Introduction
Bipolar disorder (BD) is a recurrent, chronic and severe disease, which is characterized by episodes of depression and mania/hypomania that cause serious harm to patients, families and society (Merikangas et al., Reference Merikangas, Akiskal, Angst, Greenberg, Hirschfeld, Petukhova and Kessler2007). It is one of the main causes of disability among young people, leading to cognitive impairment and increased mortality, especially deaths by suicide. Although numerous structural and functional neuroimaging studies have revealed differences in specific brain regions and connections in patients with BD, the neurobiology of BD using neuroimaging is not fully understood.
Over the past decade, blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging (fMRI) has developed rapidly and become a promising tool to investigate the different brain activity regions in patients with BD, greatly improving our understanding of the neurobiological mechanisms of BD (Chen, Suckling, Lennox, Ooi, & Bullmore, Reference Chen, Suckling, Lennox, Ooi and Bullmore2011; Gong et al., Reference Gong, Wang, Qiu, Chen, Luo, Wang and Wang2020; Hajek, Alda, Hajek, & Ivanoff, Reference Hajek, Alda, Hajek and Ivanoff2013). A task-based meta-fMRI study of neurocognitive performance reported that BD patients showed under-activation in the right inferior frontal gyrus (IFG) and hyper-activation in the left superior temporal gyrus (Hajek et al., Reference Hajek, Alda, Hajek and Ivanoff2013). Another cognitive and emotional task-based meta-analysis (Chen et al., Reference Chen, Suckling, Lennox, Ooi and Bullmore2011) demonstrated IFG differences during both cognitive and emotional processing, and limbic hyper-activation during emotional processing. However, the results of task-based fMRI studies may be highly heterogeneous due to the differences in experimental design and the tasks (Bennett & Miller, Reference Bennett and Miller2010). Resting-state functional imaging provides a non-invasive and task-free approach that eliminates some of the performance-related confounders and provides a reliable method to measure baseline brain activities (Biswal, Yetkin, Haughton, & Hyde, Reference Biswal, Yetkin, Haughton and Hyde1995). Resting-state functional imaging methods include multiple imaging modalities, such as single-photon emission computed tomography (SPECT), positron emission tomography (PET), arterial spin labeling (ASL), amplitude of low-frequency fluctuation (ALFF), fractional ALFF, and regional homogeneity (ReHo). In SPECT and PET, radiotracers are used to measure cerebral blood flow (CBF) or brain metabolism. The BOLD signal does not directly measure the neuronal activity. In contrast, the BOLD effect is sensitive to the changes in CBF, the cerebral metabolic rate of oxygen, and cerebral blood volume, which are collectively referred to as activated hemodynamic responses (Buxton, Uludağ, Dubowitz, & Liu, Reference Buxton, Uludağ, Dubowitz and Liu2004; Liu, Zhuo, & Yu, Reference Liu, Zhuo and Yu2016). ASL technology can simultaneously identify BOLD signal and CBF (Buxton et al., Reference Buxton, Uludağ, Dubowitz and Liu2004). The advantage of ASL is that it is a completely non-invasive MRI method that uses magnetically labeled water as an endogenous tracer to measure CBF. ALFF/fALFF quantifies the intensity of low-frequency oscillations in spontaneous neural activity. ReHo reflects the similarity of spontaneous neural activity between adjacent brain tissues. Previous resting-state functional imaging studies and related meta-analyses (Gong et al., Reference Gong, Wang, Qiu, Chen, Luo, Wang and Wang2020; Toma, MacIntosh, Swardfager, & Goldstein, Reference Toma, MacIntosh, Swardfager and Goldstein2018; Vargas et al., Reference Vargas, Lopez-Jaramillo and Vieta2013) found different spontaneous brain activities in BD. However, these results are inconsistent. On one hand, different directions of activity of the same brain regions were reported between studies. For example, (Brooks, Hoblyn, & Ketter, Reference Brooks, Hoblyn and Ketter2010) and (Brooks, Hoblyn, Woodard, Rosen, & Ketter, Reference Brooks, Hoblyn, Woodard, Rosen and Ketter2009) reported significant hypometabolism in the prefrontal gyrus in BD while (Liu et al., Reference Liu, Ma, Li, Wang, Tie, Li and Wang2012b) and (Xu et al., Reference Xu, Liu, Li, Tang, Womer, Jiang and Wang2014a) reported increased activities in the prefrontal gyrus in BD. (Bøen, Hjørnevik, & Hummelen, Reference Bøen, Hjørnevik and Hummelen2019) reported hypometabolism in the striatum in BD. (Zhang et al., Reference Zhang, Liu, Cao, Yang, Xu, Xu and Yang2017) and (Zhang et al., Reference Zhang, Bo, Li, Zhao, Wang, Liu and Zhou2020b) reported increased activities in the striatum in BD. On the other hand, different brain regions were reported in different studies, such as different functional activities in the prefrontal gyrus (Brooks et al., Reference Brooks, Hoblyn, Woodard, Rosen and Ketter2009, Reference Brooks, Hoblyn and Ketter2010; Liu et al., Reference Liu, Li, Li, Wang, Tie, Wu and Wang2012a; Xu et al., Reference Xu, Liu, Li, Tang, Womer, Jiang and Wang2014), insula (Liu et al., Reference Liu, Li, Li, Wang, Tie, Wu and Wang2012a), striatum (Bøen et al., Reference Bøen, Hjørnevik and Hummelen2019; Zhang et al., Reference Zhang, Liu, Cao, Yang, Xu, Xu and Yang2017; Zhang et al., Reference Zhang, Bo, Li, Zhao, Wang, Liu and Zhou2020b), temporal gyrus (Brooks et al., Reference Brooks, Hoblyn, Woodard, Rosen and Ketter2009, Reference Brooks, Hoblyn and Ketter2010; Liu et al., Reference Liu, Li, Li, Wang, Tie, Wu and Wang2012a; Qiu et al., Reference Qiu, Zhang, Mellor, Shi, Wu, Huang and Peng2018), amygdala (Brooks et al., Reference Brooks, Hoblyn, Woodard, Rosen and Ketter2009), parahippocampal gyrus (Brooks et al., Reference Brooks, Hoblyn, Woodard, Rosen and Ketter2009, Reference Brooks, Hoblyn and Ketter2010), precuneus (Brooks et al., Reference Brooks, Hoblyn and Ketter2010; Qiu et al., Reference Qiu, Zhang, Mellor, Shi, Wu, Huang and Peng2018), parietal gyrus (Liu et al., Reference Liu, Ma, Li, Wang, Tie, Li and Wang2012b; Qiu et al., Reference Qiu, Zhang, Mellor, Shi, Wu, Huang and Peng2018), and lingual gyrus (Qiu et al., Reference Qiu, Zhang, Mellor, Shi, Wu, Huang and Peng2018) in BD. These differences may be related to gender, age range, different subtypes and BD states (i.e. manic, depressed, mixed or euthymic), drug status, and methodology. There is no meta-analysis on the spontaneous neural activity in resting-state in BD by combining various functional imaging techniques.
In addition to functional alterations, morphological differences in the brains of patients with BD were found in previous structural MRI studies. Voxel-based morphometrics (VBM) is the most widely used analytical method to investigate structural differences in BD. VBM is an automatic whole-brain method that can calculate the local volume of gray matter in an unbiased manner. Existing VBM studies of BD have reported inconsistent results showing reduced gray matter volume (GMV) in the prefrontal gyrus (Almeida et al., Reference Almeida, Akkal, Hassel, Travis, Banihashemi, Kerr and Phillips2009; Alonso-Lana et al., Reference Alonso-Lana, Goikolea, Bonnin, Sarro, Segura, Amann and McKenna2016; Altamura et al., Reference Altamura, Maggioni, Dhanoa, Ciappolino, Paoli, Cremaschi and Brambilla2018; Ambrosi et al., Reference Ambrosi, Rossi-Espagnet, Kotzalidis, Comparelli, Del Casale, Carducci and Girardi2013; Brown et al., Reference Brown, Lee, Strigo, Caligiuri, Meloy and Lohr2011; Cai et al., Reference Cai, Liu, Zhang, Liao, Zhang, Wang and Li2015; Eker et al., Reference Eker, Simsek, Yilmazer, Kitis, Cinar, Eker and Gonul2014), temporal gyrus (Ambrosi et al., Reference Ambrosi, Rossi-Espagnet, Kotzalidis, Comparelli, Del Casale, Carducci and Girardi2013; Brown et al., Reference Brown, Lee, Strigo, Caligiuri, Meloy and Lohr2011; Chen, Wen, Malhi, Ivanovski, & Sachdev, Reference Chen, Wen, Malhi, Ivanovski and Sachdev2007; Cui et al., Reference Cui, Li, Deng, Guo, Ma, Huang and Li2011), cingulate gyrus(Cai et al., Reference Cai, Liu, Zhang, Liao, Zhang, Wang and Li2015; Oertel-Knochel et al., Reference Oertel-Knochel, Reinke, Feddern, Knake, Knochel, Prvulovic and Linden2014), parietal gyrus (Cui et al., Reference Cui, Li, Deng, Guo, Ma, Huang and Li2011; Li et al., Reference Li, Cui, Deng, Ma, Huang, Jiang and Li2011), precuneus/ cuneus (Oertel-Knochel et al., Reference Oertel-Knochel, Reinke, Feddern, Knake, Knochel, Prvulovic and Linden2014), hippocampus(Hajek et al., Reference Hajek, Cullis, Novak, Kopecek, Hoschl, Blagdon and Alda2012, Reference Hajek, Calkin, Blagdon, Slaney, Uher and Alda2014), increased GMV in the putamen (Chen et al., Reference Chen, Cui, Li, Jiang, Deng, Ma and Li2012; Cui et al., Reference Cui, Li, Deng, Guo, Ma, Huang and Li2011; Frangou, Reference Frangou2012), and no difference in GMV (Bruno, Barker, Cercignani, Symms, & Ron, Reference Bruno, Barker, Cercignani, Symms and Ron2004; Kandilarova, Stoyanov, Sirakov, & Maes, Reference Kandilarova, Stoyanov, Sirakov and Maes2019). Consequently, several VBM meta-analyses have been conducted to summarize the existing evidence to map brain regions associated with BD (Ellison-Wright & Bullmore, Reference Ellison-Wright and Bullmore2010; Ganzola & Duchesne, Reference Ganzola and Duchesne2017; Houenou et al., Reference Houenou, Frommberger, Carde, Glasbrenner, Diener, Leboyer and Wessa2011; Lu et al., Reference Lu, Zhong, Ma, Wu, Fox, Zhang and Wang2019; McCarthy, Liang, Spadoni, Kelsoe, & Simmons, Reference McCarthy, Liang, Spadoni, Kelsoe and Simmons2014; Selvaraj et al., Reference Selvaraj, Arnone, Job, Stanfield, Farrow, Nugent and McIntosh2012; Wang et al., Reference Wang, Tian, Wang, Cheng, Qiu, He and Jia2018a). However, the results of these meta-analyses were inconsistent, which may be attributed to certain factors. First, these meta-analyses included small number of trials, and most of these included fewer than 15 experiments (Ellison-Wright & Bullmore, Reference Ellison-Wright and Bullmore2010; Ganzola & Duchesne, Reference Ganzola and Duchesne2017; Houenou et al., Reference Houenou, Frommberger, Carde, Glasbrenner, Diener, Leboyer and Wessa2011; McCarthy et al., Reference McCarthy, Liang, Spadoni, Kelsoe and Simmons2014; Selvaraj et al., Reference Selvaraj, Arnone, Job, Stanfield, Farrow, Nugent and McIntosh2012). In addition, the inclusion and exclusion criteria of the study were not strict, due to which unrelated variables such as repeated inclusion of subjects, periodicity of disease, and primary diagnosis of disease influenced the results (Ellison-Wright & Bullmore, Reference Ellison-Wright and Bullmore2010; Ganzola & Duchesne, Reference Ganzola and Duchesne2017; Houenou et al., Reference Houenou, Frommberger, Carde, Glasbrenner, Diener, Leboyer and Wessa2011; Lu et al., Reference Lu, Zhong, Ma, Wu, Fox, Zhang and Wang2019; McCarthy et al., Reference McCarthy, Liang, Spadoni, Kelsoe and Simmons2014; Selvaraj et al., Reference Selvaraj, Arnone, Job, Stanfield, Farrow, Nugent and McIntosh2012; Wang et al., Reference Wang, Tian, Wang, Cheng, Qiu, He and Jia2018a). Recently, Lu et al., investigated gray matter deficits in the temporal and prefrontal cortex, and increased GMV in the putamen, cingulate cortex, and precuneus (46 studies with 56 experiments including 1720 subjects)(Lu et al., Reference Lu, Zhong, Ma, Wu, Fox, Zhang and Wang2019). Given that several VBM studies(Chen et al., Reference Chen, Kao, Chang, Tu, Hsu, Huang and Bai2020b; Lee et al., Reference Lee, Lee, Park, Joh, Kim and Ryu2020; Li et al., Reference Li, Cui, Cao, Zhang, Liu, Deng and Zhou2020; Sun et al., Reference Sun, Li, Zhang, Yang, Liu, Liu and Zhang2020; Vai et al., Reference Vai, Parenti, Bollettini, Cara, Verga, Melloni and Benedetti2020) have been conducted in recent years, it is necessary to update the meta-analyses to expand and /or modify the findings of previous studies. In addition, most studies adopted a separate structural MRI to investigate BD. More efforts should be made to focus on the overlap of structural and functional imaging modalities to explore the neuropathological mechanisms in BD.
Hence, we aimed to conduct a whole-brain voxel-wise meta-analysis to explore the most robust findings across numerous published resting-state functional imaging studies and VBM studies, and to investigate whether there is a structural basis for functional impairment in some specific brain regions in BD. We hypothesized that BD patients have local convergence of GM decrease and increase and/or decrease in brain function compared with the control group. Moreover, we speculated that spontaneous functional activity and structural differences in BD are mainly located in the frontal and temporal gyri. The findings of this meta-analysis could facilitate further original research and help to further understand the pathophysiological mechanism of BD.
Methods
Data sources, study selection, and quality assessment
We followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses international guidelines (Moher, Liberati, Tetzlaff, & Altman, Reference Moher, Liberati, Tetzlaff and Altman2009) (registration number is: CRD42021270700). A comprehensive search of studies published was conducted between January 1990 and January 2021 in the PubMed, Embase, Web of Science, SinoMed, Chinese National Knowledge Infrastructure, and WanFang databases using the following keywords: (‘bipolar disorder’ OR ‘bipolar disorders’ OR ‘disorder, bipolar’ OR ‘psychosis, manic-depressive’ OR ‘psychosis, manic depressive’ OR ‘manic-depressive psychosis’ OR ‘manic depressive psychosis’ OR ‘affective psychosis, bipolar’ OR ‘bipolar affective psychosis’ OR ‘psychoses, bipolar affective’ OR ‘psychosis, bipolar affective’ OR ‘psychoses, manic-depressive’ OR ‘manic-depressive psychoses’ OR ‘psychoses, manic depressive’ OR ‘mania’ OR ‘manias’ OR ‘manic state’ OR ‘manic states’ OR ‘state, manic’ OR ‘states, manic’ OR ‘depression, bipolar’ OR ‘bipolar depression’ OR ‘manic disorder’ OR ‘disorder, manic’ OR ‘manic disorders’) AND (‘neuroimag*’ OR ‘functional magnetic resonance imaging’ OR ‘fMRI’ OR ‘cerebral blood flow’ OR ‘CBF’ OR ‘positron emission tomography’ OR ‘PET’ OR ‘single photon emission computed tomography’ OR ‘SPECT’ OR ‘arterial spin labeling’ OR ‘ASL’ OR ‘amplitude of low-frequency fluctuation’ OR ‘ALFF’ OR ‘fractional ALFF’ OR ‘fALFF’ OR ‘regional homogeneity’ OR ‘ReHo’ OR ‘resting-state’ OR ‘Voxel-based morphometry’ OR ‘VBM’ OR ‘morphometry’). In addition, we also checked the reference list of included studies and related review articles to identify other relevant studies.
Studies that satisfied the following conditions were included in the meta-analysis. (1) Subjects were aged from 18 to 60 years old. (2) They compared resting-state functional imaging or GMV between BD and healthy controls (HCs). (3) Three-dimensional coordinates [Talairach or Montreal Neurological Institute (MNI)] were reported for whole-brain resting-state functional imaging or GMV analysis. (4) The study was published as an original article (not as an abstract or a letter) in a peer-reviewed English or Chinese language journal. (5) Significant results were reported using thresholds for significance corrected for multiple comparisons or uncorrected with spatial extent thresholds. And (6) When the original manuscripts' details were not reported, it could be available by making a reasonable request to the corresponding author.
Studies were excluded if (1) patients with BD with comorbid neurological diseases; (2) the data overlapped with those of another included publication; (3) the data were unavailable (e.g. missing neuroanatomical coordinates) even after the authors were contacted by telephone or email; (4) a region-of-interest approach was used.
We used a 10-point checklist involved in the previous meta-analysis of neuroimaging studies to assess the quality of each study selected for this meta-analysis (online Supplementary Table S1 in Supplementary materials) (Chen et al., Reference Chen, Du, Zhao, Huang, Li, Lui and Gong2015; Shepherd, Matheson, Laurens, Carr, & Green, Reference Shepherd, Matheson, Laurens, Carr and Green2012). Literature search, study evaluation, and selection were independently performed by three investigators (G.J.Y., Q.S.J., and C.P.). Any discrepancies were resolved by a third investigator (W.Y.) for a final decision. The current study was conducted with reference to the Meta-analysis of Observational Studies in Epidemiology guidelines for the meta-analyses of observational studies(Stroup et al., Reference Stroup, Berlin, Morton, Olkin, Williamson, Rennie and Grp2000).
Data analysis
Voxel-wise meta-analysis for functional differences
A meta-analysis of functional (i.e. Reho, ALFF, fALFF, and CBF) differences between BD and HCs was conducted using the Seed-based d Mapping with Permutation of Subject Images (SDM-PSI version 6.21) software package in a standard process (www.sdmproject.com). The SDM-PSI is a voxel-based meta-analysis software using peak coordinates and their t values as reported from the original studies, to impute multiple effect-size maps (Hedges' effect size) of contrast results (increased and decreased activations) for each study. Thereafter, the maps are combined in a standard random-effects model based on sample size, intra-study variability and inter-study heterogeneity (Radua et al., Reference Radua, Mataix-Cols, Phillips, El-Hage, Kronhaus, Cardoner and Surguladze2012b), and multiple imputations are pooled using Rubin's rules(Albajes-Eizagirre, Solanes, Vieta, & Radua, Reference Albajes-Eizagirre, Solanes, Vieta and Radua2019b). The familywise error rate (FWER) of the results is calculated using a subject-based permutation test. SDM-PSI uses MetaNSUE (Albajes-Eizagirre, Solanes, & Radua, Reference Albajes-Eizagirre, Solanes and Radua2019a) to estimate the maximum likely effect size within the lower and upper limits of possible effect sizes for each study separately, and then adds realistic noise (Albajes-Eizagirre et al., Reference Albajes-Eizagirre, Solanes, Vieta and Radua2019b). We performed the analysis as described in the SDM tutorial and related publications, using MRIcron software (www.mricro.com/mricron/) to visualize the SDM maps.
Briefly, whole-brain meta-analyses were performed to assess functional differences between BD and HCs. Residual heterogeneity (I 2 statistic) of included studies was examined to assess the robustness of results (I 2 > 50% commonly indicates serious heterogeneity). Funnel plots were created to visually examine if findings had been driven by a small subset of studies or by studies with small sample sizes. We reported results using an uncorrected p < 0.005 threshold with a cluster extent = 10 voxels, since it was found to optimally balance sensitivity and specificity(Radua et al., Reference Radua, Borgwardt, Crescini, Mataix-Cols, Meyer-Lindenberg, McGuire and Fusar-Poli2012a, Reference Radua, Mataix-Cols, Phillips, El-Hage, Kronhaus, Cardoner and Surguladze2012b). Complementary analyses were conducted using FWER-corrected p < 0.05 with the threshold-free cluster enhancement approach and 5000 permutations(Smith & Nichols, Reference Smith and Nichols2009).
In addition, for the thresholded meta-analytic results, meta-regression analysis was performed to assess the effects of illness duration, age, gender and FWHM in data-preprocessing, which could potentially influence the analytic results. In this step, we also used an uncorrected p < 0.005 threshold with a cluster extent = 10 voxels.
To further examine the specific functional differences between BD and HCs, subgroup meta-analyses were conducted for indices of Reho and ALFF, as well as in the unmedicated and depressed subgroups. There were not sufficient studies [minimum of 10 studies recommended for SDM meta-analyses (Carlisi et al., Reference Carlisi, Norman, Lukito, Radua, Mataix-Cols and Rubia2017; Radua & Mataix-Cols, Reference Radua and Mataix-Cols2009) of fALFF, PET, ASL, and patients in a manic state to conduct subgroup analyses.
Voxel-wise meta-analysis for structural differences
Similar to the meta-analyses for functional differences, we also conducted a meta-analysis for structural (i.e. VBM) differences between BD and HCs. However, meta-regression analysis was not conducted due to the limited number of studies (<10 studies).
Functional and structural overlap
To reveal both the functional and structural differences in BD, we investigated the overlap of functional and structural differences via overlapping thresholded meta-analytic results-maps.
Results
Included studies and sample characteristics
A flow diagram of the identification and exclusion of studies was presented in Fig. 1. For the functional meta-analysis, 57 experiments from 51 studies comprising 1842 patients with BD and 2190 HCs were selected. No significant differences were observed between patients with BD and HCs with respect to age [95% confidence interval (CI) = −3.444 to 2.755, t = −0.220, p = 0.826] or gender distribution (based on totals across all studies) (χ2 = 1.431, p = 0.232).
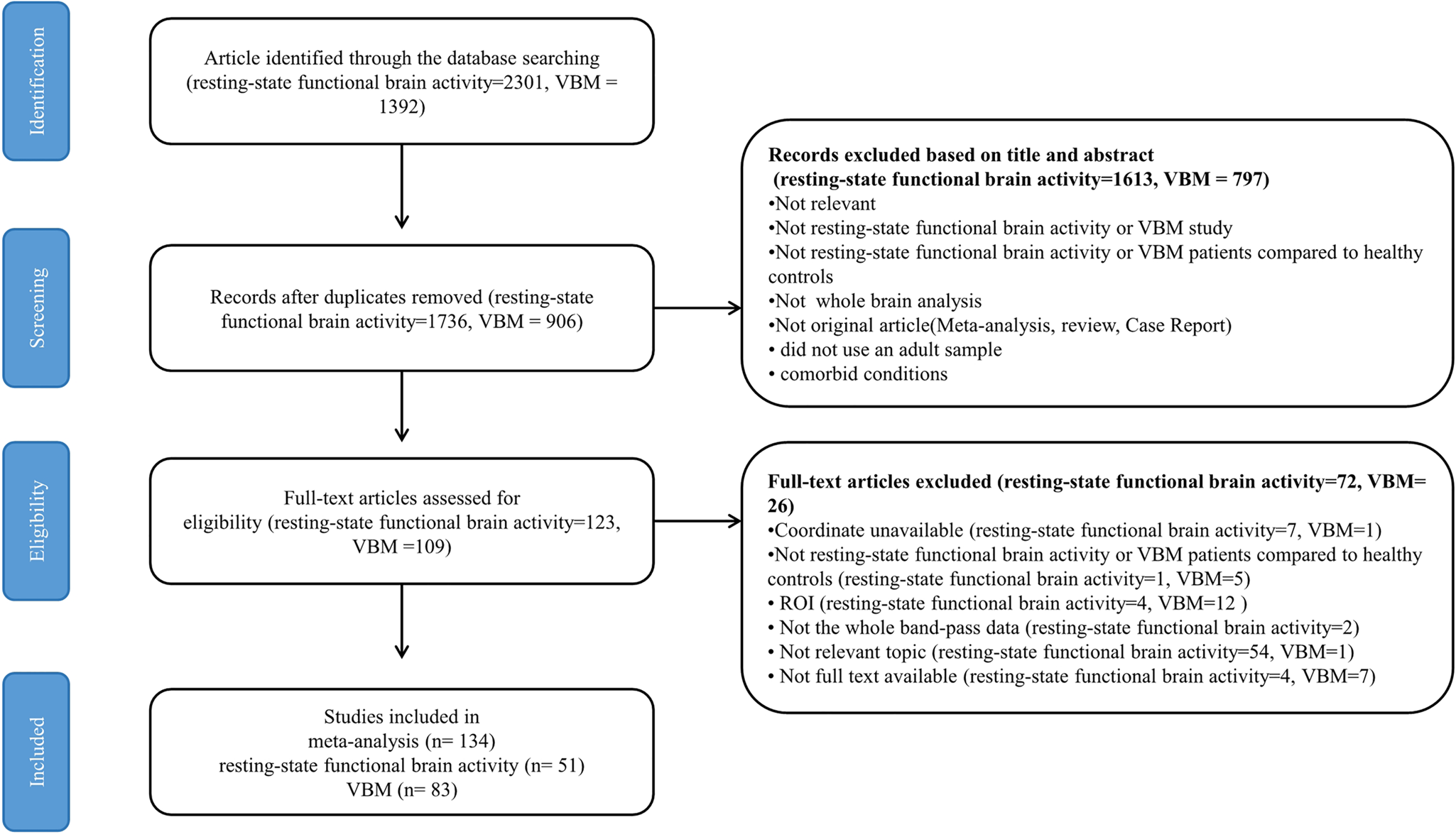
Fig. 1. Flow diagram for the identification and exclusion of resting-state functional imaging and VBM studies of patients with BD. Abbreviations: VBM, voxel-based morphometry; ROI, region of interest; BD, bipolar disorder.
Further, for the specific analysis, 13 studies from 13 publications comprising 441 patients with BD and 462 HCs were selected for the unmedicated functional meta-analysis. 20 studies from 20 publications comprising 644 patients with BD and 890 HCs were selected for the depressed functional meta-analysis. 22 studies from 20 publications comprising 751 patients with BD and 1188 HCs were selected for the Reho meta-analysis. 20 studies from 20 publications comprising 722 patients with BD and 969 HCs were selected for the ALFF analysis.
For the structural meta-analysis, 92 experiments from 83 studies comprising 2790 patients with BD and 3690 HCs were selected. No significant difference was observed between patients with BD and HCs with respect to age [95% confidence interval (CI) = −0.466 to 3.685, t = 1.530, p = 0.128] or gender distribution (based on totals across all studies) (χ2 = 0.791, p = 0.374).
The demographic, clinical, imaging characteristics, and quality scores of the included studies in this meta-analysis were well described in online Supplementary Tables S2 and S3 in Supplementary materials.
Functional meta-analysis
In the functional meta-analysis, patients with BD displayed increased resting-state functional activity in the left middle frontal gyrus, right IFG (extending to the right insula), right superior frontal gyrus and bilateral striatum, as well as decreased resting-state functional activity in the left middle temporal gyrus (extending to the left superior temporal gyrus and post-central gyrus), left cerebellum, and bilateral precuneus (Fig. 2a and Table 1). In addition to the right striatum, the inter-study heterogeneity for each significant peak was low (I 2 < 50%). Funnel plots suggested that the results were not driven by small or noisy studies, and the potential publication bias was not statistically significant (p > 0.05). In the complementary analyses, persistent increased resting-state functional activity in the left middle frontal gyrus and decreased resting-state functional activity in the left middle temporal gyrus (extending to the left superior temporal gyrus and post-central gyrus) were observed in BD after FWE correction (online Supplementary Fig. S1a and Table S4). Additionally, we did not detect any significant result for the meta-regression analyses.

Fig. 2. Meta-analyses results regarding (a) resting-state functional activity difference between BD and HCs, (b) GMV difference between BD and HCs, (c) conjunction of resting-state functional activity differences and GMV differences. Areas with decreased value are displayed in blue, and areas with increased value are displayed in red. The color bar indicates the maximum and minimum SDM-Z values. Abbreviations: BD, bipolar disorder; HCs, healthy controls; GMV, gray matter volume; SDM, signed differential mapping.
Table 1. Meta-analyses results regarding the functional difference between BD and HCs

Abbreviations: BD, bipolar disorder; HCs, healthy controls; MNI, Montreal Neurological Institute; SDM, signed differential mapping; BA, Brodmann area.
In the subgroup analyses, patients with BD in a depressed state displayed increased resting-state functional activity in the left IFG, and decreased resting-state functional activity in the left post-central gyrus (online Supplementary Fig. S2a and Table S5). The inter-group heterogeneity for the left IFG was high (I 2 > 50%), while for the left post-central gyrus was low (I 2 < 50%). Funnel plots suggested that the results were not driven by small or noisy studies, and the potential publication bias was not statistically significant (p > 0.05).
In the unmedicated functional meta-analysis, BD patients without medication showed increased resting-state functional activity in the bilateral superior frontal gyrus and right post-central gyrus (online Supplementary Fig. S2b and Table S6). The inter-study heterogeneity for each significant peak was low (I 2 < 50%). Funnel plots suggested that the results were not driven by small or noisy studies, and the potential publication bias was not statistically significant (p > 0.05).
In the Reho meta-analysis, patients with BD displayed increased Reho in the left middle frontal gyrus, and decreased Reho in the left middle temporal gyrus (online Supplementary Fig. S3a and Table S7). The inter-study heterogeneity for each significant peak was low (I 2 < 50%). Funnel plots suggested that the results were not driven by small or noisy studies, and the potential publication bias was not statistically significant (p > 0.05).
In the ALFF meta-analysis, patients with BD displayed increased ALFF in the right insula, bilateral IFG, bilateral striatum, bilateral caudate nucleus, right inferior temporal gyrus, as well as decreased ALFF in the left cerebellum (online Supplementary Fig. S3b and Table S8). The inter-study heterogeneity for each significant peak was low (I 2 < 50%). Funnel plots suggested that the results were not driven by small or noisy studies, and the potential publication bias was not statistically significant (p > 0.05).
Structural meta-analysis
In the VBM meta-analysis, we found that patients with BD displayed decreased VBM in the right IFG (extending to the right insula, temporal pole and superior temporal gyrus), left superior temporal gyrus (extending to the left insula, temporal pole, and IFG), anterior cingulate cortex (ACC), left superior frontal gyrus (medial prefrontal cortex, mPFC), left thalamus, and right fusiform gyrus (Fig. 2b and Table 2). In addition to the left ACC inter-study heterogeneity for each significant peak was low (I 2 < 50%). Funnel plots suggested that the results were not driven by small or noisy studies, and the potential publication bias was not statistically significant (p > 0.05). In the complementary analyses, decreased VBM persisted in the left temporal pole after FWER correction (online Supplementary Fig. S1b and Table S9).
Table 2. Meta-analyses results regarding VBM difference between BD and HCs

Abbreviations: BD, bipolar disorder; HCs, healthy controls; VBM, voxel-based morphometry; MNI, Montreal Neurological Institute; SDM, signed differential mapping; BA, Brodmann area.
Overlap of functional and VBM differences
After overlapping the functional and VBM differences based on the above meta-analyses, patients with BD showed increased resting-state functional activity and decreased VBM in the right insula (Fig. 2c).
Discussion
To the best of our knowledge, this is the first multimodal neuroimaging meta-analysis including a large number of whole brain studies investigating spontaneous functional activity (51 studies with 57 experiments including 4032 subjects) as well as studies investigating structural gray matter (83 studies with 92 experiments including 6480 subjects) to more consistently localize the neural substrates of BD. In the resting-state functional imaging meta-analysis, BD showed increased spontaneous functional activity in the bilateral ventrolateral prefrontal cortex (extending to the insula) and bilateral striatum, and decreased functional activity in the left middle temporal gyrus (extending to the superior temporal gyrus), precuneus and cerebellum. In the VBM meta-analysis, BD showed decreased GMV in the bilateral ACC, mPFC, bilateral temporal pole (extending to the insula, superior temporal gyrus, and IFG), left thalamus, and right fusiform gyrus. Furthermore, there were large overlapping functional and anatomical changes in the right insula in BD. These functional and structural alterations provide important insights into the pathophysiological substrate of BD.
The insula is a cortical structure with extensive connections to the frontal cortex, ACC, parietal lobe, temporal lobe, amygdala, dorsal thalamus and other limbic areas(Augustine, Reference Augustine1996), which is implicated in various cognitive, affective, and regulatory functions, including interoceptive awareness, emotional responses, and empathic processes (Menon & Uddin, Reference Menon and Uddin2010). Many deficits observed in BD involve these functions and may be related to insula pathology, such as emotional dysregulation and cognitive impairment. The results of the two meta-analyses converged on the right insula in this study, which showed both spontaneous activity increase and gray matter reduction in BD compared to HCs. Meta-regression analyses indicated that this difference was not significantly affected by age, sex or illness duration. These findings are consistent with results from previous meta-analyses of volumetric measurements in BD (Bora, Fornito, Yuecel, & Pantelis, Reference Bora, Fornito, Yuecel and Pantelis2012; Ellison-Wright & Bullmore, Reference Ellison-Wright and Bullmore2010; Selvaraj et al., Reference Selvaraj, Arnone, Job, Stanfield, Farrow, Nugent and McIntosh2012; Wang et al., Reference Wang, Luo, Tian, Cheng, Qiu, Wang and Jia2019; Wise et al., Reference Wise, Radua, Via, Cardoner, Abe, Adams and Arnone2017; Yu et al., Reference Yu, Meng, Li, Zhang, Liang, Li and Li2019). Previous studies assumed that regional volume changes are associated with changes in glial cell density, neuronal size, and dendritic branching (Anderson, Reference Anderson2011; Rajkowska, Reference Rajkowska2000; Zaremba et al., Reference Zaremba, Dohm, Redlich, Grotegerd, Strojny, Meinert and Dannlowski2018), which are known to influence the function of neural circuitries in these regions. Moreover, several brain structural studies found different GMVs in the insular cortex and IFG in individuals at high genetic risk for BD (Kempton et al., Reference Kempton, Haldane, Jogia, Grasby, Collier and Frangou2009; Matsuo et al., Reference Matsuo, Kopecek, Nicoletti, Hatch, Watanabe, Nery and Soares2012; Nery, Monkul, & Lafer, Reference Nery, Monkul and Lafer2013), which suggested that the morphologies of these regions are associated with genetic susceptibility to BD. Similar to our functional results, a recent resting state fMRI meta-analysis also found increased ALFF in the insula extending into the striatum in BD (Gong et al., Reference Gong, Wang, Qiu, Chen, Luo, Wang and Wang2020). Task-based fMRI studies found hypo-activation in the right anterior insula during cognitive control tasks (McTeague et al., Reference McTeague, Huemer, Carreon, Jiang, Eickhoff and Etkin2017). A structural network study reported normalization of altered nodal centralities in the insula after treatment with quetiapine or lithium for six weeks in patients with BD (Lei et al., Reference Lei, Li, Tallman, Patino, McNamara, Strawn and DelBello2021). Another study found that the left insula increased after lithium treatment in patients with BD (Germana et al., Reference Germana, Kempton, Sarnicola, Christodoulou, Haldane, Hadjulis and Frangou2010). The bilateral insula is one of the therapeutic targets. After multiple injections of ketamine and cardiac stabilizers, the global functional connectivity density of the bilateral insula in BD patients was reduced and accompanied by relief of depressive symptoms (Zhuo et al., Reference Zhuo, Ji, Tian, Wang, Jia, Jiang and Zhu2020). The activity at the site of stimulation as well as insula increased by transcranial magnetic stimulation (rTMS) in patients with depression (Li et al., Reference Li, Nahas, Kozel, Anderson, Bohning and George2004; Speer et al., Reference Speer, Kimbrell, Wassermann, Repella, Willis, Herscovitch and Post2000). Although the direction of alteration of the insula is inconsistent across modalities (functional neuroimaging and VBM), previous evidence suggests that increases or reductions in GMV may not directly correspond to functional neural activation or deactivation (Calhoun et al., Reference Calhoun, Adali, Giuliani, Pekar, Kiehl and Pearlson2006). A reduction in gray matter could be accompanied by a compensatory hyper-functionality of the remaining gray matter, which could involve a higher vascularization and thus show as higher spontaneous activation (Radua et al., Reference Radua, Borgwardt, Crescini, Mataix-Cols, Meyer-Lindenberg, McGuire and Fusar-Poli2012a). Therefore, the findings of increased activity in the insula could be a compensatory response to structural deficits in BD, which might contribute to the emotional and cognitive impairments of BD.
In this study, neural alterations in the prefrontal cortex were identified in both the functional and structural neuroimaging meta-analyses. Resting-state functional neuroimaging analysis showed increased spontaneous activity in the bilateral ventrolateral prefrontal/orbitofrontal cortex (mainly in the IFG) in BD. In the subgroup analyses, both increased ReHo and ALFF were found in the bilateral ventrolateral prefrontal/orbitofrontal cortex (mainly in the IFG) in BD, suggesting consistency both in the main result and the different functional subgroups. Structural meta-analysis showed decreased GMV in the bilateral mPFC, ACC and IFG in BD. These findings were partially consistent with previous functional(Gong et al., Reference Gong, Chen, Jia, Zhong, Zhao, Luo and Wang2019) and structural(Lu et al., Reference Lu, Zhong, Ma, Wu, Fox, Zhang and Wang2019; Selvaraj et al., Reference Selvaraj, Arnone, Job, Stanfield, Farrow, Nugent and McIntosh2012; Wang et al., Reference Wang, Luo, Tian, Cheng, Qiu, Wang and Jia2019; Yu et al., Reference Yu, Meng, Li, Zhang, Liang, Li and Li2019) meta-analyses of BD. Postmortem studies showed a reduction in neuronal size and packing density in the lateral prefrontal cortex and orbitofrontal cortex in BD (Rajkowska, Reference Rajkowska2000). The prefrontal regions are crucial in behavioral and emotional control (Passingham, Toni, & Rushworth, Reference Passingham, Toni and Rushworth2000; Phillips, Ladouceur, & Drevets, Reference Phillips, Ladouceur and Drevets2008). In particular, the IFG, which is the ventrolateral part of the prefrontal cortex, plays an important role in executive functions, such as cognitive inhibition(Ganzola & Duchesne, Reference Ganzola and Duchesne2017). Task-based fMRI meta-analysis found aberrant activation in the ventrolateral prefrontal regions in BD during emotional processing (McTeague et al., Reference McTeague, Rosenberg, Lopez, Carreon, Huemer, Jiang and Etkin2020) and cognitive control tasks (McTeague et al., Reference McTeague, Huemer, Carreon, Jiang, Eickhoff and Etkin2017). The ventrolateral prefrontal cortex was shown to be central to affective and non-affective inhibitory control among the same participants, and to show concurrent disruptions in psychopathology (McTeague et al., Reference McTeague, Rosenberg, Lopez, Carreon, Huemer, Jiang and Etkin2020; Tabibnia et al., Reference Tabibnia, Monterosso, Baicy, Aron, Poldrack, Chakrapani and London2011). Additionally, our subgroup analysis showed increased spontaneous activity in the bilateral superior frontal gyrus (mainly in the mPFC) in unmedicated patients with BD. These results indicated that abnormal mPFC is not caused by medication effects, mood stabilizers or illness chronicity, further indicating that increased activity in the mPFC is due to primary changes. The mPFC is associated with decision making, more precisely, with risk assessment of choices (Rogers et al., Reference Rogers, Ramnani, Mackay, Wilson, Jezzard, Carter and Smith2004). Muhlert and Lawrence (Reference Muhlert and Lawrence2015) found that individuals who have smaller volumes of mPFC exhibit riskier behavior during negative emotional states (Muhlert & Lawrence, Reference Muhlert and Lawrence2015). Therefore, these findings of gray matter deficits with increased activity in the mPFC may be related to reward hypersensitivity and impaired decision-making in patients with BD (Lu et al., Reference Lu, Zhong, Ma, Wu, Fox, Zhang and Wang2019). A previous meta-analysis of structural and functional neuroimaging studies reported increased GMV and activation in the right IFG in unaffected relatives of patients with BD compared with HCs (Cattarinussi, Di Giorgio, Wolf, Balestrieri, & Sambataro, Reference Cattarinussi, Di Giorgio, Wolf, Balestrieri and Sambataro2019). These results suggested that the IFG difference may be a heritable vulnerability factor for developing BD, which may be necessary but not sufficient to cause the condition. In addition, several studies found that functional activities in the bilateral prefrontal cortex and right orbital frontal cortex were normalized by rTMS treatment in depressed patients (Li et al., Reference Li, Nahas, Kozel, Anderson, Bohning and George2004; Speer et al., Reference Speer, Kimbrell, Wassermann, Repella, Willis, Herscovitch and Post2000). Taken together, reliable identification of the prefrontal cortex through large-scale multimodal meta-analysis strongly supports this region's role in BD. The abnormalities of the prefrontal gyrus (especially IFG) were found by different methodologies, and may be an early predictor and important target for early intervention in BD.
This study identified increased spontaneous activity in the bilateral striatum (putamen and caudate) and GMV deficits in the left thalamus in BD. In the subgroup analyses, increased ALFF was found in the striatum and thalamus in BD. Consistent with our findings, a recent VBM meta-analysis also reported smaller GMV in the thalamus in euthymic patients with BD relative to HCs (Wang et al., Reference Wang, Luo, Tian, Cheng, Qiu, Wang and Jia2019). After lithium treatment, the GMV of the thalamus increased in patients with BD (Abramovic et al., Reference Abramovic, Boks, Vreeker, Bouter, Kruiper, Verkooijen and van Haren2016). McDonald and colleagues (McDonald et al., Reference McDonald, Zanelli, Rabe-Hesketh, Ellison-Wright, Sham, Kalidindi and Kennedy2004) had reported significant heterogeneity among studies on the thalamus but included fewer studies (26 studies). Several studies have been published since then and we included 83 studies in our meta-analysis. The thalamus plays a key role in the integration and coordination of information as it passes through various brain regions, and its disturbance could explain the resultant psychotic symptoms (Cronenwett & Csernansky, Reference Cronenwett and Csernansky2010). In addition, previous neuroimaging studies reported increased functional activity (Blumberg et al., Reference Blumberg, Stern, Martinez, Ricketts, de Asis, White and Silbersweig2000), blood flow (He et al., Reference He, Sheng, Lu, Long, Han, Pang and Chen2019), metabolism (Ketter et al., Reference Ketter, Kimbrell, George, Dunn, Speer, Benson and Post2001), and dynamic functional connectivity (Chen et al., Reference Chen, Chen, Gong, Jia, Zhong, Chen and Wang2020a) in the striatum of BD patients. Anatomically, the prefrontal cortex projects heavily to the striatum as part of a circuit that loops through the basal ganglia and thalamus, and then back to the prefrontal cortex (Ding, Reference Ding2015). Fronto-striatal-thalamic regions and circuitry have been implicated in complex cognitive functions including response inhibition(Morein-Zamir & Robbins, Reference Morein-Zamir and Robbins2015), working memory(Clemensson, Clemensson, Riess, & Huu Phuc, Reference Clemensson, Clemensson, Riess and Huu Phuc2017; Darki & Klingberg, Reference Darki and Klingberg2015), and reward processing(de Leeuw, Kahn, & Vink, Reference de Leeuw, Kahn and Vink2015). Therefore, these findings, in conjunction with functional and structural changes of the prefrontal cortex, suggest a wider dysfunction of the fronto-striatal-thalamic circuitry, which could result in psychopathological and cognitive symptoms of BD.
The temporal cortex is involved in the processing of auditory information, language comprehension, semantic memory, visual perception, and sensory integration (Selvaraj et al., Reference Selvaraj, Arnone, Job, Stanfield, Farrow, Nugent and McIntosh2012). Our meta-analysis found decreased spontaneous activity in the left middle temporal gyrus extending to the superior temporal gyrus, and decreased GMV in the bilateral temporal pole extending to the superior temporal gyrus in BD. The subgroup analyses also found decreased ReHo in the left middle temporal gyrus in BD. Previous meta-analyses consistently found reduced GMV in the superior temporal gyrus in patients with BD (Selvaraj et al., Reference Selvaraj, Arnone, Job, Stanfield, Farrow, Nugent and McIntosh2012; Wang et al., Reference Wang, Luo, Tian, Cheng, Qiu, Wang and Jia2019; Wise et al., Reference Wise, Radua, Via, Cardoner, Abe, Adams and Arnone2017). Deficits in the superior temporal gyrus are associated with a wide array of symptoms including auditory hallucinations (Barta, Pearlson, Powers, Richards, & Tune, Reference Barta, Pearlson, Powers, Richards and Tune1990) and errors in facial emotion perception (Hanford, Nazarov, Hall, & Sassi, Reference Hanford, Nazarov, Hall and Sassi2016; Hoffman & Haxby, Reference Hoffman and Haxby2000). Similarly, studies also reported more widespread cortical thinning across the temporal lobes in BD, including the superior, middle and inferior gyri, and temporal pole (Hanford et al., Reference Hanford, Nazarov, Hall and Sassi2016). Functional MRI studies found different activation levels in the superior and middle temporal gyri during emotional processing in BD patients (Mitchell, Elliott, Barry, Cruttenden, & Woodruff, Reference Mitchell, Elliott, Barry, Cruttenden and Woodruff2004; Pavuluri, O'Connor, Harral, & Sweeney, Reference Pavuluri, O'Connor, Harral and Sweeney2007). Taken together, these findings are consistent with a role of temporal cortex alterations in BD.
This study also found that small parts of some brain regions exhibited either structural or functional changes in BD, such as decreased spontaneous activity in the precuneus and cerebellum, and decreased GMV in the fusiform gyrus extending to the cerebellum. We did not find any significant differences between patients with BD and HCs in lithium subgroup meta-analyses, which may be because patients with BD often take lithium and other mood stabilizers, and GMV is affected by lithium and other mood stabilizers. The precuneus/PCC and mPFC are major hub nodes of the default-mode network (DMN), which is involved in multiple cognitive and affective functions such as emotional processing, self-referential mental activity, distraction and recollection of experiences, and possibly exerts a modulatory role during attention-requiring tasks (Raichle, Reference Raichle2015). Aberrant activity and connectivity within the DMN are well-documented in different mood states of BD (Syan et al., Reference Syan, Smith, Frey, Remtulla, Kapczinski, Hall and Minuzzi2018; Vargas et al., Reference Vargas, Lopez-Jaramillo and Vieta2013; Wang et al., Reference Wang, Gao, Tang, Lu, Zhang, Bu and Huang2020; Zovetti et al., Reference Zovetti, Rossetti, Perlini, Maggioni, Bontempi, Bellani and Brambilla2020). For example, previous studies found that hypo-connectivity between the anterior and posterior DMN in manic BD patients might be related to an attention pattern that is excessively focused on external stimuli at the expense of internal reflection (Magioncalda et al., Reference Magioncalda, Martino, Conio, Escelsior, Piaggio, Presta and Amore2015; Wang et al., Reference Wang, Gao, Tang, Lu, Zhang, Bu and Huang2020). In contrast, hypo-connectivity within the posterior DMN in depressive BD patients might be related to rumination and working memory impairment (Gong et al., Reference Gong, Chen, Jia, Zhong, Zhao, Luo and Wang2019). The cerebellum is known to be connected to cortical areas involved in the pathophysiology of psychiatric disorders such as BD (Lupo, Sicilianob, & Leggio, Reference Lupo, Sicilianob and Leggio2019). Recently, a meta-analysis of VBM studies found decreased cerebellar gray matter in relatives of patients with BD, schizophrenia and major depressive disorder, which suggest that the cerebellum might be associated with shared risk for psychiatric disorders. Studies showed structural and functional alterations in the cerebellum in BD patients during the manic state (Sani et al., Reference Sani, Chiapponi, Piras, Ambrosi, Simonetti, Danese and Spalletta2016; Shaffer et al., Reference Shaffer, Johnson, Fiedorowicz, Christensen, Wemmie and Magnotta2018), depressed state (Wang et al., Reference Wang, Zhong, Jia, Sun, Wang, Liu and Huang2016; Zhao et al., Reference Zhao, Wang, Jia, Zhong, Sun, Zhou and Huang2016), and remitted state (Wang et al., Reference Wang, Zhong, Chen, Liu, Zhao, Sun and Huang2018b). Taken together, these findings imply that the cerebellum might be a key structure involved in the regulation of mood in BD (Zhang et al., Reference Zhang, Sweeney, Yao, Li, Zeng, Xu and Tallman2020a).
This study had some limitations. First, we could not determine whether these functional and anatomical alterations were part of the pathogenesis or a consequence of these disorders because of the nature of cross-sectional studies. Second, clinical details were often insufficiently reported in the studies for a comprehensive and powerful subgroup or meta-regression analyses. Including a range of clinical variables in future neuroimaging studies would be highly beneficial for future meta-analyses. Relevant clinical information could include bipolar subtype (e.g. I, II, with mainly mixed-episodes, or with psychotic features), mental state (e.g. depressive, manic, and euthymic), rating scale scores, medication status, type of medication administered (e.g. antidepressant, mood stabilizer and antipsychotics) and duration of administration, details of comorbidities, age of onset (geriatric or adolescent BD), disease duration or severity, and number of previous episodes. Third, the method used in this study is based on the peak coordinates and their effect size, rather than the original statistical brain map, which reduced the accuracy of the results (Radua et al., Reference Radua, Mataix-Cols, Phillips, El-Hage, Kronhaus, Cardoner and Surguladze2012b). Fourth, we used an uncorrected threshold of p < 0.005. Although previous studies have shown that the threshold can fully control the false positive rate (Radua et al., Reference Radua, Mataix-Cols, Phillips, El-Hage, Kronhaus, Cardoner and Surguladze2012b), it is an approximate value of the correction result. After FWE correction, the statistically significant results were significantly reduced. Following reasons may account for the differences: (1) the heterogeneity was not captured in this meta-analysis (e.g. type of medication administered), which may reduce the ability to observe the results after conservative statistical threshold, and (2) case-control studies usually included a small number of cases. Fifth, multimodal analysis conducted in this study did not directly detect correlations between structural and functional differences, but showed brain regions in which BD is associated with structural and functional changes. Future studies should investigate the spatial and temporal relationships between the structure and function of the brain regions detected in this meta-analysis. Finally, though several studies (Chen et al., Reference Chen, Wen, Malhi, Ivanovski and Sachdev2007; Molina et al., Reference Molina, Galindo, Cortés, de Herrera, Ledo, Sanz and Hernández-Tamames2011) revealed GMV reductions associated with lithium treatment, only five studies (< 10 studies) compared GMV between BD patients taking lithium and HCs, which did not meet the criteria for subgroup analysis.
In summary, the study findings suggested that BD exhibits similar differences in both function and structure of the insula (extending to the temporal cortex). In addition, fronto-striatal-thalamic and DMN regions exhibited functional or structural differences in BD. These results further expand the growing literature exploring anatomical and functional measures in BD, which provide useful insights for understanding the underlying pathophysiology of BD, and developing more targeted and efficacious treatment and intervention strategies. Further studies were needed with targeted interventions such as TMS therapy on the different brain regions, particularly the insula and prefrontal gyrus.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291722002392
Acknowledgements
The study was supported by grants from the National Natural Science Foundation of China (81671670, 81971597 and 82102003); National Key Research and Development Program of China (2020YFC2005700); Project in Basic Research and Applied Basic Research in General Colleges and Universities of Guangdong, China (2018KZDXM009); Key-Area Research and Development Program of Guangdong Province (2020B1111100001); Medical Science and Technology Research Foundation of Guangdong Province (A2021109). The funding organizations played no further role in study design, data collection, analysis and interpretation and paper writing.
Author contributions
Guanmao Chen: conception and design, acquisition of data, analysis and interpretation of data; drafted the article; gave final approval of the version to be published. Junjing Wang: conception and design, analysis and interpretation of data, gave final approval of the version to be published. Jiaying Gong: analysis and interpretation of data; drafted the article; gave final approval of the version to be published. Zhangzhang Qi: revised the manuscript critically for important intellectual content, gave final approval of the version to be published. Siying Fu: revised the manuscript critically for important intellectual content, gave final approval of the version to be published. Guixian Tang: acquisition of data, gave final approval of the version to be published. Pan Chen: acquisition of data, gave final approval of the version to be published. Li Huang: revised the manuscript critically for important intellectual content, gave final approval of the version to be published. Ying Wang: conception and design, acquisition of data, analysis and interpretation of data; revised the manuscript critically for important intellectual content, gave final approval of the version to be published.
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.







