Patients with postural tachycardia syndrome characteristically have problems maintaining an upright position in the face of a gravitational challenge. Orthostatic symptoms of postural tachycardia syndrome may occur after a postural change from the supine to an upright position. The diagnostic criterion in children is orthostatic symptom with an increment ⩾40 beats/minute (bpm) (or a heart rate that exceeds 125 bpm) within 10 minutes of starting a standing test or a head-up tilt test.Reference Freeman, Wieling and Axelrod 1 , Reference Singer, Sletten and Opfer-Gehrking 2 Symptoms such as dizziness, headache, palpitations, weakness, tremulousness, nausea, or even syncope can be found in these children.Reference Li, Zhang and Hao 3 , Reference Stewart 4 Postural tachycardia syndrome is increasingly recognised in children and adolescents. A negative influence on physical and emotional well-being has been reported.Reference Li, Deng and Zang 5 Even daily activities may exacerbate symptoms.
The pathophysiological mechanisms for postural tachycardia syndrome are not completely understood. Several mechanisms have been proposed including peripheral denervation, β-receptor hypersensitivity, hypovolaemia, and impaired cerebral autoregulation.Reference Mar and Raj 6 The purpose of this manuscript was to review recent advances in our understanding of the pathophysiological mechanisms underlying postural tachycardia syndrome in children.
Volume dysregulation
In healthy children and adolescents with normal vascular structure, intact vasomotor sensors, muscle pump ability, sufficient blood volume, and oxygen-carrying capacity, “standing up”-related reduced blood volume is usually well tolerated.Reference Streeten, Anderson and Richardson 7 , Reference Chen, Li and Todd 8 Circulatory deficits in postural tachycardia syndrome patients were first reported by MacLean and Allen,Reference Maclean and Allen 9 and evidences for excessive venous pooling and anatomical or functional abnormalities of lower limb veins in postural tachycardia syndrome children were reported by Tanaka et al.Reference Tanaka, Yamaguchi and Matushima 10 During orthostasis, low 24-hour urinary sodium excretion may contribute to a change in blood volume.Reference Chen, Du and Wang 11 , Reference Qingyou, Ying and Chaoshu 12 Postural tachycardia syndrome patients were divided into three groups according to the pattern of blood flow measured in their lower extremities: low, high, and normal flow.
Low flow
In this group, increased peripheral resistance and reduced cardiac output were observed.Reference Sheldon, Ii and Olshansky 13 Typical signs and symptoms include paleness, acrocyanosis, and cool extremities. These symptoms increase when patients stand up.Reference Stewart and Weldon 14 Decreased peripheral blood flow and increased angiotensin II in low-flow postural tachycardia syndrome patients occur as a consequence of angiotensin II/angiotensin II type-1 binding and superoxide radical formation. This interaction leads to an important vasoconstrictive response through a reduction in bioavailable nitric oxide.Reference Gamboa, Okamoto and Raj 15 Frequencies of nitric oxide synthase-related genotypes (786CC and 298DD) were significantly lower in postural tachycardia syndrome patients than in control subjects.Reference Garland, Robert and Williams 16
High flow
While standing up, this group of postural tachycardia syndrome children has decreased total peripheral resistance, which increases blood volume in lower extremities.Reference Zhang, Du and Qinglin 17 Local oedema is frequently observed, whereas acrocyanosis is rare.Reference Zhang, Du and Qinglin 17 Hyperkinetic circulation is an essential feature of this group;Reference Pianosi, Goodloe and Soma 18 moreover, symptoms are frequently exacerbated by a viral infection, suggesting that autoimmunity may play a role in this situation.
Normal flow
Splanchnic pooling is the main vascular change in normal-flow postural tachycardia syndrome children. Failure of venous and arterial constriction to increase splanchnic vascular compliance has been proposed as a causal mechanism.
Abnormal vascular endothelial function
Relative hypovolaemia can occur in children with postural tachycardia syndrome because of venous pooling, capillary leakage, and impaired vascular function. Postural tachycardia syndrome patients have an abnormal response to hypovolaemia, which can be ascribed to renin–angiotensin–aldosterone axis abnormalities.Reference Yozgat, Karadeniz and Ozdemir 19
Angiotensin II
Angiotensin II in postural tachycardia syndrome children was significantly higher than that of healthy subjects (105±50 versus 84±28 ng/L, p=0.041), whereas impaired plasma renin and aldosterone responses were found in postural tachycardia syndrome children.Reference Li, Du and Zhang 20 The so-called renin–aldosterone paradox could be a response to direct hormonal effect, low blood flow of the juxtaglomerular apparatus, sensor problem of the macula densa, or missing transmission of signal to the juxtaglomerular apparatus.Reference Seiji, Hidetaka and Ryota 21
Angiotensin-converting enzyme 2
Angiotensin 1–7 can be formed from angiotensin II through the angiotensin-converting enzyme. In postural tachycardia syndrome patients, high levels of angiotensin II did not cause any increase in angiotensin 1–7, suggesting the possibility of decreased activity of angiotensin-converting enzyme 2.Reference Mustafa, Garland and Italo 22
Nitric oxide dysfunction
Nitric oxide is well recognised for its function in endothelial development. Liao confirmed that flow-dependent nitric oxide release was reduced in some children with postural tachycardia syndrome, suggesting an abnormal endothelial function.Reference Ying, Stella and Xueqin 23
Plasma urotensin II and plasma intermedin
Low levels of urotensin II and plasma intermedin in postural tachycardia syndrome children leading to impaired vasoconstrictive response may play a role in the pathogenesis of postural tachycardia syndrome.Reference Liao, Jun bao and Tang 24 , Reference Li, Liao and Han 25
Neuropathy
Index of valsalva manoeuver, heart rate change with deep respiration, and QT interval dispersion can be evidences for impaired autonomic nervous function in postural tachycardia syndrome children and adolescents.Reference Zhu, Zhang and Zhang 26 Postural tachycardia syndrome may be a mild form of autonomic neuropathy caused by a preceding infection or vaccination history.Reference Blitshteyn 27 This was confirmed in different studies. Auto-antibodies against cardiac lipid raft-associated proteins were found in postural tachycardia syndrome patients;Reference Wang, Ling and Charlesworth 28 moreover, the TT genotype (GNB3-C825T) encoding the G-proteinb3 subunit was more frequently found in postural tachycardia syndrome children.Reference Nakao, Tanaka and Takitani 29 α1-Adrenergic receptor activation, leading to vasoconstriction and concurrent β adrenergic receptor-mediated tachycardia, is present in autoimmune situations.Reference Hongliang, Xichun and Campbell 30 Acetylcholine acts as an important neurotransmitter in the autonomic nervous system.Reference Vernino, Lindstrom and Hopkins 31 Antibodies for acetylcholine receptor were positive in 24.39% of postural tachycardia syndrome children, and their symptoms were significantly more severe than in patients who were acetylcholine receptor antibodies negative. This may be caused by broken immune tolerance, abnormal immune reaction, or sensitised acetylcholine receptors of thymus cells.Reference Li, Zhang and Liao 32
Hyperadrenergic status
Several studies have shown that postural tachycardia syndrome children and healthy subjects have similar levels of plasmatic norepinephrine and epinephrine at rest, whereas orthostatic plasma norepinephrine levels are different.Reference Zhang, Xia and Li 33 Dizziness, headache, fatigue, and tremulousness were frequent in hyperadrenergic postural tachycardia syndrome children and adolescents.Reference Qingyou, Xia and Jiawei 34 Pharmacological norepinephrine transporter blockade drugs produce postural tachycardia syndrome-like symptoms.Reference Christoph, Jens and Michael 35 Increased synaptic norepinephrine concentrations can be observed in norepinephrine transporter deficiency families. Norepinephrine transporter deficient mice exhibited elevated blood pressure and increasing tachycardia when engaged in wakeful activities.Reference Keller, André and Martin 36 Ala457Pros, which can cause elevated heart rate and plasma norepinephrine transporter levels in the supine position, is the result of a mutation in the norepinephrine transporter SLC6A2. In the analysis of SLC6A2 gene polymorphisms, the norepinephrine transporter dysfunction of postural tachycardia syndrome children is still unexplained.Reference Ivancsits, Heider and Rudiger 37 In 2012, Bayles described that epigenetic modification of this gene could decrease expression of the norepinephrine transporter protein, thus constituting a mechanism of postural tachycardia syndrome.Reference Bayles, Harikrishnan and Lambert 38 On the other hand, as excessive tachycardia is a main feature, treatment with β-adrenergic blockers is used. There is a large variation in the level of β-blockage efficiency, suggesting the existence of hyperadrenergic subgroups.Reference Zhang, Xia and Li 33
Muscle pump defects
Against orthostatic challenge, muscle blood flow increases, and contraction of leg and gluteal muscles drive venous blood back to the heart, so that arterial pressure can be maintained in healthy children.Reference Tschakovsky, Sujirattanawimol and Ruble 39 Some researchers have stressed the importance of muscle pump activity in postural tachycardia syndrome patients. Decreased calf circumference, calf ejection fraction, and calf venous capacity may play a role in this type.Reference Stewart, Medew and Montgomery 40 Some scholars take the presence of venous valves that are incomplete or congenitally absent into consideration.
Conclusions
In recent years, an in-depth understanding has accumulated on the underlying mechanisms of postural tachycardia syndrome in children. These can be divided into five categories: volume dyregulation, abnormal vascular endothelial function, neuropathy, hyperadrenergic state, and muscle pump defects. When deciding treatment strategies for patients with postural tachycardia syndrome, it is useful to try and understand which pathophysiological mechanism or combination of pathophysiological mechanisms are contributing to the symptoms (Table 1 and Fig 1).Reference Raj, Italo and Yamhure 41 – Reference Khalil, Bilal and Beverly 43 Non-pharmacological treatments should be recommended at first.Reference Khalil, Bilal and Beverly 43 If possible, healthcare providers should consider discontinuing medications that exacerbate the tachycardia. A regular structured exercise programme, oral rehydration saline, and a multidisciplinary approach with β-blockers or midodrine should be selected, depending on the pathophysiological status of the patients (Fig 1).Reference Khalil, Bilal and Beverly 43 Common mechanisms such as volume dysregulation, disturbed vascular endothelial function, abnormal vasodilation, and hyperadrenergic state require further research; moreover, more research is necessary to fully understand autoimmune and inflammation mechanisms to facilitate treatment options. It is unclear whether there are postural tachycardia syndrome patients who might have mixed underlying mechanisms or subtypes in various proportions, which would be worthy of investigation.
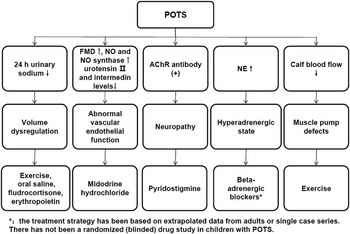
Figure 1 Laboratory investigation, pathophysiology and therapeutic options for postural tachycardia syndrome in children. AChR, acetylcholine receptor; POTS, postural tachycardia syndrome.
Table 1 Mechanisms for postural tachycardia syndrome and related symptoms and laboratory findings.

Acknowledgement
None.
Financial Support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflicts of Interest
None.





