grain of Ni–(Co) arsenides.
Table 8. Compositions of Co-rich maucherite and associated orcelite in a zoned grain from the Ognit complex.

Note: Results of WDS (#1–5) and SEM/EDS (#6, 7) analyses are listed in the top part of the table in wt.%. Analyses #1–6 pertain to maucherite. Analysis #7 pertains to orcelite. Atomic proportions, are based on a total of 19 atoms per formula unit (apfu) for maucherite, and on two apfu S for orcelite.
‘–’ = not detected.
Our results of the synchrotron micro-Laue diffraction followed by a monochromator energy scan yielded the following cell parameters for the Co-rich maucherite at Ognit: a = 6.85(2) and c = 21.83(5) Å. Thus, these data agree well with those known for the ‘normal’ phase of maucherite (Co-free; Fleet, Reference Fleet1973a).
The orcelite rim corresponds to Ni5As2 (Table 8, #7). The structure of orcelite [Ni5–xAs2; x = 0.25] represents a distorted variant of the Pd5Sb2 structure (Bindi et al., Reference Bindi, Tredoux, Zaccarini, Miller and Garuti2014).
As noted, the Co-rich maucherite–orcelite crystal is hosted by a matrix of fine intergrowths of serpentine and clinochlore (Fig. 2). Five grains of serpentine (WDS data) yielded the composition (Mg2.69–2.74Fe0.09–0.11Al0.02–0.08Cr0–0.02Mn<0.01Ni<0.01)Σ2.83–2.90Si2.03–2.08O5(OH)4 (calculated for O = 7 apfu). The Mg# index [100Mg/(Mg + Fe2+ + Mn)] is high: ~97.
Compositions of six grains of clinochlore analysed (WDS) in this sample are uniformly rich in Mg, and in Cr, with 2.93–3.21 wt.% Cr2O3, and correspond to (Mg4.57–4.70Fe0.21–0.24Mn<0.01Ni≤0.01)Σ4.80–4.96Al1.0(Si3.00–3.09Al0.73–0.80Cr0.22–0.25Ti≤0.01)Σ4.01–4.10O10(OH)8 (for O = 14 apfu). Their values of the Mg# index are correspondingly high: up to ~96.
The skeletal grains of chromite associated with the zoned grain of Co-rich maucherite–orcelite (Fig. 2a) are weakly Mg-rich, with Mg# in the range 17–22, and are low in aluminium (≤6.5 wt.% Al2O3); they display very high values of Cr# [100Cr/(Cr + Al + Fe3+]: 85–92 (Table 1). In addition, elevated levels of Ti (0.7–1.4 wt.% TiO2), V (≤0.3 wt.% V2O3), Mn (0.9–1.0 wt.% MnO), and Zn (0.5–0.7 wt.% ZnO) are present. The chromite grains have a narrow rim of virtually pure magnetite (Fig. 3, Table 1, #10).
Discussion and conclusions
We have described two highly unusual species of Ni ore minerals found in mineralised zones in the Ognit ultramafic complex, Eastern Sayans, Russia. The anomalously high level of Bi in ognitite [Ni1.1Bi0.9Te] evidently cannot be explained by incorporation of the merenskyite and moncheite components in solid solution (Table 3). There is no evidence that the proximity to granodiorite (Fig. 1b) is an important factor, as there is no evidence of assimilation of xenoliths. And efforts to synthesise the phase NiBiTe in long-duration experiments at 200°, 400° and 600°C were not successful; the bismuth present in the charge crystallised to Bi + Bi2Te (Barkov et al., Reference Barkov, Shvedov, Flemming, Vymazalová and Martin2017a). Thus, we suggest that special conditions were important to stabilise the markedly Bi-enriched ognitite structure instead of that of common melonite.
The uniqueness of the Co-rich variant of maucherite also implies the existence of strongly atypical conditions. The high values of Mg# (95–97) of serpentine and clinochlore reflect the highly Mg-rich compositions of primary grains of olivine and pyroxene crystallised in the primitive ultramafic cumulates. We propose that the skeletal texture of the associated grains of chromite (Fig. 2a) indicates difficulties in their nucleation. They could well provide an indication of rapid cooling in the system, possibly related to the degassing of the magma at an advanced stage of crystallisation of the complex, reflected in the prominent metasomatic aureole at the contact (Fig. 1b). Effects of supercooling and metastable crystallisation were observed in the Pados-Tundra ultramafic complex, Kola Peninsula, Russia (Barkov et al., Reference Barkov, Nikiforov, Halkoaho and Konnunaho2016, Reference Barkov, Nikiforov and Martin2017b), for example.
We thus suggest a mechanism of metastable crystallisation to explain the anomalous compounds at Ognit. An elevated fluid pressure could have existed in the ore-forming system. The anomalously Bi-rich composition of ognitite could well represent a late-crystallising phase formed in a fluid-saturated environment from a droplet of residual melt enriched in Ni, Bi and Te. These elements are incompatible in the host chalcopyrite, as are Ag and Pb, present in the coexisting hessite and altaite. The shared boundary with native Bi (Fig. 4) is consistent with various occurrences of native elements and alloys (i.e. native silver, Ag–Au alloy, auricupride, awaruite, native copper and graphite), which indicate the existence at Ognit of reducing conditions (Shvedov and Barkov, Reference Shvedov and Barkov2017).
These conditions point to a probable stability-field at Ognit of the reduced form, As3+, rather than As5+, which seems to be typical of mantle-related serpentinites (Hattori et al., Reference Hattori, Takahashi, Guillot and Bo2005). The Ni-rich arsenides, including their grains with their original shapes, are quite common accessory phases in the ore zones at Ognit; these formed from droplets of As-rich melts (Shvedov and Barkov, Reference Shvedov and Barkov2017). The documented occurrences of Ni arsenides thus reflect arsenide saturation of the sulfide liquid (cf., Piña et al., Reference Piña, Gervilla and Barnes2014); an initial content of ≥0.1 wt.% As is considered sufficient to ensure saturation in an arsenide (Fleet et al., Reference Fleet, Chryssoulis, Stone and Weisener1993). The zonation is documented in the grain composed of the Co-rich maucherite in the core and Co-depleted orcelite in the rim (Figs 2b; 6a,b). In other layered complexes, zoned grains of Ni–Co–(PGE) sulfarsenides are developed instead (e.g. Barkov et al., Reference Barkov, Thibault, Laajoki, Melezhik and Nilsson1999).
If the equilibrium crystallisation is postulated at Ognit, results of experimental studies are then applicable. The melting point of synthetic Ni5As2 (orcelite equivalent) is 998°C; the upper thermal stability limit of Ni11As8 (maucherite) is 830°C (Yund, Reference Yund1961; Singleton and Nash, Reference Singleton and Nash1987). These values suggest that orcelite is expected to crystallise before maucherite if they coexist under normal conditions. In contrast, we observe that the Co-enriched maucherite occurs in the core; thus, it presumably nucleated first and before the orcelite phase developed in the rim. If so, we can reasonably propose that the incorporation of Co increases, to a notable extent, the melting point of Ni11As8, in contrast to the effect observed from the incorporated Pd (cf., Gervilla et al., Reference Gervilla, Makovicky, Makovicky and Rose-Hansen1994). This possibility requires an experimental confirmation, however. The Co analogue of maucherite does not seem to have been reported, and thus was probably not obtained by synthesis. However, synthetic Co5As2 (space group P63cm; i.e. prototype of Pd5Sb2), related to orcelite, exists in the system Co–As, in which it is a high-temperature phase; it undergoes a disproportionation transformation to (α-Co) + β-Co2As at 867°C (Ishida and Nishizawa, Reference Ishida and Nishlzawa1990, and references therein). Note that the orcelite phase is essentially devoid of Co in the rim, in spite of the existence of the cobalt analogue of orcelite. This feature also implies that Co was preferentially incorporated into maucherite as the early phase, a reflection of the relatively high temperature of its crystallisation.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2019.31
Acknowledgements
We are grateful to an anonymous referee, Drs. Peter Leverett, Anna Vymazalová, Irina Galuskina, Roger Mitchell, and to the editorial staff for their valuable comments. This research required access to beamline 12.3.2 at the Advanced Light Source, which is a DOE Office of Science User Facility under contract #DE-AC02-05CH11231. A.Y.B. gratefully acknowledges a partial support of this investigation by the Russian Foundation for Basic Research (projects #RFBR 16-05-00884 and # RFBR 19-05-00181). C.J.S. acknowledges Natural Environment Research Council grant NE/M010848/1 “Tellurium and Selenium Cycling and Supply”.