Introduction
There is now clear evidence demonstrating that psychotic experiences (PEs) in the absence of psychotic disorders are common in the general population (Linscott & van Os, Reference Linscott and van Os2013; McGrath et al. Reference McGrath, Saha, Al-Hamzawi, Alonso, Bromet and Bruffaerts2015). Recent research showing that those with PEs are at increased risk of premature mortality (Sharifi et al. Reference Sharifi, Eaton, Wu, Roth, Burchett and Mojtabai2015) heightens interest in the relationship between PEs and general medical conditions (GMCs). Although this is a relatively new topic of investigation, evidence is accruing that PEs are associated with a range of GMCs such as heart disease, diabetes, arthritis, asthma, dental problems, hearing loss and chronic pain conditions (Saha et al. Reference Saha, Scott, Varghese and McGrath2011a; Moreno et al. Reference Moreno, Nuevo, Chatterji, Verdes, Arango and Ayuso-Mateos2013; Oh & DeVylder, Reference Oh and DeVylder2015; Koyanagi & Stickley, Reference Koyanagi and Stickley2015a; Stubbs et al. Reference Stubbs, Koyanagi, Veronese, Vancampfort, Solmi and Gaughran2016; Koyanagi et al. Reference Koyanagi, Stickley and Haro2016b; Koyanagi et al. Reference Koyanagi, Oh, Stubbs, Haro and DeVylder2017). Several studies have also found associations between PEs and sleep problems (Thompson et al. Reference Thompson, Lereya, Lewis, Zammit, Fisher and Wolke2015; Koyanagi & Stickley, Reference Koyanagi and Stickley2015b; DeVylder & Kelleher, Reference DeVylder and Kelleher2016; Oh et al. Reference Oh, Singh, Koyanagi, Jameson, Schiffman and DeVylder2016; Andorko et al. Reference Andorko, Mittal, Thompson, Denenny, Epstein and Demro2017), and a recent randomized controlled trial found that treatment of insomnia reduced the prevalence of PEs (Freeman et al. Reference Freeman, Sheaves, Goodwin, Yu, Nickless and Harrison2017).
Two questions arise in relation to these PE–GMC associations. The first question is whether they are independent of comorbid common mental disorders given that (i) PEs are associated with common mental disorders (DeVylder et al. Reference DeVylder, Burnette and Yang2014; McGrath et al. Reference McGrath, Saha, Al-Hamzawi, Andrade, Benjet and Bromet2016b) and (ii) that common mental disorders are associated with GMCs (Lawrence et al. Reference Lawrence, Hancock and Kisely2013; Scott et al. Reference Scott, Lim, Al-Hamzawi, Alonso, Bruffaerts and Caldas-de-Almeida2016). Only a very small number of studies have examined this question and the results are equivocal. In general, these studies find that associations between PEs and GMCs are attenuated after adjustment for comorbid mental disorders but that some associations do persist (Saha et al. Reference Saha, Scott, Varghese and McGrath2011a; Moreno et al. Reference Moreno, Nuevo, Chatterji, Verdes, Arango and Ayuso-Mateos2013; Oh & DeVylder, Reference Oh and DeVylder2015). In a study of PEs and pain conditions, however, the associations lost significance after adjustment for comorbid mental disorders (Koyanagi et al. Reference Koyanagi, Stickley and Haro2016b).
The second important question concerns the temporal direction of associations. Prior studies have not been able to consider the temporal sequencing of PEs and GMCs; that is, whether temporally prior PEs are associated with subsequent onset of GMCs, and/or whether GMCs are associated with subsequent first onset of PEs. Mindful that certain GMCs typically have an early onset (e.g. asthma), while many have later onset (e.g. heart disease, cancer), understanding the extent to which these PEs and GMCs associations follow a specific temporal sequence has important theoretical, clinical and public health significance. Ideally, this kind of investigation of temporal sequence should be undertaken in prospective studies. However, this would require very large cohorts with information on PEs, mental disorders and GMCs followed for decades (given the typically early onset of PEs and mental disorders, and later onset of most GMCs). To our knowledge, no such data are presently available. Therefore, in order to examine the temporal direction of PEs and GMCs, we have used retrospectively collected data from the World Mental Health (WMH) Surveys (Kessler & Üstün, Reference Kessler and Üstün2004) on age of onset (AOO) of PEs, mental disorders and GMCs.
The specific aims of this study were to examine: (a) whether PEs are associated with subsequent onset/diagnosis of GMCs; (b) whether GMCs are associated with subsequent onset of PEs; (c) whether these associations are independent of a wide range of antecedent mental disorders; and (d) the role of severity of PEs (number of PE types, annualized frequency of PEs) in the association with GMCs.
Method
Samples
The WHO WMH surveys are a coordinated set of community surveys generally administered to adult respondents (18 years and over) in countries throughout the world (Kessler & Üstün, Reference Kessler and Üstün2004). Data for this study were drawn from the 16 WMH surveys that included both the psychosis module and the items related to GMCs (N = 28 002). These 16 surveys are distributed across North and South America (Argentina, Colombia, Mexico, Peru, USA); the Middle East (Iraq); Asia (Shenzhen in the People's Republic of China); the South Pacific (New Zealand) and Europe (Belgium, France, Germany, Italy, the Netherlands, Portugal, Romania, Spain). The majority of these surveys were based on multi-stage, clustered area probability household sampling designs, the exceptions being Belgium, Germany and Italy, which used municipal resident registries to select respondents (online Supplementary Table S1). The weighted (by sample size) average response rate across the 16 surveys was 71.3%.
In order to focus on the correlates of PEs in those without psychotic disorders, we made the a priori decision to exclude individuals who had PEs but who also screened positive for possible schizophrenia/psychosis and manic-depression/mania. Thus, in keeping with the previous studies of PEs (Saha et al. Reference Saha, Scott, Johnston, Slade, Varghese and Carter2011b; McGrath et al. Reference McGrath, Saha, Al-Hamzawi, Alonso, Bromet and Bruffaerts2015; McGrath et al. Reference McGrath, Alati, Clavarino, Williams, Bor and Najman2016a; McGrath et al. Reference McGrath, Saha, Al-Hamzawi, Andrade, Benjet and Bromet2016b), we excluded respondents who (a) reported (1) schizophrenia/psychosis or (2) manic-depression/mania in response to the question ‘What did the doctor say was causing (this/these) experiences?’ (respondents with these disorders who did not report PEs were not excluded); and (b) those who ever took an antipsychotic medication for these symptoms. This resulted in the exclusion of 124 respondents (0.4%), leaving 28 002 respondents for this study.
Procedures
WMH interviews were conducted in the homes of respondents by trained lay interviewers. Informed consent was obtained before beginning interviews in all countries. Procedures for obtaining informed consent and protecting individuals (ethical approvals) were approved and monitored for compliance by the institutional review boards of the collaborating organizations in each country. Standardized interviewer training and quality control procedures were used consistently in the surveys. Full details of these procedures are described elsewhere (Kessler et al. Reference Kessler, Haro, Heeringa, Pennell and Ustun2006; Kessler & Üstün, Reference Kessler, Üstün, Kessler and Üstün2008).
Interviews were administered face to face in two parts. Part 1, which assessed a core set of mental disorders, was administered to all respondents. Part 2 of the interview, which assessed additional mental disorders, questions about PEs, and GMCs, was administered to respondents who met lifetime criteria for any part 1 disorder, and a random proportion of the remaining sample of those without any part 1 disorders. Part 2 respondents were weighted by the inverse of their probability of selection to adjust for differential sampling, and therefore, provide representative data on the target adult general population. Details about sampling methods are available elsewhere (Kessler & Üstün, Reference Kessler and Üstün2004). Additional weights were used to adjust for differential probabilities of selection within households, non-response and to match the samples to population socio-demographic distributions in each country.
Measures
The WMH surveys administered the WHO Composite International Diagnostic Interview (CIDI) (Kessler & Üstün, Reference Kessler, Üstün, Kessler and Üstün2008), a validated fully structured diagnostic interview designed to assess the prevalence and correlates of a wide range of mental disorders according to the definitions and criteria of both the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) and the International Classification of Mental Disorders, Tenth Edition (ICD-10) diagnostic systems. Translation, back-translation and harmonization protocols were used to adapt the CIDI for use in each participating country.
General medical conditions
GMCs were assessed based on a series of questions adapted from the US National Health Interview Survey. Twelve conditions were assessed in this study. Respondents were asked if they had a lifetime history of symptom-based conditions (i.e. arthritis or rheumatism, chronic back or neck pain, frequent or severe headaches, any other chronic pain or stroke) and whether they were ever told by a doctor or other health professional that they had a series of medical conditions (e.g. heart disease, cancer, diabetes mellitus, hypertension, asthma, other chronic lung diseases or peptic ulcer). For all of these conditions reported, respondents were also asked how old they were when they were first diagnosed with the condition or first experienced the symptomatic condition. Prior research has demonstrated good concordance between self-reported illness and medical records (Baumeister et al. Reference Baumeister, Kriston, Bengel and Harter2010).
Psychotic experiences
The CIDI psychosis module included questions about six PE types – two related to hallucinatory experiences and four related to delusional experiences. We excluded PEs experienced while dreaming, half-asleep or under the influence of alcohols or drugs (online Supplementary Tables S2a, S2b). In this paper, we present estimates of GMCs for ‘any PEs’ only (i.e. not individual types of PEs). In addition, we included two key PE variables: (a) number of PE types, and (b) an annualized frequency metric based on the frequency of PE episodes per year. We derived the latter by dividing the total number of PE episodes by the time since onset of the first PE (age at interview minus age-at onset plus 1 in order to avoid zero as a denominator). We used the median threshold to dichotomize this metric. Age-at-onset of respondents with PEs was also assessed.
Mental disorders
The WMH version of the CIDI assessed lifetime history of 21 mental disorders broadly classified into mood disorders, anxiety disorders, behaviour disorders, eating disorders and substance use disorders (see online Supplementary Table S2c). Full details are given in several WMH publications including two of our recent papers (McGrath et al. Reference McGrath, Saha, Al-Hamzawi, Andrade, Benjet and Bromet2016b; McGrath et al. Reference McGrath, McLaughlin, Saha, Aguilar-Gaxiola, Al-Hamzawi and Alonso2017). Clinical reappraisal studies indicate that lifetime diagnoses based on the CIDI have good concordance with diagnoses based on blinded clinical interviews (Haro et al. Reference Haro, Arbabzadeh-Bouchez, Brugha, de Girolamo, Guyer and Jin2006). In keeping with our previous research, standardized diagnostic hierarchy rules among the disorders assessed were applied where appropriate (McGrath et al. Reference McGrath, Saha, Al-Hamzawi, Andrade, Benjet and Bromet2016b).
Statistical analysis
Descriptive statistics were used for the lifetime prevalence of PEs, GMCs and GMCs among respondents with and without PEs. Respondents with GMCs that developed after PEs and GMCs that developed before PEs were compared using weighted Rao–Scott χ2 test. Discrete-time survival models with person-year as the unit of analysis were used to investigate the temporal sequencing of associations between PEs and GMCs. When examining the predictive relationship between temporally prior PEs and the subsequent onset of GMCs, PEs that occurred in the same year as GMCs or following GMCs were excluded. Those without GMCs were censored at their age at interview. We used any PEs, number of PE types and annualized frequency of PEs in base models as predictors of subsequent GMCs adjusting for age at interview, sex, person-year and country. For multivariate models, we additionally adjusted for 21 antecedent mental disorders (i.e. disorders occurring antecedent or intervening between PEs and GMCs). Likewise, when examining the relationship between temporally prior GMCs and subsequent onset of PEs, we excluded GMCs that occurred in the same year as PEs onset or following PEs. Those without PEs were censored at their age at interview. To examine the associations between GMCs and subsequent onset of PEs, a series of base and multivariate models (M1–M5) was developed. For the base models, we incorporated one GMC at a time to predict for subsequent PEs adjusting for age at interview, gender, person-year and country. For multivariate models, we incorporated: (a) all GMC types simultaneously (model M1), (b) number of GMCs without any information about type (model M2), (c) type and number of GMCs (model M3), and (d) type and number of GMCs with adjustments for antecedent mental disorders (model M4) in predicting subsequent PE onset. Models M3 and M4 allow for non-additive relationships between type and number such that the effect of number of GMCs may vary across types. Finally, as there are associations between smoking and both (a) GMCs (Doll, Reference Doll1998; Keto et al. Reference Keto, Ventola, Jokelainen, Linden, Keinanen-Kiukaanniemi and Timonen2016) and (b) PEs (Koyanagi et al. Reference Koyanagi, Stickley and Haro2016a), we undertook post hoc analyses where we adjusted for lifetime occurrence of tobacco use (yes/no) as an additional covariate with all other adjustments.
As the WMH data are both clustered and weighted, the design-based Taylor series linearization implemented in SUDAAN software (RTI International, 1999) was used to estimate the standard errors and evaluate the statistical significance of the coefficients. Survival coefficients and their standard errors were exponentiated to generate odds ratios (ORs) and 95% confidence intervals (CIs). All significance tests were evaluated using 0.05-level two-sided tests.
Results
Characteristics of the sample
The age range of the combined cross-national sample was 18–99 years (online Supplementary Table S1), with a weighted mean age of 41.9 years (s.e. = 0.2) and median age of 38.2 years (IQR = 26.5–53.3). Females accounted for 51.6% of the sample (s.e. = 0.4).
Prevalence of GMCs
The weighted prevalence of PEs in this sample was 5.0% (s.e. = 0.2). The lifetime prevalence of GMCs ranged from 1.3% (s.e. = 0.1) for other chronic lung diseases to 24.3% (s.e. = 0.4) for back or neck pain (Table 1). When divided into those with or without PEs, the prevalence of GMCs was consistently higher for those with lifetime PEs compared with those without. Among the subset of respondents with both lifetime GMC and PEs, a large proportion of respondents experienced PEs prior to GMCs, consistent with the later age of onset of most of the GMCs examined in this study (Table 1; see also online Supplementary Table S4).
Table 1. Prevalence of general medical conditions (GMC) among respondents with and without lifetime psychotic experiences (PEs) (n = 28 002)

s.e., standard error.
* Significant at the 0.05 level, two-sided test.
a χ2 tests comparing the proportion of respondents with PE onset prior to GMC onset v. the proportion of respondents with GMC onset prior to PE onset.
b Estimates are based on weighted data.
c Unstable estimates due to small design effect.
Associations between PEs and subsequent onset of GMCs
We first examined the associations between PEs and subsequent onset of GMCs (Table 2). Temporally prior PEs were significantly associated with subsequent first onset of eight of the 12 GMCs (i.e. arthritis, back or neck pain, other chronic pain, frequent or severe headache, heart disease, high blood pressure, diabetes and peptic ulcer). The ORs ranged from 1.4 (95% CI 1.2–1.7) for high blood pressure to 2.3 (95% CI 1.8–3.0) for other chronic pain. There was a significant dose–response relationship between the number of PE types and all the eight GMCs indicating increasing odds of GMCs with increasing number of PE types (χ2 ranged between 10.5 and 73.3). Those with more frequent annualized PEs (>0.3 episodes per year) compared with those with ⩽0.3 episodes per year had approximately twofold increased odds of developing subsequent GMCs. After adjusting for antecedent mental disorders, the majority of the associations between PEs and these eight GMCs lessened in magnitude but remained statistically significant.
Table 2. Associations between temporally prior psychotic experiences (PEs) and the subsequent onset of general medical conditions (GMCs)

OR, odds ratio; CI, Confidence interval.
*Significant at the 0.05 level, two-sided test.
a PE (any PE, number of PE types and PE frequency metric) was used as a predictor of general medical condition outcomes in separate discrete-time survival models. These models control for age at interview, sex, person-year and country.
b Unstable estimates due to small cell counts.
c These models additionally control for 21 antecedent mental disorders. See online Supplementary Table S2c for the list of mental disorders.
Associations between temporally prior GMCs and subsequent onset of PEs
Next, we examined the associations between temporally prior GMCs and the subsequent onset of PEs (Table 3). In the base model, seven of the 12 GMCs (arthritis, back or neck pain, frequent or severe headache, other chronic pain, high blood pressure, asthma and peptic ulcer) were significantly associated with increased odds of any PEs. The ORs ranged between 1.7 and 2.7 with the highest OR for other chronic pain (OR 2.7, 95% CI 2.1–3.5) followed by frequent or severe headache (OR 2.1, 95% CI 1.7–2.5). When we adjusted for comorbidity of other GMCs (M1), five of the GMCs (arthritis, back or neck pain, frequent or severe headache, other chronic pain and asthma) remained statistically significant. Next, after fitting the model that included number as well as type of GMCs (M3), the significant associations of these conditions with PE onset persisted. Finally, when we adjusted for antecedent mental disorders (M4), only three of the five GMCs previously associated with PEs (frequent or severe headache, other chronic pain and asthma) remained statistically significant (OR 1.5, 95% CI 1.2–1.9; OR 1.7, 95% CI 1.2–2.4; OR 1.6, 95% CI 1.2–2.1, respectively).
Table 3. Associations between temporally prior general medical conditions (GMCs) and the subsequent onset of psychotic experiences (PEs)
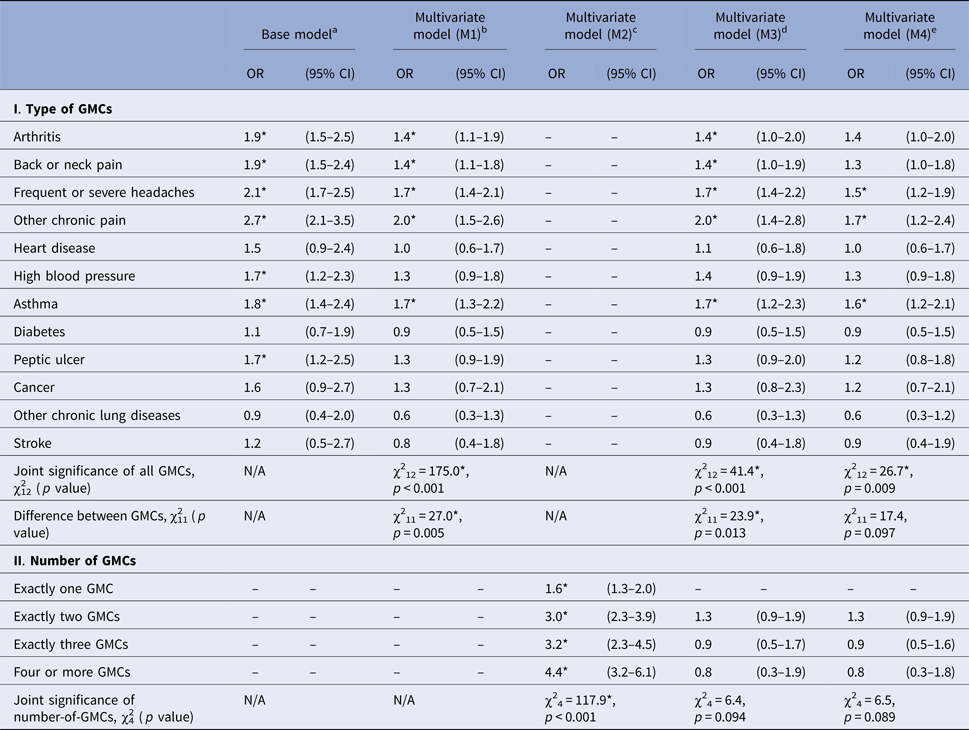
OR, odds ratio; CI, confidence interval.
*Significant at the 0.05 level, two-sided test.
a Each model was estimated with one GMC entered at a time as predictor of PE onset controlling for age at interview, sex, person-year and country.
b M1: Model was estimated with dummy variables for all GMCs entered simultaneously as predictors of PE onset including the controls specified in (a).
c M2: Model was estimated with dummy variables for all number of GMCs without any information about type entered simultaneously as predictors of PE onset including the controls specified in (a).
d M3: Model was estimated with dummy variables for type and number of GMCs (exactly two GMCs,.., four or more GMCs) entered simultaneously as predictors of PE onset including the controls specified in (a).
e M4: Model was estimated with dummy variables for type and number of GMCs (exactly two GMCs,.., four or more GMCs) entered simultaneously as predictors of PE onset including the controls specified in (a) and 21 antecedent mental disorders.
When we repeated the main analyses using additional adjustment for smoking, there was no substantial change in the results except for other chronic lung diseases (as expected) in which the ORs attenuated substantially in both analyses (online Supplementary Tables S3a, S3b). However, in the final adjusted models, the associations between PEs and other chronic lung diseases (whether PEs as the predictor for GMCs or vice versa) were not significant.
There was a significant dose–response relationship between the number of GMCs and subsequent first-onset PEs, with ORs monotonically increasing with increasing number of GMCs (χ24 = 117.9, p < 0.001) indicating that multimorbidity of GMCs predicts first-onset PEs (M2, Table 3). This model, however, assumes an additive relationship between the number of GMCs and onset of PEs. In the next models (M3 and M4) that take type of GMCs into account and remove the additive assumption about the effect of number of GMCs, the ORs for the association of GMCs with odds of subsequent PEs became progressively smaller with each additional GMC. These sub-additive interactions need to be interpreted with caution, however, as number-of-GMCs as a set fell short of significance.
Discussion
In this large, population-based dataset from 16 countries, we found that temporally prior PEs were significantly associated with subsequent first onset of eight of the 12 GMCs studied (i.e. arthritis, back or neck pain, other chronic pain, frequent or severe headache, heart disease, high blood pressure, diabetes and peptic ulcer). PEs were not significantly associated with the subsequent onset of asthma, cancer, other chronic lung disease or stroke. After adjustment for mental disorders antecedent to the GMCs, all previously significant associations remained significant, although all were somewhat attenuated, which suggests that a portion of these PE–GMC associations are explained by comorbid mental disorders. These results extend findings from prior studies (Saha et al. Reference Saha, Scott, Varghese and McGrath2011a; Moreno et al. Reference Moreno, Nuevo, Chatterji, Verdes, Arango and Ayuso-Mateos2013; Oh & DeVylder, Reference Oh and DeVylder2015) by providing description of associations between PEs and a range of GMCs in the general population independent of comorbid mental disorders. In addition, our temporally ordered survival analyses based on a large cross-national sample provide new insights into the temporal direction of associations between PEs and GMCs. For while, we found that prior onset PEs were predictive of eight of the 12 GMCs; conversely, we also found that three of the 12 GMCs (i.e. frequent or severe headache, other chronic pain and asthma) were significantly associated with subsequent first onset of PEs.
We were also able to undertake examination of how associations between PEs and GMCs varied by PE severity, as indicated by the number of PE types and frequency of annualized PEs. For the associations of PEs with subsequent GMCs, we observed a dose–response pattern for many (although not all) of the associations, whereby those with a higher number of PE types and higher frequency of annualized PEs were more likely to develop subsequent GMCs. For many associations though, especially for those of PEs with subsequent pain conditions (with the exception of headache), this pattern was no longer evident after adjustment for mental disorders, suggesting that some of the increased risk of GMCs associated with a higher burden of prior PEs may be mediated by comorbid mental disorders. In considering the reverse direction of associations of GMCs with subsequent PEs, we similarly observed a dose–response pattern, whereby a higher count of temporally prior GMCs was associated with higher odds of subsequent first onset of PEs, although the added risk became smaller with each additional GMC. While it is widely accepted that people with psychotic disorders are more likely to develop a range of chronic GMCs (Stubbs et al. Reference Stubbs, Koyanagi, Veronese, Vancampfort, Solmi and Gaughran2016) and have reduced life expectancy (Laursen et al. Reference Laursen, Nordentoft and Mortensen2014; Hjorthoj et al. Reference Hjorthoj, Sturup, McGrath and Nordentoft2017), our findings suggest that those with PEs, even in the absence of known psychotic disorders, also warrant close scrutiny in relation to physical health.
The observational nature of this study precludes any firm conclusions about whether these associations reflect causal mechanisms. That said, there are plausible mechanisms that could be involved. The fact that the strongest temporal sequence observed in this study was from prior PEs to subsequent onset of GMCs is consistent with the now substantial evidence for associations between antecedent common mental disorders and subsequent onset of GMCs (Thurston et al. Reference Thurston, Rewak and Kubzansky2013; Whooley & Wong, Reference Whooley and Wong2013; Scott, Reference Scott2014; Scott et al. Reference Scott, Lim, Al-Hamzawi, Alonso, Bruffaerts and Caldas-de-Almeida2016). This raises the possibility that some of the same mechanisms that mediate the relationship between the common mental disorders and GMCs may be contributing to the associations between PEs and GMCs, and one such mechanism may be deleterious health behaviours associated with PEs such as smoking (Moreno et al. Reference Moreno, Nuevo, Chatterji, Verdes, Arango and Ayuso-Mateos2013). However, when we repeated the main analysis controlling for smoking, there was no substantial change in the results indicating that the association between PEs and GMCs was not affected substantially by smoking history. It may also be relevant that PEs have a well-established relationship with sleep disturbance (Thompson et al. Reference Thompson, Lereya, Lewis, Zammit, Fisher and Wolke2015; Koyanagi & Stickley, Reference Koyanagi and Stickley2015b; DeVylder & Kelleher, Reference DeVylder and Kelleher2016; Oh et al. Reference Oh, Singh, Koyanagi, Jameson, Schiffman and DeVylder2016; Andorko et al. Reference Andorko, Mittal, Thompson, Denenny, Epstein and Demro2017); poor sleep could therefore be part of a nexus of adverse health behaviours associated with PEs that mediate associations with poor health outcomes, but this requires further study before any conclusions can be drawn. To the extent that PEs serve as a marker of general psychological distress (Yung et al. Reference Yung, Buckby, Cotton, Cosgrave, Killackey and Stanford2006; Saha et al. Reference Saha, Scott, Varghese and McGrath2011c), it is also theoretically conceivable that PEs could be associated with subsequent GMCs via chronic elevation or dysregulation of the physiological stress response pathways given that considerable evidence has accrued for these biological mechanisms in the associations of depression and anxiety with subsequent cardio-metabolic conditions (Davies et al. Reference Davies, Esler and Nutt2010; Stetler & Miller, Reference Stetler and Miller2011). However, this suggestion remains speculative in the absence of any known evidence for such biological perturbations in people with PEs; this is an important area for future research. In addition to these potential causal explanations, it should also be noted that there are also several potential shared determinants of PEs and GMCs such as genetics, low birth weight and adverse early circumstances, and indeed, sleep disturbances. On the basis of this study, therefore, we can say that PEs appear to be risk markers for a range of subsequent poor health outcomes, but determining whether they are causal risk factors will require more definitive study designs that can control for a range of potential confounding factors.
After adjustment for comorbid mental disorders, the only GMCs that were associated with subsequent onset of PEs were asthma and two of the pain-related conditions (frequent/severe headaches and other chronic pain). This suggests the possibility that inflammatory mechanisms related to GMCs could contribute to the subsequent emergence of PEs. For example, a birth cohort study reported an association between childhood asthma and/or eczema and the onset of PEs by age 13 years (Khandaker et al. Reference Khandaker, Zammit, Lewis and Jones2014). The fact that frequent/severe headaches and other chronic pain conditions were significantly associated with PEs in both temporal directions is interesting in light of the robust evidence from prior studies of the relationship between PEs and pain conditions (Koyanagi & Stickley, Reference Koyanagi and Stickley2015a; Koyanagi et al. Reference Koyanagi, Stickley and Haro2016b). Of note is that while one of those prior studies (Koyanagi et al. Reference Koyanagi, Stickley and Haro2016b) found that the significant association between PEs and pain was fully accounted for by depression and anxiety disorders, the present study found these associations between PEs and pain conditions (in both temporal directions) to persist despite comprehensive adjustment for 21 comorbid mental disorders. The well-established connections between chronic pain and sleep disturbance (Smith & Haythornthwaite, Reference Smith and Haythornthwaite2004), taken together with the several reports cited above of sleep disturbance in association with PEs, suggest that the present study's findings may reflect complex reciprocal relationships between chronic pain, sleep disturbance and psychological distress, particularly in the context of multimorbidity. It should also be noted that people with sleep disturbance may take hypnotic medications, which may influence the association between sleep disturbance and PEs – this topic warrants additional research.
While the current study has many strengths (e.g. range of PE types, large sample size, range of countries, uniform methodology for data collection), the methodological limitations are also considerable. Perhaps chief among these is the fact that the analyses were dependent on retrospective recall of the occurrence of mental disorders and PEs. Lifetime recall of mental disorders is unreliable and recall of the onset timing of mental disorders is known to be subject to bias (Simon & Von Korff, Reference Simon and Von Korff1995; Moffitt et al. Reference Moffitt, Caspi, Taylor, Kokaua, Milne and Polanczyk2010). Although the probing strategy employed in the WMH surveys has been shown to reduce this bias to some extent (Knäuper et al. Reference Knäuper, Cannell, Schwarz, Bruce and Kessler1999), it is likely that some inaccuracy in onset timing remains and that mild disorders in particular will be under-recalled (Wells & Horwood, Reference Wells and Horwood2004). Recall of AOO of the GMCs is generally more reliable (Pattaro et al. Reference Pattaro, Locatelli, Sunyer and de Marco2007), but the assessment of GMCs in this study was less rigorous than the assessment of mental health problems, and self-report of physician-assigned diagnoses will miss some conditions that are asymptomatic in early stages. These limitations will undoubtedly have resulted in some individuals with a true history of PE, mental disorder and/or GMC being misclassified as non-cases. It is important to note though that this kind of misclassification tends to bias associations towards the null, making the results conservative. Survival bias may also contribute to the conservative nature of these findings. We excluded those who screened positive for possible psychotic disorders, but it is possible that some respondents who reported PEs had an untreated psychotic disorder. Finally, it should be noted that many of the GMCs examined in this study are chronic conditions of ageing (e.g. heart disease, arthritis, cancer, stroke). While the median age of onset for PEs is 26 years (McGrath et al. Reference McGrath, Saha, Al-Hamzawi, Alonso, Andrade and Borges2016c), later-onset PEs have also been noted. It is feasible that the mechanisms underlying later-onset PEs may be influenced by the neurobiological correlates of these GMCs.
In summary, in this large community-based cross-sectional study, we found that individuals with prior onset of PEs were more likely to self-report subsequent development or diagnosis of a wide range of GMCs (i.e. arthritis, back or neck pain, other chronic pain, frequent or severe headache, heart disease, high blood pressure, diabetes and peptic ulcer) independent of comorbid mental disorders. A higher burden of PEs was associated with higher odds of subsequent GMC onset. In addition, three of the 12 GMCs studied (i.e. other chronic pain, frequent or severe headache, asthma) were significantly associated with subsequent onset of PEs. Although the temporal directions of the associations observed here will need to be confirmed in prospective designs, this study contributes substantial evidence in support of the proposition that PEs are associated with GMCs even after controlling for mental disorder comorbidity. It remains to be determined whether these associations are causal, but there are several plausible causal mechanisms. Behavioural and biological mechanisms that could mediate these associations between PEs and physical morbidity are an important area for future research. Clinicians should be aware that psychotic symptoms, independent of comorbid psychotic or other mental disorder, may be risk markers for a range of adverse health outcomes.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718000363
Acknowledgements
The World Health Organization World Mental Health (WMH) Survey Initiative is supported by the United States National Institute of Mental Health (NIMH; R01 MH070884), the John D. and Catherine T. MacArthur Foundation, the Pfizer Foundation, the United States Public Health Service (R13-MH066849, R01-MH069864, and R01 DA016558), the Fogarty International Center (FIRCA R03-TW006481), the Pan American Health Organization, Eli Lilly and Company, Ortho-McNeil Pharmaceutical Inc., GlaxoSmithKline, and Bristol-Myers Squibb. The authors thank the staff of the WMH Data Collection and Data Analysis Coordination Centres for assistance with instrumentation, fieldwork and consultation on data analysis. None of the funders had any role in the design, analysis, interpretation of results or preparation of this paper. The views and opinions expressed in this report are those of the authors and should not be construed to represent the views of the World Health Organization, other sponsoring organizations, agencies or governments.
The Argentina survey – Estudio Argentino de Epidemiología en Salud Mental (EASM) – was supported by a grant from the Argentinian Ministry of Health (Ministerio de Salud de la Nación). The Colombian National Study of Mental Health (NSMH) is supported by the Ministry of Social Protection. The ESEMeD surveys were funded by the European Commission [Contracts |QLG5-1999-01042; SANCO 2004123, and EAHC 20081308), the Piedmont Region (Italy)], Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Spain (FIS 00/0028), Ministerio de Ciencia y Tecnología, Spain (SAF 2000-158-CE), Departament de Salut, Generalitat de Catalunya, Spain, Instituto de Salud Carlos III (CIBER CB06/02/0046, RETICS RD06/0011 REM-TAP), and other local agencies and by an unrestricted educational grant from GlaxoSmithKline. Implementation of the Iraq Mental Health Survey (IMHS) and data entry were carried out by the staff of the Iraqi MOH and MOP with direct support from the Iraqi IMHS team with funding from both the Japanese and European Funds through United Nations Development Group Iraq Trust Fund (UNDG ITF). The Mexican National Comorbidity Survey (MNCS) is supported by The National Institute of Psychiatry Ramon de la Fuente (INPRFMDIES 4280) and by the National Council on Science and Technology (CONACyT-G30544- H), with supplemental support from the PanAmerican Health Organization (PAHO). Te Rau Hinengaro: The New Zealand Mental Health Survey (NZMHS) is supported by the New Zealand Ministry of Health, Alcohol Advisory Council, and the Health Research Council. The Peruvian World Mental Health Study was funded by the National Institute of Health of the Ministry of Health of Peru. The Portuguese Mental Health Study was carried out by the Department of Mental Health, Faculty of Medical Sciences, NOVA University of Lisbon, with collaboration of the Portuguese Catholic University, and was funded by Champalimaud Foundation, Gulbenkian Foundation, Foundation for Science and Technology (FCT) and Ministry of Health. The Shenzhen Mental Health Survey is supported by the Shenzhen Bureau of Health and the Shenzhen Bureau of Science, Technology, and Information. The Romania WMH study projects ‘Policies in Mental Health Area’ and ‘National Study regarding Mental Health and Services Use’ were carried out by National School of Public Health & Health Services Management (former National Institute for Research & Development in Health, present National School of Public Health Management & Professional Development, Bucharest), with technical support of Metro Media Transilvania, the National Institute of Statistics – National Centre for Training in Statistics, SC. Cheyenne Services SRL, Statistics Netherlands and were funded by Ministry of Public Health (former Ministry of Health) with supplemental support of Eli Lilly Romania SRL. The US National Comorbidity Survey Replication (NCS-R) is supported by the National Institute of Mental Health (NIMH; U01-MH60220) with supplemental support from the National Institute of Drug Abuse (NIDA), the Substance Abuse and Mental Health Services Administration (SAMHSA), the Robert Wood Johnson Foundation (RWJF; Grant 044708), and the John W. Alden Trust. Dr McGrath received John Cade Fellowship APP1056929 from the Australian National Health and Medical Research Council and Niels Bohr Professorship from the Danish National Research Foundation.
A complete list of all within-country and cross-national WMH publications can be found at http://www.hcp.med.harvard.edu/wmh/.
Conflict of interest
Competing interests: In the past 3 years, Dr Kessler received support for his epidemiological studies from Sanofi Aventis; was a consultant for Johnson & Johnson Wellness and Prevention, Shire, Takeda; and served on an advisory board for the Johnson & Johnson Services Inc. Lake Nona Life Project. Kessler is a co-owner of DataStat, Inc., a market research firm that carries out healthcare research.
Role of the funder/sponsor
The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.





