Introduction
Stroke is the leading cause of disability and one of the leading causes of death. It also accounts for up to 11% of epilepsy.Reference Hauser, Annegers and Kurland1 Population-based data have shown that stroke is the underlying cause of epilepsy in older adults in more than 30%.Reference Kim, Sila, Koubeissi, Alshekhlee and Mehndiratta2
Seizures due to ischemic stroke (IS) are generally categorized into early seizures (ESs) (≤1–4 weeks) and late seizures (LSs) (≥1–4 weeks) based on different studies, but no consensus exists; however, ESs were defined by the International League Against Epilepsy as those occurring within 7 days of stroke onset.Reference Camilo and Goldstein3, 4
Tissue plasminogen activator (tPA) thrombolysis remains the gold standard treatment for IS within a limited window (∼4–5 h in most countries in Europe).Reference Knecht, Story and Liu5 Stroke mimics (including postseizure palsies) can account for approximately one of five clinically diagnosed acute strokes and the rate of stroke mimics (ictal or postictal paralysis, migraine, hysteria, hypoxic hemiplegia, and hypoglycemia) who are thrombolyzed can be as high as 17%.Reference Hacke, Kaste and Fieschi6 Based on recent trials, single-center and case-control studies stroke mimics are most likely identified as seizures or conversion disorder.Reference Winkler, Fluri and Fuhr7, Reference Scott and Silbergleit8
On the other hand, the recent AHA guideline states that seizure at onset with postictal residual neurological impairments is a relative exclusion criterion based on the findings of low-quality evidence (case series), suggesting that these residual deficits are attributable to ischemia rather than the postictal state.Reference Jauch, Saver and Adams9
Moreover, experimental studies suggested that recombinant tissue-type plasminogen activator (rt-PA) may play a role in the development of seizures/epilepsy. In rodent models, t-PA expression was increased after seizures and mediated kainic acid-induced seizure propagation, whereas t-PA/−/ mice are resistant to chemoconvulsant-induced seizures.Reference Tan, Ng and Pandher10 Recent clinical studies showed its possible association with ESs.Reference Alvarez, Rossetti and Papavasileiou11
Multimodal patient imaging (perfusion computer tomograph (CT) and magnetic resonance imaging (MRI) techniques) are important parts of acute stroke imaging to delineate the infarct from ischemic penumbra in specific situations such as stroke of unknown time of onset (SUTO) or in endovascular intervention with extended time window.Reference Balogun, Brown and Bertoni12, Reference Vilela13 Perfusion techniques play a vital role in diagnosing stroke mimics in stroke/thrombolysis pathway when the etiology is doubtful or in patients with SUTO .Reference Hedna, Shukla and Waters14
The aim of our review was to summarize the findings of different studies focusing on the pathogenesis, prevalence, risk factors, detection, management, and clinical outcome of ESs in IS and in stroke/thrombolysis situations. We also collected articles focusing on the association of rt-PA treatment and epileptic seizures.
We searched PubMed, MEDLINE, and the Cochrane Library restricted to English language publications to April, 2017. We used these search items in the following combinations: stroke, IS, seizure, ES, poststroke epilepsy (PSE), thrombolysis, rt-PA, alteplase, imaging, perfusion imaging, CT, MRI, electroencephalogram (EEG), outcome, and mortality. After reviewing the abstracts, we obtained and reviewed the full text and reference lists of relevant articles.
Pathogenesis of Early Ischemic Seizures
Mechanisms of early and late poststroke seizures are relatively poorly studied, mainly due to limited developments in animal modeling.Reference Reddy, Bhimani and Kuruba15 PSE has different pathophysiologic mechanisms based on these experimental data; transient cellular biochemical dysfunctions play an important role in the development of ESs, while gliotic scarring with persistent changes can initiate LSs.Reference Ferlazzo, Gasparini and Beghi16
Acute arterial occlusion and hypoperfusion lead to the loss of neurovascular unit integrity. Albumin enters the brain due to increased vessel wall permeability, binds directly to the astrocytes, and activates transforming growth factor beta (TGFβ). The activation of TGFβ signalling pathway leads to extracellular accumulation of potassium and glutamate due to reduced astrocytal uptake, with consequent hyperexcitability and low seizure threshold.Reference Tanaka and Ihara17 Glutamate is a major excitotoxic neurotransmitter and acts through various receptors including alpha-amino3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), kainate, and N-methyl-D-aspartate (NMDA). The activation of these receptors results in intracellular inflow of calcium and sodium lowering the seizure threshold for depolarization and leading to neuronal damage and death.Reference Tanaka and Ihara17, Reference Xie, Dong and Liu18 Furthermore, the excitatory neurotransmitter glutamate (Glu) and the inhibitory neurotransmitter γ-aminobutyric acid (GABA) have a close inverse relationship.Reference Xie, Dong and Liu18
Extravasated thrombin can bind the astrocytes to protease-activated receptor-1.Reference Reddy, Bhimani and Kuruba15–Reference Tanaka and Ihara17 Activated microglial cells and astrocytes release proinflammatory cytokines such as interleukin (IL)-1b, high-mobility group box 1 (HMGB1), tumor necrosis factor a (TNFa), IL-6, and IL-1b, worsening vessel injury and promoting PSE.Reference Reddy, Bhimani and Kuruba15–Reference Tanaka and Ihara17
In their clinical study, Xie et al. aimed to identify early predictors of PSE by measuring changes in blood levels of GABA, Glu, and calcium (Ca2+). They showed that patients with poststroke seizures had higher glutamate and lower calcium in their plasma compared to seizure-free cerebrovascular patients underlying the possible clinical importance of these molecules.Reference Xie, Dong and Liu18
ESs after Stroke
Included studies
We have identified 37 studies with 36,775 participantsReference Alvarez, Rossetti and Papavasileiou11, Reference Kim, Park and Choi19–53 (Table 1). Thirteen retrospective, single-center (15,190 patients), 15 prospective, single-center (9593 patients), 5 prospective, observational, population-based (7710 patients), and 4 prospective, multicenter (4282 patients) studies were identified (Table 1).
TABLE 1. Included studies

CVT, cerebral venous thrombosis; HS, hemorrhagic stroke; IS, ischemic stroke; SAH, subarachnoid hemorrhage; TIA, transient ischemic attack.
Cut-off values for the occurrence of ESs were different, from 24 h to 30 days. Twenty-one studies applied 1 week, 13 studies applied 2 weeks, 2 studies applied 30 days, and 1 study applied 24 h cut-off point.
Fourteen studies reported ES rates in IS, and the remaining studies also included patients with hemorrhagic stroke (HS)/subarachnoid hemorrhage (SAH) or with cerebral venous thrombosis (CVT) (Table 1).
Early seizure rates
ES rate was 1633/36,775 (4.4%) in the whole population. These rates were 3.4% in retrospective, single-center (519/15,190), 6.2% in prospective, single-center (597/9593), 3.5% in prospective, observational, population-based (272/7710), and 5.7% in multicenter studies (245/4282). As vast majority of these studies included patients with hemorrhagic stroke syndromes (HS, SAH) or CVT, we also calculated ES rates in IS (if reported).
ES rate was 3.8% (1012/26,409) overall in patients with IS; 3% (303/10,106) in retrospective, single-center, 5.4% (376/6991) in prospective, one-center, 2.5% (137/5400) in prospective, population-based, observational, and 4.3% (196/3912) in multicenter studies (Table 1).
ES rates were 3.5% (104/2959) in 5 studies from Northern America overall (84/2487, 3.3% in IS), 3.4% (304/8808) in 4 studies from the Far East (97/1117, 8.7% in IS), 9.5% in 4 studies from South Asia (40/324, 12.3% in IS), 10.6% (200/1880) in 1 study published from the Near East (Turkey) (138/1302, 10.6% in IS), 12% (72/603) in 2 studies from Africa (47/352, 13% in IS), 3.8% (795/20,575) in 20 studies (including all multicenter studies and 3 prospective, observational population-based studies) from Europe (532/16,720, 3.2% in IS).
Risk factors of early seizures
Cortical involvement (especially anterior) (13 studies), severe stroke (10 studies), hemorrhagic transformation (6 studies), age <65 (4 studies), large lesion (3 studies), and atrial fibrillation (3 studies) were the most important risk factors of ESs (Table 3). Hemorrhagic stroke syndromes (both HS and SAH) were also important predictors.Reference Wang, Jia and Chen22, Reference Conrad, Pawlowski and Dogan30, Reference Bladin, Alexandrov and Bellavance41, Reference Panitchote and Tiamkao44, Reference Goswami, Karmakar and Ghosh45, Reference Beghi, D’Alessandro and Beretta50
Metabolic disturbances such as elevated blood glucose or low sodium levels can also precipitate seizures.Reference Wang, Jia and Chen22, Reference Conrad, Pawlowski and Dogan30, Reference Serafini, Gigli and Gregoraci42, Reference Procaccianti, Zaniboni and Rondelli48 Low blood pressure and normal lipid levels seem to have protective effect.Reference Hundozi, Shala and Boshnjaku29, Reference Panitchote and Tiamkao44, 53 The other risk factors were low ASPECTS score, alcoholism, previous stroke, male gender, and impaired consciousnessReference Chen, Churilov and Koome28, Reference Hamidou, Aboa-Eboulé and Durier31, Reference Cheung, Tsoi and Au-Yeung36, Reference Goswami, Karmakar and Ghosh45, Reference De Reuck and Van Maele54 (Table 2).
TABLE 2. Risk factors of early and LSs

ASPECTS, Alberta Stroke Program Early CT Score; BP, blood pressure; ES, early seizure; HS, hemorrhagic stroke; IS, ischemic stroke; ICH, intracerebral hemorrhage; NIHSS, NIH Stroke Scale; SAH, subarachnoid hemorrhage.
Only 13 studies reported risk factors of late/recurrent seizures and epilepsy.Reference Kim, Park and Choi19, Reference Wang, Jia and Chen22, Reference Arntz, Rutten-Jacobs and Maaijwee23, Reference Chen, Churilov and Koome28, Reference Kammersgaard and Olsen37, Reference Lamy, Domigo and Semah39–Reference Serafini, Gigli and Gregoraci42, Reference Dhanuka, Misra and Kalita52–Reference De Reuck and Van Maele54 Severe and cortical strokes were the most important risk factors; ESs were associated with recurrent seizures/epilepsy in two studiesReference Kammersgaard and Olsen37, Reference Lamy, Domigo and Semah39 (Table 2).
The impact on clinical outcome is controversial: eight studies reported ESs as predictors of worse clinical outcome, while seven studies showed neutral effectReference Bryndziar, Sedova and Kramer20–Reference Arntz, Rutten-Jacobs and Maaijwee23, Reference Hamidou, Aboa-Eboulé and Durier31, Reference Couillard, Almekhlafi and Irvine32, Reference Labovitz, Hauser and Sacco38, Reference Bladin, Alexandrov and Bellavance41–Reference Goswami, Karmakar and Ghosh45, Reference Pezzini, Grassi and Del Zotto47, Reference Beghi, D’Alessandro and Beretta50, Reference Dhanuka, Misra and Kalita52, 53 (Table 2).
Early seizure type
Twenty studies reported clinical data with regard to ES type.Reference Kim, Park and Choi19, Reference Bryndziar, Sedova and Kramer20, Reference Wang, Jia and Chen22–Reference Jung, Schindler and Findling24, Reference Cordonnier, Hénon and Derambure27, Reference Conrad, Pawlowski and Dogan30, Reference Couillard, Almekhlafi and Irvine32, Reference Stefanidou, Das and Beiser35, Reference Cheung, Tsoi and Au-Yeung36, Reference Labovitz, Hauser and Sacco38–Reference Cordonnier, Hénon and Derambure40, Reference Aiwansoba and Chukwuyem46–Reference Procaccianti, Zaniboni and Rondelli48, Reference Beghi, D’Alessandro and Beretta50, Reference Misirli, Ozge and Somay51, 53, Reference De Reuck and Van Maele54 Based on their results, 63.8% (725/1137) seizures were partial and 35.2% (400/1137) were general at onset. Twelve seizures cannot be classified due to lack of adequate clinical data in five from the above-mentioned studies (12/1137, 1%).Reference Arntz, Rutten-Jacobs and Maaijwee23, Reference Cordonnier, Hénon and Derambure27, Reference Conrad, Pawlowski and Dogan30, Reference Labovitz, Hauser and Sacco38, Reference Cordonnier, Hénon and Derambure40 Status epilepticus (SE) occurred in 22.9% (130/567)Reference Kim, Park and Choi19–Reference Mohamed and Kissani21, Reference Cordonnier, Hénon and Derambure27, Reference Conrad, Pawlowski and Dogan30, Reference Cheung, Tsoi and Au-Yeung36, Reference Labovitz, Hauser and Sacco38–Reference Cordonnier, Hénon and Derambure40, Reference Serafini, Gigli and Gregoraci42, Reference Pezzini, Grassi and Del Zotto47–Reference Mecarelli, Pro and Randi49, Reference Dhanuka, Misra and Kalita52, Reference De Reuck and Van Maele54 (Table 3).
TABLE 3. Early seizure type
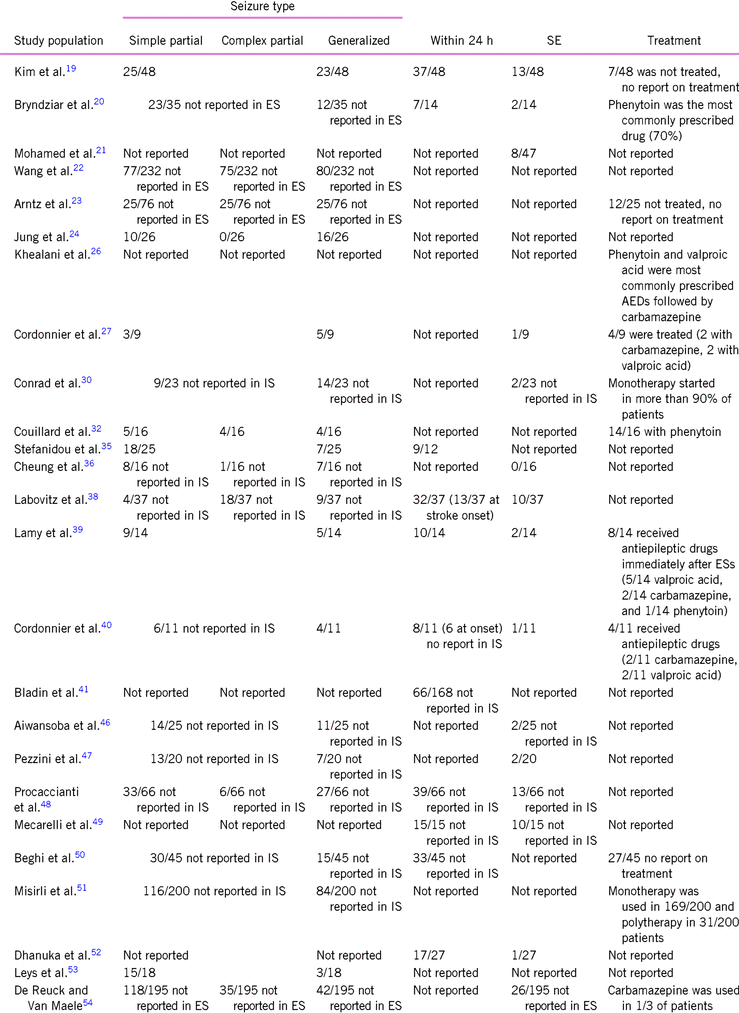
AED, antiepileptic drug; ES, early seizure; IS, ischemic stroke.
Partial seizures were simplex partial in 63.8% (245/384) and complex partial in 36.2% (139/245) based on the results of six studiesReference Wang, Jia and Chen22,32,36,38,48,54 (Table 3).
59.7% (273/457) of ESs occurred within 24 h,Reference Kim, Park and Choi19, Reference Bryndziar, Sedova and Kramer20, Reference Stefanidou, Das and Beiser35, Reference Labovitz, Hauser and Sacco38–Reference Bladin, Alexandrov and Bellavance41, Reference Procaccianti, Zaniboni and Rondelli48–Reference Beghi, D’Alessandro and Beretta50, Reference Dhanuka, Misra and Kalita52 and 39.6% was presented at the onset of stroke (19/48) based on the results of two studies.Reference Labovitz, Hauser and Sacco38, Reference Cordonnier, Hénon and Derambure40
As vast majority of these studies included patients with different stroke syndromes (IS, HS, SAH, and CVT), we also calculated ES type rates in IS (if reported).
Only six studies reported data on seizure type in IS.Reference Kim, Park and Choi19, Reference Cordonnier, Hénon and Derambure27, Reference Couillard, Almekhlafi and Irvine32, Reference Stefanidou, Das and Beiser35, Reference Lamy, Domigo and Semah39, 53 Sixty-one percent were partial (79/130) and 39% were general (47/130), and four patients had inadequate clinical data.Reference Cordonnier, Hénon and Derambure27, Reference Couillard, Almekhlafi and Irvine32 Partial seizures were simplex partial in 55.6% (5/9) and complex partial in 44.4% (4/9) based on the result of only one study.Reference Couillard, Almekhlafi and Irvine32 SE occurred in 16.3% (32/196).Reference Kim, Park and Choi19, Reference Bryndziar, Sedova and Kramer20, Reference Cordonnier, Hénon and Derambure27, Reference Cheung, Tsoi and Au-Yeung36, Reference Labovitz, Hauser and Sacco38–Reference Cordonnier, Hénon and Derambure40, Reference Pezzini, Grassi and Del Zotto47, Reference Dhanuka, Misra and Kalita52 73.6% (112/152) had an onset within 24 h.Reference Kim, Park and Choi19, Reference Bryndziar, Sedova and Kramer20, Reference Stefanidou, Das and Beiser35, Reference Labovitz, Hauser and Sacco38, Reference Lamy, Domigo and Semah39, Reference Dhanuka, Misra and Kalita52 Due to the lack of clinical data, we could not calculate seizure rate at stroke onset.
EEG findings
Eight studies reported EEG findings in poststroke seizures and the data of four additional studies were also included.Reference Kim, Park and Choi19, Reference Bryndziar, Sedova and Kramer20, Reference Wang, Jia and Chen22, Reference Khealani, Ali and Baig26, Reference Conrad, Pawlowski and Dogan30, Reference Beghi, D’Alessandro and Beretta50, Reference Dhanuka, Misra and Kalita52, Reference De Reuck and Van Maele54–Reference Velioğlu, Ozmenoğlu and Boz58 11.9% (88/739) had normal EEG, 49.3% (389/739) had diffuse/focal slowing, and 35% (259/739) had epileptiform discharge/periodic lateralized epileptiform discharge (PLED). Data of three patients were missing (0.3%).Reference Bryndziar, Sedova and Kramer20
EEG findings in IS/ES were properly reported in three studies.Reference Gupta, Naheedy and Elias55–Reference De Reuck, De Groote and Van Maele57 Based on their results, 19.8% (19/96) had normal EEG, diffuse slowing can be detected in 24% (23/96), focal slowing could be found in 37.5% (36/96), and 18.8% (18/96) had PLED/epileptiform discharge (Table 4).
TABLE 4. EEG findings in ES

PLED, periodic lateralized epileptiform discharge.
Brain imaging
Plain CT is the cornerstone of CT imaging to rule out hemorrhage; however, it has very limited role in the differentiation of stroke mimics.Reference Austein, Huhndorf and Meyne59
CT angiography (CTA) can be helpful to pick up large artery occlusion (but not in other conditions, for example, lacunar stroke syndromes), and only one study showed its contribution to decision making for thrombolysis.Reference Sylaja, Dzialowski and Krol60
We found 20 studies focusing on MRI diffusion-weighted imaging (DWI) changes in seizure patients.Reference Hufnagel, Weber and Marks61–Reference Jabeen, Cherukuri and Mridula80 Five case reports, 1 case series, and 16 single-center studies cover overall 355 patients (Table 5). Based on their results, most often DWI or ADC abnormalities could be detected in the hippocampus, thalamic/pulvinar region, and in the corpus callosum in the vast majority of the studies (Table 5). DWI changes may occur in various cortical and cerebellar locations helping in the identification of epileptic foci (for example, aphasic seizure seemed to be associated with temporoparietal DWI changes based on the results of two case reports).Reference Hong, Cho and Lee65, Reference Maalouf and Keyrouz72 Multiple DWI changes could be associated with longer and recurrent seizures in IS patients as reported by Kumral et al.Reference Kumral, Uncu and Dönmez71
TABLE 5. MRI diffusion-weighted imaging findings in seizure

ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging.
Twelve studies covering 188 patients including four case reports and eight single-center studies reported CTP changes in seizuresReference Hedna, Shukla and Waters14, Reference Austein, Huhndorf and Meyne59, Reference Wiest, von Bredow and Schindler81–Reference Payabvash, Oswood and Truwit90 (Table 6). Their results are relatively homonymous: focal hypo- or hyperperfusion or large hemispheral hypo –or hyperperfusion (usually sparing the basal ganglia) in atypical vessel pattern/non-corresponding vessel distribution could be detected. Comparing the CTP results of 17 stroke and 12 seizure patients, time to peak (TTP) parameter can be helpful in the differentiation of ischemia vs. seizure.Reference Kubiak-Balcerewicz, Fiszer and Nagańska88
TABLE 6. CT perfusion findings in seizure

There are limited reports in association with MRI perfusion and seizures. Three case reports detected DWI hyperintensity and hyperperfusion in the temporal lobe of patients with SE.Reference Calistri, Caramia and Bianco91–Reference Warach, Levin and Schomer93 Aphasic SE is also associated with temporoparietal DWI hyperintensity with corresponding hyperperfusion.Reference Toledo, Munuera and Sueiras68 Arterial spin labeling (ASL) MRI seems to be a promising technique in the identification of epileptic foci.Reference Gaxiola-Valdez, Singh and Perera94, Reference Chen, Lei and Ren95
Seizures and thrombolysis
In their retrospective study, Couillard et al. reported predictors of ESs, analyzing the clinical data of 400 thrombolyzed patients.Reference Couillard, Almekhlafi and Irvine32 The detected low seizure rate which was comparable to previous studies suggested tPA in itself is not a risk factor. ES rate was also low in two studies confirming their findings.Reference Jung, Schindler and Findling24, Reference Chen, Churilov and Koome28 De Reuck and Van Maele also could not find association between treatment modality and occurrence of seizures.Reference De Reuck and Van Maele34 However, systemic thrombolysis was a significant predictor of ESs in two studies including a multicenter trialReference Alvarez, Rossetti and Papavasileiou11, 53 (Table 1).
Treatment of early seizures
Twelve studies reported on antiepileptic treatment of ESs.Reference Kim, Park and Choi19, Reference Bryndziar, Sedova and Kramer20, Reference Arntz, Rutten-Jacobs and Maaijwee23, Reference Cordonnier, Hénon and Derambure27, Reference Conrad, Pawlowski and Dogan30, Reference Couillard, Almekhlafi and Irvine32, Reference Lamy, Domigo and Semah39, Reference Cordonnier, Hénon and Derambure40, Reference Misirli, Ozge and Somay51, Reference De Reuck and Van Maele54 Therapy was started in 79.3% (276/348).Reference Kim, Park and Choi19, Reference Arntz, Rutten-Jacobs and Maaijwee23, Reference Cordonnier, Hénon and Derambure27, Reference Couillard, Almekhlafi and Irvine32, Reference Lamy, Domigo and Semah39, Reference Cordonnier, Hénon and Derambure40, Reference Beghi, D’Alessandro and Beretta50, Reference Misirli, Ozge and Somay51 This rate was 37.9% (33/87) in IS patients.Reference Kim, Park and Choi19, Reference Cordonnier, Hénon and Derambure27, Reference Couillard, Almekhlafi and Irvine32, Reference Lamy, Domigo and Semah39 Carbamazepine, valproic acid, and phenytoin were the most commonly prescribed drugsReference Bryndziar, Sedova and Kramer20, Reference Khealani, Ali and Baig26, Reference Cordonnier, Hénon and Derambure27, Reference Couillard, Almekhlafi and Irvine32, Reference Lamy, Domigo and Semah39, Reference Cordonnier, Hénon and Derambure40, Reference De Reuck and Van Maele54 (Table 3).
Discussion
Based on our review article analyzing the data of 37 studies including more than 36,000 patients, we can conclude that ES rate was pretty low (4.4% overall). As vast majority of these studies included patients with IS and other stroke subtypes, we calculated ES rate in IS if reported. This was 3.8%, which was comparable to the existing literature.Reference Camilo and Goldstein3, Reference Wang, Vyas and Saposnik96 However, significant geographical differences could be found in the occurrence of ES, and there was a three-fold increase in Asian and African populations compared to European and Northern-American studies, which has been previously unreported. Despite extensive literature search, we could not find obvious explanation to these phenomena. Possibly, differences in stroke services and the more frequent occurrence of concomitant diseases (especially infections and metabolic changes) can elucidate these findings.Reference Wang, Jia and Chen22, Reference Hamidou, Aboa-Eboulé and Durier31, Reference Serafini, Gigli and Gregoraci42, Reference Procaccianti, Zaniboni and Rondelli48
Cortical involvement, large lesion, severe stroke, hemorrhagic transformation, younger age (<65 years), and atrial fibrillation were the most important risk factors of ES in IS. Metabolic disturbances such as low sodium or elevated plasma glucose can also precipitate seizures lowering the threshold. A recent meta-analysis (including all types of strokes) concluded that hemorrhagic stroke (significantly higher occurrence compared to IS) and cortical involvement are the most important risk factors of poststroke seizures.Reference Zou, Wu and Zhu97 It is a matter of debate whether an ES is a marker of stroke severity or not.Reference Jung, Schindler and Findling24
ES tends to be focal (more likely simplex partial than complex partial) at onset in about two-thirds of all patients and general in the remaining one-third. Sixty percent occur within 24 h and it is possible that 40% of all seizures are presented at the onset of stroke symptoms raising the possibility of other etiology, requiring an extensive diagnostic workup.Reference Labovitz, Hauser and Sacco38, Reference Cordonnier, Hénon and Derambure40, Reference Karunaratne, Bertoni and Balogun98 Aphasic SE or postictal confusion with paresis is indistinguishable from IS in patients with dementia or aphasia.Reference Toledo, Munuera and Sueiras68
EEG is not routinely available in the emergency rooms and the procedure takes time causing significant delay in acute stroke treatment. Furthermore, EEG is not really helpful apart from the detection of seizure patterns or non-convulsive SE as based on our results, about one-fifth of all ES patients had negative EEG findings and seizure-like activity could be detected only in approximately 18%. On the other hand, abnormal EEG findings can be detected in vast majority of acute stroke patients and seizures are presented in about one-third in patients with PLED/epileptiform discharge.Reference Mecarelli, Pro and Randi49, Reference Onder, Arsava and Topcuoglu99, Reference Finnigan and van Putten100
Plain brain CT has no place in the detection of seizures vs. ischemia. In patients with severe neurological symptoms, CTA can be helpful to pick up large-vessel occlusion but unable to detect hyperacute small vessel pathology or lacunar strokes.Reference Sylaja, Dzialowski and Krol60, Reference Coutts, Lev and Eliasziw101 Nevertheless, it only takes 10 additional minutes to perform to diagnose an intracranial occlusion and also gives information of the vascular status and extent of parenchymal ischemic injury, extracted from CTA source imaging.Reference Sylaja, Dzialowski and Krol60, Reference Schramm, Schellinger and Fiebach102, Reference González, Schaefer and Buonanno103
MRI DWI is the most sensitive and specific imaging technique for the visualization of an acute infarct, with excellent sensitivity and specificity within minutes after the onset of symptoms.Reference González, Schaefer and Buonanno103 MRI DWI seems to be helpful to distinguish between stroke and stroke mimics, which can be responsible for one quarter of all stroke ward admissions.Reference González, Schaefer and Buonanno103–Reference Dawson, Cloud and Pereira105 However, diffusion restriction can be detected in the hippocampal, thalamic, and callosal region in a considerable amount of seizure patients and in various cortical regions based on our literature search, but usually these lesions are not restricted to vascular territories.Reference Quenardelle, Lauer-Ober and Zinchenko106, Reference Förster, Griebe and Gass107 Finally, MRI DWI can be negative in a small (∼7%), but significant percentage of stroke patients, especially in strokes involving the posterior circulation.Reference Edlow, Hurwitz and Edlow108
CT and MR perfusions are promising imaging techniques in the detection of regional differences in blood flow, thereby able to differentiate the ischemic penumbra from the infarct core, which can be useful in patients with SUTO or patients with extended time window who are usually opted out from treatment.Reference Balogun, Brown and Bertoni12 They are potentially useful imaging techniques helping to determine candidates for systemic thrombolysis or revascularization.Reference Balogun, Brown and Bertoni12, 109
CTP involves a continuous imaging over a minute during the administration of a relatively large amount of bolus of contrast by monitoring the first pass of the contrast through the cerebral vasculature.Reference Saba, Anzidei and Piga110 Its advantage includes wide availability and short detection time, while it is associated with relatively large radiation dose and potential contrast-related side effects. It visualizes different derived color maps such as cerebral blood volume (CBV -- the total volume of blood flowing through a voxel of interest in a given unit of time), cerebral blood flow (CBF -- the total blood volume within the voxel of interest), mean transit time (MTT -- mean time taken for a contrast to pass through the voxel of interest and is equivalent to CBV/CBF), and TTP(time taken to reach maximal contrast enhancement). In IS, the core has a decrease in both CBF and CBV, whereas the penumbra demonstrates a reduction in CBF with a relatively maintained CBV with corresponding vessel distribution. Tissue at risk of infarction will also have elevated MTT.Reference Saba, Anzidei and Piga110, Reference Mair and Wardlaw111 In contrast, seizures are associated with focal or large hemispheral hypo- or hyperperfusion on CBV/CBF sequences (usually sparing the basal ganglia) in non-corresponding vessel distribution without MTT or TTP changes, which can be helpful in the differentiation of ischemia vs. seizure.Reference Shelly, Maggio and Boxer87, Reference Kubiak-Balcerewicz, Fiszer and Nagańska88
MR perfusion includes T1 signal intensity from baseline also through the period of contrast inflow to estimate the same parameters.Reference Mair and Wardlaw111 If intravenous contrast is contraindicated, an MR technique called ASL may obtain perfusion mapping for acute stroke.Reference Mair and Wardlaw111 The same changes can be detected as in the case of CTP, and it can also identify the tissue at risk which can be salvageable. There is very limited evidence which suggests that seizures can be accompanied by temporal hyperperfusion detected by MR perfusion technique.
Rodan et al. raised the possibility of thrombolysis-induced seizures in their case report.Reference Rodan, Aviv and Sahlas112 We found six studies with controversial results. A recent meta-analysis (including four out of the mentioned six studies) found no association between the application of rt-PA and the occurrence of seizures.Reference Lekoubou, Awoumou and Kengne113 However, the recent multicenter OPHELIE trial suggested its association with ES.53 Rt-PA has many properties beyond its thrombolytic activity, including control of neuronal survival, homeostasis of the blood–brain barrier, inflammation, axonal damage, and demyelination.Reference Lekoubou, Awoumou and Kengne113 A specific cleavage at the Arg275-Ile276 peptide bound converts the single-chain (sc) form of tPA into a two-chain (tc) form, which have similar thrombolytic properties, but the sc form promotes NMDA receptor signaling (with increased neurotoxicity, potentially lowering seizure threshold), while the tc form downregulates this signaling pathway.53, Reference Docagne, Parcq and Lijnen114 The above-mentioned OPHELIE trial raised the possibility that the ratio of sc/(sc 1 tc) rtPA >80.5% can be associated with ES.53 This trial was not included in the recent meta-analysis.Reference Lekoubou, Awoumou and Kengne113
The impact of ES on clinical outcome is also controversial: eight studies reported ESs as predictors of worse clinical outcome, while seven studies showed neutral effect. However, the meta-analysis of Xu et al. suggested higher risks of both mortality and disability in a large cohort of poststroke patients.Reference Xu, Ou and Liu115 It is possible that ESs are the markers of stroke severity and by this are associated with worse clinical outcome.Reference Jung, Schindler and Findling24 On the other hand, a significant proportion of patients can develop SE, which can be as high as 16% based on our results. It is well known that SE is associated with higher fatality rates and unfavorable clinical outcome.Reference Lv, Wang and Cui116
The AHA and ESO guidelines stated against the routine use of antiepileptic drugs in ES and there is also insufficient evidence with regard to the initiation and type of antiepileptics (if required).117, 118 There is no evidence of immediate primary prophylaxis in ES, but based on our results about 40% of these patients were treated. Carbamazepine, valproic acid, and phenytoin were the most commonly prescribed drugs. The only trial (although focusing on LSs) found similar efficacy between carbamazepine and levetiracetam, the latter has advantages on cognitive functions.118 However, a significant proportion of patients can develop SE and ES may be a risk factor of PSE.Reference Ferlazzo, Gasparini and Beghi16
Strzelczyk et al. assessed their PSE risk scale (PoSERS) within a follow-up of 1 year.Reference Strzelczyk, Haag and Raupach33 Data on 10 risk items concerning the stroke localization, stroke subtype, stroke severity, hemorrhagic transformation, previous vascular encephalopathy, early- and late-onset seizures were collected using a PSE risk scale (PoSERS), which showed moderate sensitivity and positive predictive value while specificity and negative predictive value were relatively high, if only ESs within 24 or 48 h were considered, maybe promoting clinicians’ decision with regard to treatment initiation.Reference Strzelczyk, Haag and Raupach33
In conclusion, we overtook a systematic and in-depth review on ESs after IS. ESs are rare complications of acute stroke with substantial burden. A significant proportion can be presented at the onset of stroke requiring an extensive diagnostic workup. Multimodal brain imaging can be helpful in the differentiation of seizure vs. ischemia. Large, multicenter trials are needed to evaluate the role of different variables on seizure risk and to evaluate the initiation and type of optimal treatment.
Availability of data and materials
The dataset supporting the conclusions of this article is available on request to the corresponding author.
Disclosures
The authors declare that they have nothing to disclose.








