Common arterial trunk – also known as truncus arteriosus – is a conotruncal anomaly consisting of a single great artery supplying both systemic and pulmonary arterial systems along with a subarterial ventricular septal defect. Surgical correction involves separation of the systemic and pulmonary arterial pathways and closure of the ventricular septal defect. Common arterial trunk was historically managed with pulmonary artery banding followed by delayed surgical repair, usually at 6 months of age. Results of delayed surgical repair were poor until the early 1980s when Ebert et alReference Ebert, Turley, Stanger, Hoffman, Heymann and Rudolph 1 described early surgical correction in infants <6 months of age with excellent results. In the late 1980s, Bove and Hanley described their experiences with neonatal repair of common arterial trunk with excellent results.Reference Bove, Lupinetti and Pridjian 2 , Reference Hanley, Heinemann and Jonas 3 Current surgical management today consists of early primary surgical repair, predominantly in the neonatal period.
Anatomy
Common arterial trunk is typically classified according to two principle classification systems (Fig 1). The Collett and Edwards system is based on a description of where the pulmonary arteries originate. Type 1 signifies a discrete main pulmonary artery segment that then bifurcates into a left and right pulmonary artery. In Type 2 truncus, the left and right pulmonary arteries arise as separate adjacent orifices from the posterior region of the truncus. In Type 3, the left and right pulmonary arteries arise as separate distant orifices from opposite sides of the truncus. Type 4 is no longer considered related to common arterial trunk and is considered part of the spectrum of pulmonary atresia with ventricular septal defect and aortopulmonary collaterals. The most common form of common arterial trunk appears to be somewhere between type 1 and 2, and therefore most surgeons will commonly call this a “type
![]() $$\[--><$>1 {1\over 2}$$$
”.
$$\[--><$>1 {1\over 2}$$$
”.
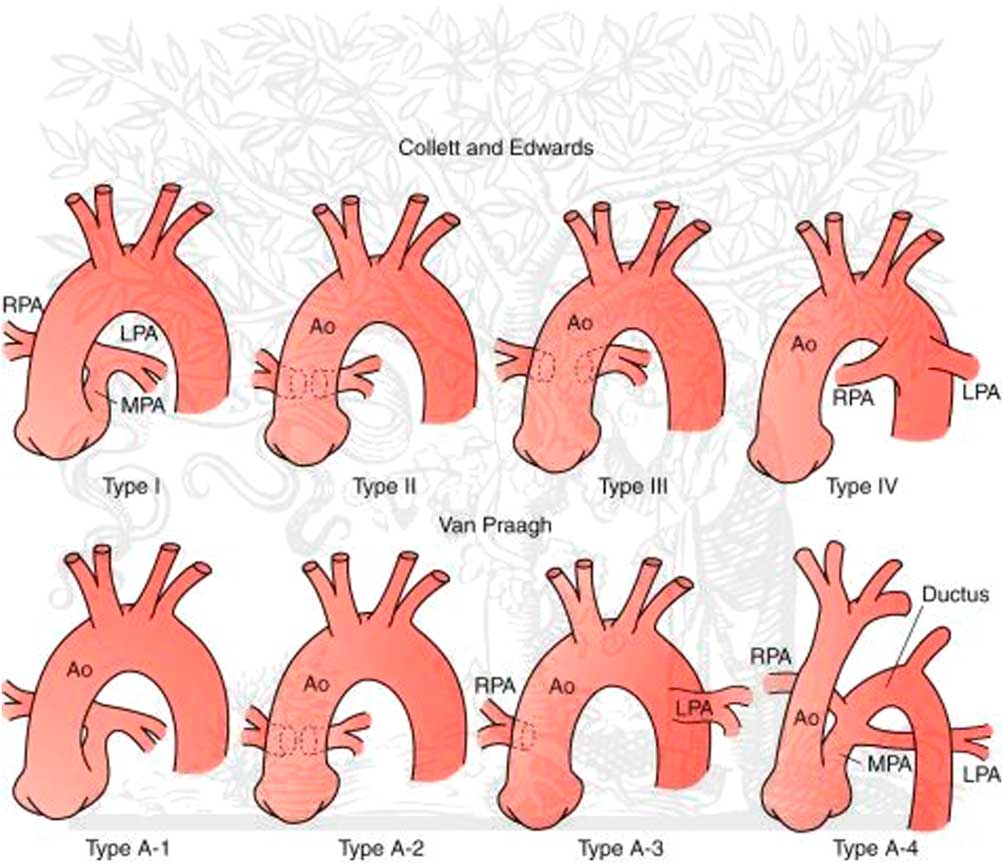
Figure 1 Classification scheme. Ao = aorta; LPA = left pulmonary artery; MPA = main pulmonary artery; RPA = right pulmonary artery.
The second major classification system is the Van Praagh system, and the first two types (A1 and A2) are essentially identical to the Collett and Edwards system, although the proximity of the left and right pulmonary orifices to each other are not specified in this system. The third type (A3) signifies ductal or aortic origin of one of the pulmonary arteries. In the fourth type (A4), the aorta is interrupted or hypoplastic. The Van Praagh system also specifies the presence (type A) or absence (type B) of a ventricular septal defect.
Pre-operative evaluation
Echocardiography is diagnostic. A complete description of the truncal valve should be made for a complete surgical pre-operative evaluation. A description of the number of leaflets, degree of dysplasia, and functional abnormalities such as degree of stenosis or regurgitation – if present – are necessary and significantly relevant for a complete surgical pre-operative evaluation. Cardiac catheterisation is rarely needed. If a child presents late beyond the newborn period, evaluation of the pulmonary vascular resistance may be necessary.
Surgical management (Supplementary material – video)
In general, surgery should be performed within the 1st week of life. Cardiopulmonary bypass is instituted via bicaval cannulation and moderate hypothermia. Snares should be tightened around the right and left pulmonary arteries as soon as cardiopulmonary bypass is started to prevent distal run-off into the pulmonary circulation, as well as potential left heart distention.
Cardioplegia is administered antegrade. We use a single-dose cardioplegia infusion (del Nido solution) that obviates the need for frequent redosing. In patients with significant truncal valve regurgitation, retrograde cardioplegia or direct infusion into the coronary ostia may be necessary. The heart is vented via the atrial septal defect following a limited right atriotomy.
We recommend complete transection of the truncus for separation of the pulmonary arteries. The left coronary ostium is often located in close proximity to the take-off of the pulmonary arteries and can be easily injured if not clearly delineated. Transection of the truncus also allows for a more symmetrical reconstruction to the ascending aorta, as there is often a significant size mismatch from the truncal root to the ascending aorta. Lastly, transection of the truncus allows for significantly improved exposure for the distal conduit anastomosis to the pulmonary arteries, and we recommend performing this anastomosis before reconstitution of the truncal aortic anastomosis.
Once the pulmonary arteries are safely separated from the truncus, the aorta is left transected, as described above, and attention is turned to making the ventriculotomy. It is important that the site of the ventriculotomy be carefully chosen as this ultimately dictates how the right ventricle to pulmonary artery conduit will lie within the mediastinum. We recommend choosing a site on the right ventricular surface that allows for the conduit to eventually lie off to the side under the left hemi-sternum and not directly in the midline, where it will be at risk for future sternal re-entries. An oblique ventriculotomy incision is thus created, angling leftward. The size of the ventriculotomy should be close to the size of the homograft that will be used for the right ventricle to pulmonary artery conduit.
Once the ventriculotomy is made, the necessary length of the right ventricle to pulmonary artery conduit can be assessed. A pulmonary valve homograft is selected for the right ventricle to pulmonary artery reconstruction. Using a Hagar dilator to estimate how much space the conduit will take up in the mediastinum can be helpful in choosing the proper size for the conduit. In general, a conduit with a Z score of +2 for the child's estimated pulmonary valve size is appropriate. The conduit is cut to the desired length and the distal anastomosis of the conduit to the pulmonary artery is performed first. The initial construction of the distal anastomosis is made easier because the truncus is still separated, allowing for excellent exposure of the pulmonary artery bifurcation. Once the distal anastomosis is complete, the truncal root to aortic anastomosis is completed.
Attention is returned back to the ventriculotomy and closure of the ventricular septal defect. The ventricular septal defect is easily visualised via the ventriculotomy and is closed with a running 6-0 Prolene suture. We use a special-order 6-0 Prolene on a TF needle (Ethicon, Sommerville, New Jersey, United States of America) that facilitates this repair. There should be minimal risk for heart block because the posterior limb of the septal band separates the ventricular septal defect from the tricuspid valve and conduction tissue.
The proximal conduit anastomosis is performed last, suturing the posterior portion of the homograft to the superior portion of the ventriculotomy. The anterior portion of the anastomosis is reconstructed with a gluteraldehyde-treated pericardial hood.
Results
Results of surgery for common arterial trunk in the modern era are quite good. A number of single-centre reports publish excellent results with mortality rates at 4–5%.Reference Thompson, McElhinney, Reddy, Petrossian, Silverman and Hanley 4 , Reference Jahangiri, Zurakowski, Mayer, del Nido and Jonas 5 Many early studies cite the association of truncal valve insufficiency as a risk factor for poor outcome. Rajasinghe et alReference Rajasinghe, McElhinney, Reddy, Mora and Hanley 6 reported a long-term follow-up on patients with common arterial trunk repaired in the neonatal period and found that severe truncal insufficiency was a risk factor for late death (30% mortality). Di Donato et alReference Di Donato, Fyfe and Puga 7 in a review of 167 patients at the Mayo Clinic also found moderate to severe truncal insufficiency to be a risk factor for poor long-term survival (50% mortality).
More recent reports are starting to suggest that truncal valve regurgitation may not be associated with as high a risk for mortality as previously reported.Reference Bove and Mosca 8 , Reference Kaza, Burch, Pinto, Minich, Tani and Hawkins 9 A recent report from the Society of Thoracic Surgeons database looked at 572 cases of common arterial trunk and the impact of truncal valve surgery. In all, 23 patients underwent concomitant truncal valve surgery. Mortality for common arterial trunk repair with truncal valve surgery compared with common arterial trunk repair alone was 30% versus 10%.Reference Russell, Pasquali and Jacobs 10
Right ventricle to pulmonary artery conduit
The choice of right ventricle to pulmonary artery conduit is centre specific. Our centre currently favours homograft tissue when the appropriate size is available. In some studies, aortic homografts have been shown to have worse longevity when compared with pulmonary homografts.Reference Kalavrouziotis, Purohit, Ciotti, Corno and Pozzi 11 Other studies have shown small homograft size (<12 mm) to be a risk factor for early failure.Reference Danton, Barron and Stumper 12 , Reference Perron, Moran, Gauvreau, del Nido, Mayer and Jonas 13
One option is the use of a direct right ventricle to pulmonary artery connection. This technique has been used successfully by a number of centres;Reference Danton, Barron and Stumper 12 , Reference Chen, Glickstein and Davies 14 however, poor outcomes have been reported in patients with small-sized or distorted pulmonary arteries and those with pulmonary hypertension with the use of that technique.Reference Lacour-Gayet, Serraf and Komiya 15
The ideal right ventricle to pulmonary artery conduit remains controversial. Options include the use of homografts, xenografts – such as bovine jugular vein conduits – Gore-Tex conduits,Reference Behrendt and Dick 16 and direct anastomosis. Any of these choices can likely be used with excellent results.
Interrupted aortic arch
Interrupted aortic arch is associated with common arterial trunk in 15% of patients. The presence of interrupted aortic arch with common arterial trunk can be a significant risk factor. A recent article from the Congenital Heart Surgeons Society examined 50 neonates with common arterial trunk associated with interrupted aortic arch.Reference Konstantinov, Karamlou and Blackstone 17 Their study looked at a 10-year time period up to 1997 and found a mortality rate as high as 67%. Results and techniques, however, appear to have improved significantly since that time period. One paper that included 34 patients from the Society of Thoracic Surgeons database from 2000 to 2009 suggested a mortality rate of only 20%.Reference Russell, Pasquali and Jacobs 10 There are a number of single-centre reports with much more favourable results as well;Reference Jahangiri, Zurakowski, Mayer, del Nido and Jonas 5 , Reference Bohuta, Hussein and Fricke 18 however, small patient populations in these categories make realistic statistical analysis difficult.
The technique of homograft enlargement of the ascending aorta during repair was first described in the mid-1990s.Reference Lacour-Gayet, Serraf and Galletti 19 This allows for a much more favourable size match between the truncal root and hypoplastic ascending aorta. This technique has likely contributed to the improved success with this repair in the more recent time period.
Management of the truncal valve
The decision to intervene on a truncal valve during the neonatal period should be weighed carefully. The indications on when to intervene on truncal valve insufficiency are not well defined. The addition of truncal valve repair during the primary operation in the neonatal period can have a mortality rate as high as 30%. A recent review from the Society of Thoracic Surgeons Database looked at 23 patients who underwent truncal valve repair at the time of primary repair for common arterial trunk. Mortality rate for common arterial trunk repair with truncal valve repair was 30% compared with 10% for patients who received common arterial trunk repair alone.Reference Russell, Pasquali and Jacobs 10 A significant limitation of the study was that it could not include patients with truncal valve regurgitation that were simply followed and not repaired, as the patients were identified from a procedure-based database. This makes it difficult to draw conclusions on what subset of patients should undergo truncal valve repair.
Most groups feel that truncal valve repair is the preferred option as opposed to allograft valved root replacement due to the historically high and early – within a few months – need for reoperation for allograft insufficiency.
Some single-institution studies reviewed their experience with truncal valve interventions during primary repair with favourable results. Jahangiri et alReference Jahangiri, Zurakowski, Mayer, del Nido and Jonas 5 reported the Boston series of 6 patients who underwent truncal valve repair with excellent results in patients followed out to 2 years. Patients who received truncal valve repair were mostly those with severe regurgitation.
Kaza et alReference Kaza, Burch, Pinto, Minich, Tani and Hawkins 9 reported the results from Utah on 17 patients who underwent truncal valve repair, 14 performed at the time of primary operation. Actuarial survival was 94% at 5 and 10 years. Freedom from truncal valve reintervention was 70% at 5 years and 50% at 7 years. Truncal valve repair was performed mostly for moderate to severe truncal valve regurgitation. Repair was performed if truncal valve pre-operative peak systolic gradients were >70 mmHg.
Indications for truncal valve repair range from an aggressive approach of repairing truncal valves with moderate regurgitation to a more conservative approach of reserving truncal valve repair for patients with severe regurgitation only. Proponents of a more aggressive approach cite studies showing that moderate or greater truncal valve regurgitation is a risk factor for either death or later need for truncal valve replacement.Reference Rajasinghe, McElhinney, Reddy, Mora and Hanley 6 , Reference Henaine, Azarnoush and Belli 20 Proponents of a more conservative approach are supported by the significant mortality rates seen in multi-centre large-volume database reports.
Supplementary materials
For supplementary material referred to in this article, please visit http://dx.doi.org/doi:10.1017/S1047951112002016. This hyperlink goes to a video of the surgical technique for repair of common arterial trunk.



