Introduction
Spilonema paradoxum was described by Bornet (Reference Bornet1856) as a new genus and species to accommodate an enigmatic, thread-like, caespitose lichen occurring on granite in the mountains around Cannes in the south of France. In a lengthy discussion of the thalline and ascomatal morphology of the species, Bornet drew comparisons to the cyanobacterial genus Stigonema which was later revealed to be its photobiont, as well as the similarity to the lichen genus Ephebe, described thirty years prior by Fries (Reference Fries1825). The new species did not fit into either of these groups, nor Collema or Leptogium, as Bornet noted. Spilonema was later expanded to include a second species, S. revertens, by Nylander (Reference Nylander1865) and then two species from the Asia-Pacific region almost a century later (Henssen Reference Henssen1963). In the latter work, the genus was placed in the Peltigeralean family Coccocarpiaceae, a placement later expounded in more depth based on anatomical studies of the ascomata (Henssen & Jahns Reference Henssen and Jahns1973; Keuck Reference Keuck1977; Henssen et al. Reference Henssen, Keuck, Renner, Vobis and Reynolds1981). The family Coccocarpiaceae was, however, not nomenclaturally validated until later (Eriksson & Hawksworth Reference Eriksson and Hawksworth1986: 314).
The family Coccocarpiaceae, as defined by Henssen et al. (Reference Henssen, Keuck, Renner, Vobis and Reynolds1981), occupies a special position among Lecanoromycetidae as a hot spot of body plan innovation and photobiont affinity. Relatively few genera are assigned to Coccocarpiaceae, but those that are could hardly appear more different in gross morphology: a genus with a ‘classical’ dorsiventral, longitudinal, broadly attached body plan (Coccocarpia), a dorsiventral, umbilicate body plan (Peltularia), and two genera with thread-like thalli that form cushions (Spilonemella and Spilonema). What is more, these four genera as currently circumscribed include species that associate with photobionts deriving from four different cyanobacterial families: Nostoc (Nostocaceae, Nostocales; in Peltularia crassa), Scytonema (Scytonemataceae, Nostocales; in Coccocarpia, Spilonemella), Stigonema (Stigonemataceae, Stigonematales; in Spilonema) and Hyphomorpha (Loriellaceae, Stigonematales; in Spilonema), the last being the only known case of its occurrence in lichens (Henssen Reference Henssen1981). The genera assigned here also span the widest possible macroclimatic gradient, from polar permafrost soils to tropical rainforests. (A fifth lichen genus previously assigned to Coccocarpiaceae, Steinera, associated in part with Nostoc and Scytonema, has since been shown not to belong to Coccocarpiaceae; Spribille & Muggia Reference Spribille and Muggia2013).
Recent molecular studies have not always lent support to past classifications of fungal genera with similar body plans but different photobionts. An example of this is Polychidium, treated in the same classical work by Henssen (Reference Henssen1963) as uniting two groups of species that associated with Nostoc and Scytonema, respectively. Muggia et al. (Reference Muggia, Nelson, Wheeler, Yakovchenko, Tønsberg and Spribille2011) found in a multilocus phylogeny that the two groups are in fact only distantly related and achieved their dendroid thallus architecture through body plan convergence. This, and the finding that ascomatal ontogeny is not always a reliable predictor of relatedness, cast doubt on the circumscription of Coccocarpiaceae and the position of one of its key genera, Spilonema. Spilonema is furthermore the only genus of Peltigerales, and one of only a few Lecanoromycetes, to include species lichenized with Stigonema (illustrated in detail by Henssen Reference Henssen1963). Spilonema has not heretofore been sampled in a molecular phylogeny and fresh material can be challenging to acquire, especially in central Europe, where most collections are historical.
The present paper is the third in a series in which we test classical evolutionary hypotheses within the Peltigerales based on new molecular data, with special emphasis on small, often overlooked and poorly sampled species. In the present case, we assess three of the four described members of Spilonema, including the rare Hyphomorpha-associated species S. dendroides, as well as their relationship to the Pacific Rim endemic genus Spilonemella (Muggia et al. Reference Muggia, Nelson, Wheeler, Yakovchenko, Tønsberg and Spribille2011). The phylogenetic relationships of these species to each other, and in the context of the Peltigerales, can be expected to inform views on photobiont and body plan diversity in Coccocarpiaceae and lay the groundwork for hypothesis testing in character evolution.
Materials and Methods
Taxon sampling
We sampled a total of 51 taxa representing all ten recognized families of the Peltigerales according to recent studies (Wedin et al. Reference Wedin, Jørgensen and Wiklund2007, Reference Wedin, Wiklund, Jørgensen and Ekman2009, Reference Wedin, Jørgensen and Ekman2011; Muggia et al. Reference Muggia, Nelson, Wheeler, Yakovchenko, Tønsberg and Spribille2011; Spribille & Muggia Reference Spribille and Muggia2013). We acquired sequences for a total of 14 new isolates including one of S. paradoxum, six of S. revertens s. str., six of S. dendroides and one of what appears to be an undescribed taxon from British Columbia, Canada. The material studied is in the herbaria cited, following abbreviations used in Index Herbariorum.
DNA extraction, amplification and sequencing
DNA was extracted according to Cubero et al. (Reference Cubero, Crespo, Fatehi and Bridge1999). The phylogenetic affiliation of the lichen mycobionts was studied with sequences of the nuclear 28S, partial nuclear 18S and mitochondrial 12S ribosomal subunits (hereafter 28S, 18S and mitochondrial 12S). The 28S fragment was obtained in two pieces using primers ITS1F (Gardes & Bruns Reference Gardes and Bruns1993) and LR5 for the first half, and LR7 (Vilgalys & Hester Reference Vilgalys and Hester1990) and LR3R for the second (http://www.biology.duke.edu/fungi/mycolab/primers.htm). 18S was amplified using nuSSU0072 and nuSSU0852 (Gargas & Taylor Reference Gargas and Taylor1992), and mitochondrial 12S was obtained with mtSSU1 and mtSSU3R (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999). PCR conditions were as in previous studies (Muggia et al. Reference Muggia, Gueidan and Grube2010, Reference Muggia, Nelson, Wheeler, Yakovchenko, Tønsberg and Spribille2011). Complementary strands were sequenced by Macrogen Inc. (Korea) and were subjected to a blastn query against NCBl non-redundant nucleotide database (nr/nt) to confirm sequence similarity to Peltigerales and rule out association with other fungal groups such as Lichinomycetes and Arctomiaceae (Ostropomycetidae), species of which in the latter case have been misclassified with Peltigerales in the past (e.g., Otálora & Wedin Reference Otálora and Wedin2013). Base calls were proof-read and sequence alignments prepared in BioEdit (Hall Reference Hall1999).
Alignment and phylogenetic analysis
For a number of specimens we were unable to generate the sequences of one of the three loci, and in other instances single sequences were unavailable from GenBank. We examined the heterogeneity in phylogenetic signal between the different genetic markers (Buckley et al. Reference Buckley, Arensburger and Chambers2002). Using both Bayesian and Maximum Likelihood (ML) approaches, we first analyzed each locus separately and subsequently combined them in a multilocus alignment, as performed in previous studies (Kauff & Lutzoni Reference Kauff and Lutzoni2002; Miądlikowska et al. Reference Miądlikowska, Kauff, Hofstetter, Fraker, Grube, Hafellner, Reeb, Hodkinson, Kukwa and Lücking2006). The combined data set was used to infer the phylogenetic relationships of the taxa selected using both Bayesian and ML approaches. The optimal nucleotide substitution model was estimated with the program MrModeltest v3.7 for each locus individually. The Bayesian Markov Chain Monte Carlo (B/MCMC) algorithm of MrBayes 3.1.2 (Huelsenbeck & Ronquist Reference Huelsenbeck and Ronquist2003; Ronquist et al. Reference Ronquist, Huelsenbeck and van der Mark2005) was performed with the General Time Reversible substitution model (Rodriguez et al. Reference Rodriguez, Oliver, Marin and Medina1990), with estimation of invariant sites and assuming a gamma distribution with four categories (GTR+I+Γ). The Bayesian algorithm ran with six chains simultaneously, each starting from a random tree, for 10 million generations, and trees were sampled every 100th generation for a total sample of 100 000 trees. A burn-in sample of 500 000 generations (50 001 trees) was discarded for each run and a majority rule consensus tree calculated for the remaining 50 000 trees. The burn-in period was determined after testing for stationarity of likelihood values (Ronquist et al. Reference Ronquist, Huelsenbeck and van der Mark2005) by plotting log-likelihood scores against generation time using Tracer 1.4 (Rambaut & Drummond Reference Rambaut and Drummond2007). The program RAxML 7.0.4 (Stamatakis et al. Reference Stamatakis, Ludwig and Meier2005) was used for ML analyses and estimation of bootstrap support. The ML analyses in RAxML were performed with a GTRMIX substitution model and 1000 bootstrap replicates. Gene partitions were applied in both Bayesian and ML analyses in the three-locus data sets. The phylogenetic tree graphics were produced with TreeView (Page Reference Page1996).
Morphological analysis
We studied thallus morphology using a Leica Wild M3Z dissecting microscope. Ascus type and other microanatomical features were studied using a Zeiss Axioskop light microscope at ×1000 magnification. Photographs were taken with a ZeissAxioCam MRc5 digital camera and accompanying software (Axiovision, Axio VS40, Zeiss); images of growth habit were digitally optimized using CombineZM open source image processing software (CombineZM, www.hadleyweb.pwp.blueyonder.co.uk/CZM/).
Scanning electron microscopy
Air-dried thalli were fixed on aluminium stubs with carbon-impregnated, double-sided tape and studied with a scanning electron microscope (XL30 ESEM, FEI). We either investigated samples directly without any further preparation using the low vacuum mode and detection of backscattered electrons (Stabentheiner et al. Reference Stabentheiner, Zankel and Pölt2010), or sputter-coated them with gold (AGAR Sputter Coater) and studied them in high vacuum mode using secondary electron detection.
Results
We obtained a total of 30 new sequences for the three target loci for 14 taxa. When combined with previously sequenced taxa, this yielded an alignment of 4·8 kb of sequence and 65 taxa. Burn-in was reached after <1 000 000 generations in the Bayesian analysis, and the average standard deviation of splits with a frequency of at least 0·1 was 0·012565. The same topology was recovered in both the Bayesian and ML analyses of the combined loci.
Newly acquired sequences of Spilonema were recovered in different places in the order Peltigerales (Fig. 1). The type species, S. paradoxum, forms a strongly supported monophyletic group with the type species of Spilonemella, S. americanum, and is nested between samples of Spilonema revertens and a possibly undescribed Spilonema species from British Columbia, Canada. Together, sequences from these isolates form a strongly supported monophyletic group in Coccocarpiaceae. Likewise, all samples of S. dendroides form a strongly supported monophyletic group but, in contrast to the other Spilonema species, they are recovered within an unresolved Peltigerineae as poorly supported sister to Koerberiaceae.

Fig. 1. Multigene phylogenetic reconstruction of Peltigerales showing position of Spilonema s. str. and Spilonema dendroides (=Erinacellus). ML analysis with branch length based on the combined 28S, 18S and mitochondrial 12S data sets (Table 1 and Table 2). Bootstrap support values >70% and Bayesian PP>95% are reported above the branches.
Table 1. Newly analyzed specimens included in the phylogenetic analysis. Dashes stand for absence of sequence data

Table 2. Previously sequenced specimens included in the phylogenetic analysis, with their species name and NCBI accessions. For sequences originally produced in Graz, isolate numbers are also given. Dashes stand for absence of sequence data

* outgroups
The inclusion of Spilonema species in the present phylogenetic analysis weakens the backbone support for the major nodes of Peltigerales (Collematineae and Peltigerineae) compared to previously published analyses (Spribille & Muggia Reference Spribille and Muggia2013), and this led us to try to isolate the cause of this. Experimentally deleting S. dendroides from the alignment and re-running the concatenated data set including Spilonema s. str. restored the support for high level groupings to nearly the same levels as observed in previous studies. We accordingly analyzed the data set for intralocus signal conflict by performing both ML and Bayesian phylogenetic reconstructions of single locus data sets. Both the 28S and mitochondrial 12S data sets produced topologies broadly similar to the results of the three loci when concatenated (Fig. 2). The placement of Koerberiaceae changed in the 28S data compared to the concatenated analysis, and recovery of numerous families in 18S data, in particular, differed from that in the concatenated data set but these relationships were not supported. The position of S. dendroides was perhaps the most disparate between 28S and mitochondrial 12S: in the 28S data set, S. dendroides was recovered as sister to Pannariaceae, while in the mitochondrial 12S data set it came out as sister to Koerberiaceae, though again in both cases lacked support (Fig. 2).

Fig. 2. Single locus phylogenetic trees of Peltigerales, with terminal clades collapsed to the family level to highlight position of Erinacellus in different loci. ML analyses individually based on 28S, 18S and mitochondrial 12S loci. Bootstrap support values (>70%) are reported above branches.
Discussion
Our assessment of Spilonema paints a diverging picture of the phylogenetic position of the species studied. The core species of Spilonema, including S. paradoxum (the type species) and S. revertens, are paraphyletic to Spilonemella, a genus that differs from Spilonema in photobiont, cortex and ascomatal development (Henssen & Tønsberg Reference Henssen and Tønsberg2000). Spilonema paradoxum, for which we have only a single mitochondrial 12S sequence, is however so close to Spilonemella as to form a strongly supported sister group relationship, with S. revertens more distantly related. Taken together, Spilonema and Spilonemella form a strongly supported monophyletic group. While we did not test the hypothesis using an ancestral state reconstruction, it is possible that the switch to Scytonema as a photobiont in Spilonemella americana is no more than a reversion to the ancestral state in Coccocarpia; this is in fact likely owing to the absence of Stigonema as a photobiont throughout the rest of the known Peltigerales. Finally, at the next higher level, the close relationship of the morphologically disparate genera Spilonema and Coccocarpia, first postulated by Henssen (Reference Henssen1963) based on ascomatal ontogeny alone, is resoundingly confirmed by our analysis.
The relationships of Spilonema dendroides, by contrast, are rather more problematic. In the three-locus analysis, S. dendroides comes out with weak support in the Peltigerineae. Interestingly, the inclusion of S. dendroides in the phylogeny of the Peltigerales affects two of the main upper nodes that have been recovered as strongly supported in previous studies. The higher level relationships within Collematineae (mostly involving Collemataceae / Placynthiaceae on the one hand, and Pannariaceae on the other) are weakened by its inclusion, as is the monophyly of the Collematineae itself. In the Peltigerineae, which were recovered as monophyletic in previous analyses, the sister group relationship of Koerberiaceae to the remaining families (Vahliellaceae, Massalongiaceae, Peltigeraceae, Nephromataceae and Lobariaceae), supported in a previous Bayesian analysis (Spribille & Muggia Reference Spribille and Muggia2013), is compromised with the inclusion of our new sequence data. Interestingly, these backbone-weakening effects are reversed if S. dendroides is selectively deleted from the data set and the phylogeny is run with the remaining taxa and Spilonema s. str., using exactly the same parameters as the whole data set (data not shown).
The underlying reason for the ambiguity in placement of S. dendroides is not yet clear. There is broad concordance between the 28S and mitochondrial 12S topologies and it is easy to see how combination of the data sets leads to robust support for, for example, the monophyly of the Vahliellaceae / Peltigeraceae / Massalongiaceae / Nephromataceae / Lobariaceae clade (the core of Peltigerineae) as well as the Collemataceae / Placynthiaceae / Coccocarpiaceae / Pannariaceae clade (the core of Collematineae). These relationships have been recovered repeatedly in numerous studies, including those using additional loci (Spribille & Muggia Reference Spribille and Muggia2013). The most uncertainty appears to be associated with the sister group relationships of Koerberiaceae and S. dendroides, respectively. Although the position of S. dendroides in the single locus phylogenies is not supported in any locus, one hypothetical explanation for its ambiguous placement in the combined analysis could be an underlying incongruence between the S. dendroides 28S and mitochondrial 12S gene genealogies and those of other sampled Peltigerales. The poor resolution (and almost complete lack of topological support) achieved in 18S extends so far that Lecideaceae and Cladia are not separated from Peltigerales. That 18S has the lowest level of polymorphisms of the three loci studied probably does not play a major role in the overall topological instability of the concatenated data set. However, the single locus analysis does suggest that 18S, which has been used in phylogenetic analyses of Peltigerales by Miądlikowska & Lutzoni (Reference Miądlikowska and Lutzoni2004), Muggia et al. (Reference Muggia, Nelson, Wheeler, Yakovchenko, Tønsberg and Spribille2011) and Spribille & Muggia (Reference Spribille and Muggia2013), may not be especially informative for elucidating phylogenetic relationships in this order.
The evolutionary relationships of Spilonema dendroides are not likely to be elucidated without analysis of additional loci. That said, none of the sequence reads obtained from multiple individuals and not one of our phylogenetic reconstructions place it close to Spilonema s. str., and it can safely be excluded from that genus. It is not clear why S. dendroides should have diverging nuclear and mitochondrial sequence data compared to the rest of Peltigerales. The co-occurrence of motifs from both Peltigerineae and Collematineae may suggest the divergence of S. dendroides from a pan-Peltigeralean ancestor around the time of divergence of Peltigerineae and Collematineae; the association of S. dendroides with the rare photobiont Hyphomorpha, otherwise lacking in Peltigerales, may be further evidence of evolutionary isolation. This is the most striking case of locus incongruence known to us from several different taxon and locus samplings within the Peltigerales.
Even after our phylogenetic analysis, Coccocarpiaceae continue to represent a concentration of body plan and photobiont diversity with few parallels in Lecanoromycetidae, with a range from dorsiventral to minutely branched caespitose thalli and with three cyanobacterial families represented as photobionts. The reason for evolution of thread-like filaments and cushions, and the path by which this body plan evolves from foliose ancestors in Polychidium, Leptogidium (Muggia et al. Reference Muggia, Nelson, Wheeler, Yakovchenko, Tønsberg and Spribille2011) and likely Spilonema, is not known. The increase in surface area in a filamentose body plan compared to that of a foliose lichen may allow increased control over the wetting-drying process (e.g., Kershaw Reference Kershaw1985), probably of importance to species that, like Spilonema, grow in close association with bryophytes in rainforests and on seepage tracks. The frequency of photobiont and body plan transitions within the Coccocarpiaceae clade, compared to other clades of Lecanoromycetes where this is virtually unheard-of, suggests a lineage-specific relaxation of body plan conservatism in Coccocarpiaceae. This could be a promising target for evolutionary developmental studies in lichen symbiotic interactions.
The full range of photobiont and body plan diversity in Coccocarpiaceae is still not known. In particular, the subantarctic genus Peltularia has yet to be sampled for a molecular phylogeny and fresh material is not available (D. Galloway, pers. comm.). Further sequence diversity can be anticipated within Coccocarpia and more, yet unsampled genera may still be recognized as belonging to Coccocarpiaceae.
It is clear from sequence motifs already at the DNA alignment stage that Spilonema dendroides cannot be closely related to Coccocarpiaceae. In addition, it differs from all currently accepted species of Spilonema in its habit of producing secondary and tertiary branching, the differentiated coloration of light primary ‘trunks’ and dark outer branches (in S. dendroides), and its photobiont Hyphomorpha (Henssen Reference Henssen1981). The dense, caespitose habit is grossly similar to Spilonema revertens, but in that species the cushion interior is comprised of a dense pillow of ‘rhizines’ giving rise to a continuously regenerating outer layer of lichenized primary branches; a dendroid growth habit as in Fig. 3 is never achieved. Spilonema dendroides and the likely closely related S. schmidtii cannot be accommodated in Spilonema even in the broadest sense, and accordingly we propose here the establishment of a new genus for these species.

Fig. 3. Erinacellus dendroides. A, habit, on a branch of Pinus contorta subsp. contorta, Glacier Bay National Park, Alaska, July 2012; B, habit of dried, broken-open cushion in environmental SEM; C, branching (in H2O, light microscope); D & E, architecture and surficial properties (dry in SEM following gold sputtering). B–E from Spribille 36301 (GZU). Scales: B=500 µm; C, D & E=50 µm. In colour online.
Taxonomy
Erinacellus T. Sprib., Muggia & Tønsberg gen. nov.
MycoBank No.: MB 803465
Ascomycetes cyanobacteriis generis Hyphomorpha lichenisati. Thallus pulvinatus, ramis filiformibus minutis suberectis compositus. Rami ipsi dichotome quasi isotome ramosi, ramis primariis pallide cinereis vel fuscis, et ramis secundariis tertiariisque obscure fuscis. Hyphae circum filamentos photobionti vaginas continuas sed non corticem cellulosum formantes. Ascomata matura et pycnidia adhuc ignota.
Typus generis: Erinacellus dendroides (Henssen) T. Sprib., Muggia & Tønsberg.
Ascomycetes lichenized with the cyanobacterial genus Hyphomorpha. Thallus comprised of a dense cushion of erect, thread-like branches, differentiated into primary branches, which are light grey or dark brown, and secondary and tertiary branches which are dark brown. Terminal branching nearly isotomic dichotomous. Fungal hyphae enclosing photobiont in continuous sheath, the sheathing fungal cells rectangular. Ripe ascomata and pycnidia unknown.
Etymology
Diminutive of Erinaceus, the genus of Eurasian hedgehogs; from a fancied resemblance to the dark, cushion-forming thalli.
The relationships of Erinacellus within the Peltigerales are unclear. Multiple Bayesian and maximum likelihood analyses with different combinations of taxa have recovered it in the Peltigerineae as sister to the Koerberiaceae, or unresolved in a polytomy with Koerberiaceae and the clade that includes Vahliellaceae, Massalongiaceae and Lobariaceae (data not shown). The consensus tree we use in Fig. 1 shows it sister to Koerberiaceae but with low support and, as noted above, its inclusion affects the relationship of Koerberiaceae to the rest of the Peltigerineae. We think the genus should be treated ad interim as Peltigerales incertae sedis. Acquisition of genetic material from the second Hyphomorpha-containing species (Spilonema schmidtii, here transferred to Erinacellus) may help clarify the evolutionary relationships.
Erinacellus dendroides (Henssen) T. Sprib., Muggia & Tønsberg comb. nov.
MycoBank No.: MB 803466
Spilonema dendroides Henssen, Symb. Bot. Upsal. 18(1): 97 (Reference Henssen1963); type: New Zealand, Stewart Island, 1927, Du Rietz (UPS—holotypus!).
(Fig. 3)
Erinacellus dendroides is known from only a few sites worldwide. In addition to New Zealand, the species was first reported from Alaska by Henssen (Reference Henssen1981) and from British Columbia by Brodo & Tønsberg (Reference Brodo and Tønsberg1994). It is locally common on shore pines (Pinus contorta subsp. contorta) in blanket bogs, locally known as muskegs, in south-eastern Alaska (T. Spribille, pers. obs.). This species was referred to as Spilonema sp. 1 by Goward (Reference Goward1999).
Erinacellus schmidtii (Vain.) T. Sprib., Muggia & Tønsberg comb. nov.
MycoBank No.: MB 803467
Leptodendriscum schmidtii Vain., Ann. Acad. Scient. Fenn. Ser. A 15: 34 (1920); type: “Siam” [=Thailand], Insel Koh Chang, 1900, Schmidt 24 (TUR, seen by Henssen Reference Henssen1963).
Erinacellus schmidtii is a palaeotropical species known from Thailand and Sri Lanka (Henssen Reference Henssen1981). Henssen considered it to differ from E. dendroides in the dark brown (as opposed to light silvery grey) base of its primary branches.
Spilonema Born.
Mémoires de la Société impériale des sciences naturelles de Cherbourg 4: 226 (1856) ; typus generis: Spilonema paradoxum Born., ibidem 4: 226 (1856).
Spilonemella Henssen & Tønsberg, Bryologist 103: 108 (Reference Henssen and Tønsberg2000), syn. nov.; typus generis: Spilonemella americana Henssen & Tønsberg, Bryologist 103: 113 (Reference Henssen and Tønsberg2000).
The two core species of Spilonema were described from Europe in the 19th century. The type species, S. paradoxum Born. (Fig. 4), is a loosely appressed species with sprawling branches and resembles the unrelated Ephebe (Lichinaceae). A detailed morphological analysis was provided by Henssen (Reference Henssen1963). The only other widely distributed species is S. revertens Nyl., a dense cushion-forming species (Fig. 5). A further species, termed here “Spilonema sp. 1” has been found in coastal British Columbia (Björk 19757, UBC; isolate L944 in Fig. 1). It was originally thought to belong to S. revertens, but was found to have distinct DNA sequences in our analysis. It is broadly similar to S. revertens but consists of minute cushions only a few millimetres across (Fig. 6F–H), smaller than typically seen in S. revertens. The terminal branches are shaped like studded clubs (Fig. 6G) but the material in most other respects appears similar to S. revertens. We include photographs of the sample here in the hope that more collections can be made and a morphological and ecological characterization is eventually developed for this species.

Fig. 4. Spilonema paradoxum (Henssen 22509, GZU). A & B, habit, A in environmental SEM, B SEM after sputter-coating in gold; C & D, anatomy of branches in H2O (light microscope); E, cortical fungal cells (arrow). Scales: A=200 µm; B=100 µm; C & D=50 µm; E=10 µm. In colour online.
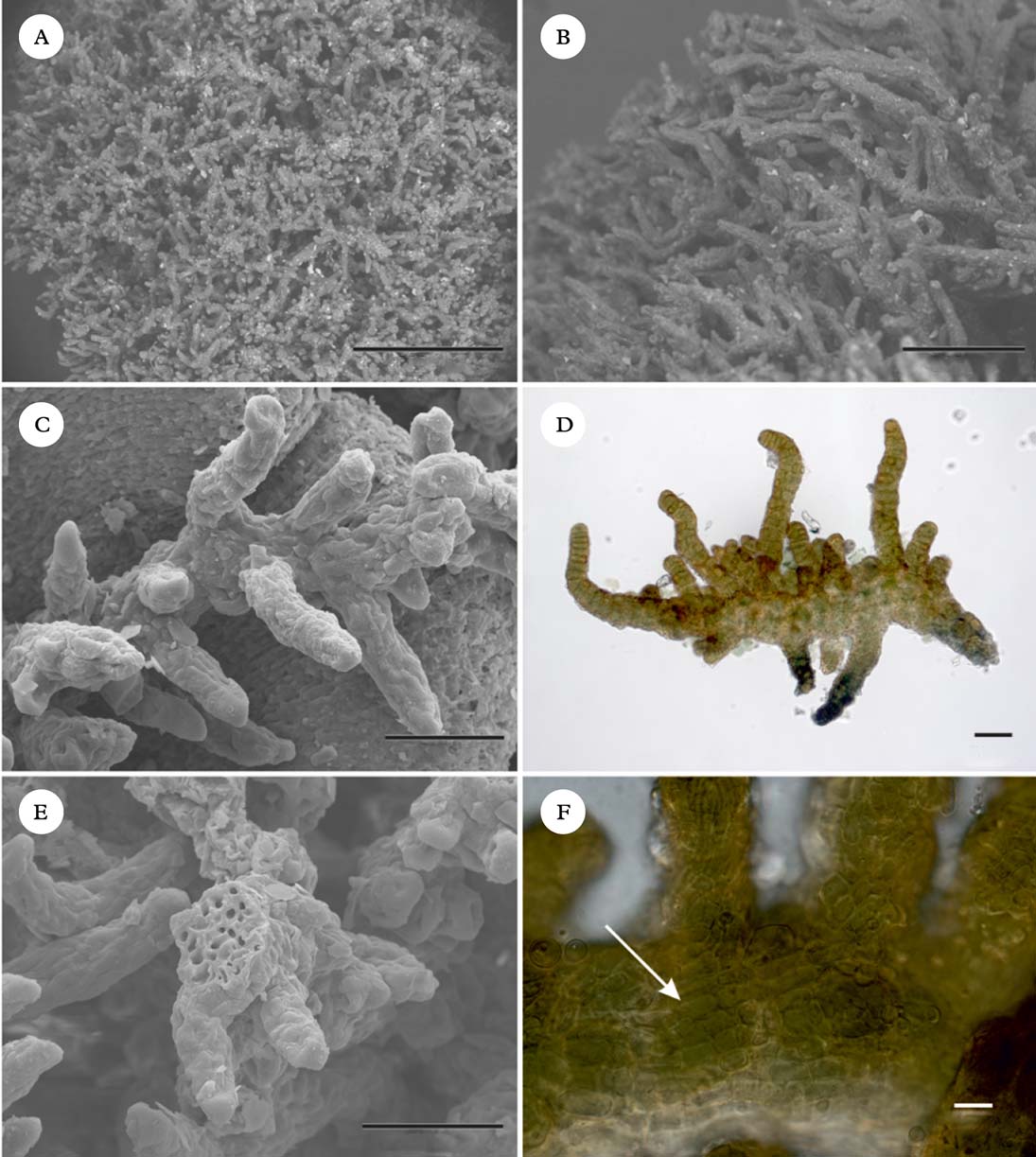
Fig. 5. Spilonema revertens (Spribille 27944, GZU). A–C, habit, dry in environmental SEM (A & B) and after gold sputtering (C); D, anatomy of branches in H2O (light microscope); E, architecture and broken branch in SEM; F, cortical fungal cells (arrow). Scales: A=500 µm; B=200 µm; C, D & E=50 µm; F=10 µm. In colour online.
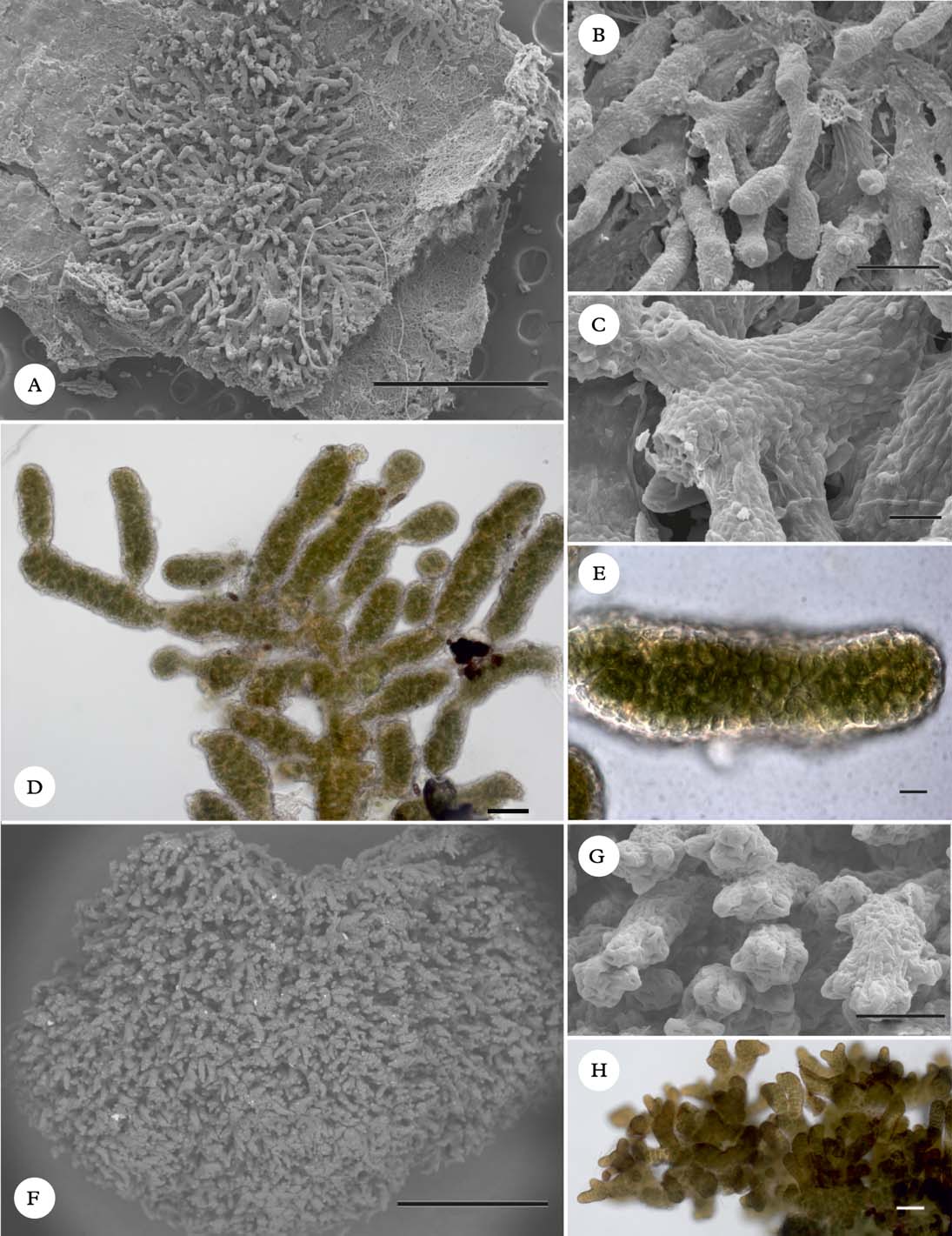
Fig. 6. A–E, Spilonema americanum (Spribille 27038, GZU). A–C, habit; D & E, cellular anatomy, in H2O (light microscope). F–H, Spilonema sp. 1 (Björk 19757, UBC). F & G, habit; H, branching pattern in H2O. All SEM images taken after sputter-coating. Scales: A=1000 µm; B=100 µm; C=20 µm; D, G & H=50 µm; E=10 µm; F=500 µm. In colour online.
Spilonema americanum (Henssen & Tønsberg) T. Sprib., Muggia & Tønsberg comb. nov.
MycoBank No.: MB 803468
Spilonemella americana Henssen & Tønsberg, Bryologist 103: 113 (Reference Henssen and Tønsberg2000); type: USA, Washington, Jefferson Co., SE of Hwy 101, 3·3 km (along road) S of Hoh River bridge, 47°47·6′N, 124°15·1′W, alt. 60 m, corticolous on trunk of Alnus rubra, 31 March 1998, T. Tønsberg 25758 [holotypus—BG; isotypi—FH, H (from hb. Henssen), TNS, WTU].
(Fig. 6)
A detailed anatomical study of this species was provided by Henssen & Tønsberg (Reference Henssen and Tønsberg2000).
Spilonema japonicum (Henssen & Tønsberg) T. Sprib., Muggia & Tønsberg comb. nov.
MycoBank No.: MB 803469
Spilonemella japonica Henssen & Tønsberg, Bryologist 103: 116 (Reference Henssen and Tønsberg2000); type: Japan, central Japan, [Honshu,] Prov. Sagami, Hakone, 1931, Sato [holotypus—TNS; isotypi—H (from hb. Henssen)].
We have not seen fresh material of Spilonema japonicum and thus cannot vouch with certainty for its phylogenetic position. However, it appears to be close to S. americanum and in any case the genus Spilonemella is no longer available to house it.
We thank Curtis Björk, Trevor Goward, Peter Nelson, Jay Scelza and Tim Wheeler for providing material for sequencing, and the curator of UPS for the loan of type material. Josef Hafellner translated the diagnosis into Latin. Support for laboratory work came from the University of Bergen, Norway. The management and staff of Klondike Gold Rush National Historical Park and Glacier Bay National Park and Preserve, Alaska, supported lichenological fieldwork during which Erinacellus was collected.










