INTRODUCTION
Reproductive strategies displayed by bivalves often vary according to the manner in which energy is provided for reproduction (Bayne, Reference Bayne and Wiley1976). Some species, such as the cockle Cerastoderma edule (Navarro et al., Reference Navarro, Iglesias and Larrañaga1989) and the scallop Nodipecten subnodosus (Arellano-Martínez et al., Reference Arellano-Martínez, Racotta, Ceballos-Vázquez and Elorduy-Garay2004), show a ‘conservative’ reproductive strategy. Energy reserves are built up over a period and later mobilized to support gamete production. This type of reproductive activity requires macromolecular transfers between tissues. In contrast, other species, such as the cockle Glycymeris glycymeris (Galap et al., Reference Galap, Leboulenger and Grillot1997) and the surf clam Donax dentifer (Riasco, Reference Riasco2005), show an opportunistic strategy. Gametes are produced during periods when food is available; otherwise, there is reproductive rest. The temperate mussel Mytilus galloprovincialis appears to follow the conservative strategy in some periods and the opportunistic strategy in others (Villalba, Reference Villalba1995).
In many scallop species, the digestive and reproductive systems are closely situated and often intertwined, although these organs are more separate than in other bivalves (Beninger & Le Pennec, Reference Beninger, Le Pennec and Shunway1991). This structure is useful when gametogenesis relies on transfer of nutrients from the digestive gland to the gonad (Beninger et al., Reference Beninger, Le Pennec and Le Pennec2003). Transfer of lipids to the gonads from the digestive gland during gametogenesis has been suggested for many scallop species, such as Placopecten magellanicus (Robinson et al., Reference Robinson, Wehling, Morse and Macleod1981), Pecten maximus (Le Pennec et al., Reference Le Pennec, Le Pennec and Beninger2001). Such transfer of lipids from digestive gland to oocytes has been confirmed using 14C as a marker in Chlamys hericia (Vassallo, Reference Vassallo1973) and Argopecten irradians (Barber & Blake, Reference Barber and Blake1985); and by using ferritin (an iron-containing transfer protein) in Pecten maximus (Beninger et al., Reference Beninger, Le Pennec and Le Pennec2003). The strategy of lipid transfer may vary with the reproductive strategy: the opportunistic strategy would lead to direct transfer of recently absorbed lipids whereas the conservative strategy would deplete the digestive gland of the specific lipids that are essential for gametogenesis.
Fatty acid profiles (FAPs) can differ according to tissue specific requirements and are influenced by the FAPs of ingested food. These conclusions are borne out by analyses of the FAPs of individual organs in the scallops Argopecten purpuratus (Caers et al., Reference Caers, Coutteau, Cure, Morales, Fajardo and Sorgeloos1999a) and N. subnodosus (Palacios et al., Reference Palacios, Racotta, Kraffe, Marty, Moal and Samainc2005) and the clam Mya truncata (Birkely et al., Reference Birkely, Grahl-Nielsen and Gulliksen2003). We reasoned that the dynamics of tissue FAPs could help elucidate the energetic strategy used by bivalves during reproductive investment. Effectively, as energy transfers between tissues take place over time, evaluation of how FAPs in different organs change through an annual cycle could help reveal how a bivalve population acquires and transfers lipids from its reserve tissues. When a species reproduces under a range of environmental conditions, as can occur with tropical species, this approach should help identify the energetic strategy(ies) followed. This has been shown in species in which FAPs differ between periods with contrasting environmental conditions, such as N. nodosus in which gonad fatty acids such as:16:0, 22:6n-3, 20:5n-3 and 16:1n-7 vary markedly between periods of upwelling and upwelling relaxation (Freites et al., Reference Freites, García, Troccoli, Maeda-Martínez and Fernández-Reiriz2010).
The upwelling ecoregion of the north-eastern coast of Venezuela is characterized by high productivity and low temperature (Miloslavich & Klein, Reference Miloslavich, Klein and Klein2008). Upwelling occurs primarily from January to July, following a period with relatively low phytoplankton levels from August to November–December. This seasonal cycle in upwelling is related to the intensity of trade winds. Maximal wind intensities over 6 m/s occur between November and May, decreasing sea surface temperatures (SST) and increasing chlorophyll-a concentrations (Muller-Karger et al., Reference Muller-Karger, Varela, Thunell, Astor, Zhang, Luerssen and Hu2004). The north-eastern coast of Venezuela shows two types of phytoplankton community structure, one characterized by high abundance and richness, dominance of some species and low equitability, leading to constant values in community diversity, and a second community with low abundance and richness, and high values of equitability (Pirela-Ochoa et al., Reference Pirela-Ochoa, Troccoli and Hernández-Ávila2008). The first community is similar to those developed during upwelling and the second community resembles that of the upwelling relaxation period (Estrada & Blasco, Reference Estrada and Blasco1979; Tilstone et al., Reference Tilstone, Figueiras and Fraga1994). This suggests that bivalve food sources vary qualitatively and quantitatively between the upwelling and upwelling relaxation periods.
The scallop N. nodosus is an epibenthic species up to 150 mm in length and is found at water depths between 10 and 100 m. In general, they live separated from one another on coral formations or at the edges of rocky and sandy substrates and, therefore, are not normally present in abundance (Lodeiros et al., Reference Lodeiros, Marín and Prieto1999). This species is distributed from North Carolina to Brazil, Ascension Island and the mid-Atlantic. Nodipecten nodosus is a functional hermaphrodite and due to the quality and commercial value of the meat, it has considerable potential for cultivation (Maeda-Martínez et al., Reference Maeda-Martínez, Lombeida, Freites, Lodeiros, Sicard and Maeda-Martínez2001). On the north coast of the Araya Peninsula, north-eastern of Venezuela, the gonadosomatic index (GSI) of N. nodosus peaks at 18%, in November and April, and the GSI and gonad dry weight decrease significantly from late October to January and again from April to May (Garcia et al., Reference García, Lodeiros, Arrieche, Prieto, Freites and Himmelman2007). This suggests two major spawning periods. Considering that these spawning events occur during periods that differ in food availability, N. nodosus is an appropriate species to test the idea that one species can use different energetic strategies to support reproductive processes according to environmental conditions and food availability.
Despite their commercial importance, there are few studies on the biochemical composition of natural populations of N. nodosus. Vélez et al. (Reference Vélez, Sotillo and Pérez1987) studied the seasonal variation of the biochemical composition of soft tissues in specimens from north-eastern Venezuela. They interpreted the abrupt increases and decreases in total lipids as continuous reproduction, with the greatest spawning activity in May and between August and December. We compared the FAPs and total lipids of three major tissues (digestive gland, muscle and mantle) to those of the female gonad (Freites et al., Reference Freites, García, Troccoli, Maeda-Martínez and Fernández-Reiriz2010) of the tropical scallop N. nodosus over an annual cycle to evaluate whether the energetic strategy of reproductive conditioning of this species changes with the marked shifts in temperature and food availability that occur with seasonal changes in upwelling.
MATERIALS AND METHODS
Collection of bivalves
Fifty individuals (>75 mm in antero-posterior axis) of Nodipecten nodosus were obtained from the field monthly for one year (August 2003–July 2004) trawling at a depth between 8 and 15 m, as in the Arca zebra fishery, on the Chacopata bank situated to the north-east of the Araya Peninsula, Sucre State, Venezuela (Figure 1). Scallops were transferred live to the laboratory at the Centre for Ecological Research (Guayacán) at the Universidad de Oriente in isothermal containers at a temperature below field values (18–20°C).

Fig. 1. Geographical location of the sampling site where Nodipecten nodosus was collected.
Analysis of total lipids and fatty acids
For all biochemical analyses of gonad, muscle, mantle and digestive gland, three replicates were analysed. Each replicate consisted of the dry tissues (oven-dried at 60°C for 72 h) of 8 individuals taken at random from 24 scallops with a range of 80–90 mm in length. The pooled tissues were pulverized and then 1 g was taken for biochemical analysis. Lipids from gonad, muscle, digestive gland and mantle dry tissue were extracted following Bligh & Dyer (Reference Bligh and Dyer1959) as modified by Fernández-Reiriz et al. (Reference Fernández-Reiriz, Perez-Camacho, Ferreiro, Blanco, Planas, Campos and Labarta1989). The lipid extract was recovered with a 1:2 mixture of chloroform:methanol. The solvent was dried by evaporation with N2 gas and the lipids were derivatized with a 5:95 mixture of hydrochloric acid:methanol at 85°C for 2.5 h. The solvents contain 0.05% butylated hydroxytoluene (BHT). Total lipids were determined gravimetrically by evaporating the solvent of 200 μl of purified extract onto pre-weighed aluminium plates on a slide warmer (60–80°C) (Rouser et al., Reference Rouser, Kritchevsky, Yamamoto and Marinetti1967).
The fraction containing the fatty acid methyl ester (FAME) of three replicates of different tissues was re-suspended in hexane and collected in tapered vials containing tricosanoic acid 23:0 as an internal standard (Sato & Murata, Reference Sato and Murata1988). The FAME samples were analysed using a Hewlett Packard G1800B gas mass chromatograph equipped with an on-column injector and a silica capillary column (omega wax TM 250 Supelco) internally covered by polyethylene glycol (30 m × 0.25 mm, 0.25 μm film thickness) with an electron ionization detector. High purity helium was used as the carrier gas at a flow rate of 0.9 ml/min. The temperature of the oven was programmed from 110°C to 165°C at 30°C min−1 and from 165°C to 209°C at 2.2°C min−1. The temperatures of the detector and injector were 260°C and 250°C, respectively.
Fatty acids in the samples were identified by comparing mass spectra with spectra contained in the mass spectral NIST/NBS library (database). They were verified by interpretation of mass spectra of methyl esters of fatty acids (McLafferty & Turecek, Reference McLafferty and Turecek1993) and by comparison of retention times of peaks in the sample, with retention times of a commercial pattern of 37 methyl esters of fatty acids (Supelco 47 855-U). The individual fatty acids, total polyunsaturated fatty acids (PUFA), Σ of the saturated, monounsaturated, PUFA n-6 and PUFA n-3 data are expressed in relative percentages of the total fatty acids (%).
Statistical analysis
Bartlett's test was employed to verify the homogeneity of variances of total lipids of each tissue. Thereafter, analysis of variance (ANOVA) was applied. The level of significance for ANOVAs was 0.05%. Values expressed as percentage of the different fatty acids were arcsine transformed before analysis (Zar, Reference Zar1984).
In their analysis of the gonadal FAPs of N. nodosus, Freites et al. (Reference Freites, García, Troccoli, Maeda-Martínez and Fernández-Reiriz2010) established two periods; one dominated by upwelling (January to July) and the other by upwelling relaxation (August to December). In the present study we examined differences in mantle, digestive gland, muscle and gonad FAPs within these periods by non-parametric multidimensional scaling analysis (MDS-ANOSIM), from the ‘Plymouth Routines in Multivariate Ecological Research’ (PRIMER) package 5.1 (Kruskal & Wish, Reference Kruskal and Wish1978). This approach evaluates similarities of the FAP, both within and among tissues and how they change with time. Standardized transformed data were used to produce a two-dimensional ordination plot of the within similarity of the FAPs of each tissue. Similarity percentage (SIMPER) analysis was used to assess the variability of tissue FAPs over time and to determine the contribution of each FA to the average Bray–Curtis similarity within each tissue FAP cluster, and to the average Bray–Curtis dissimilarity among FAPs of the different tissues using the software package PRIMER (Clarke & Warwick, Reference Clarke and Warwick1994).
RESULTS
Total lipids
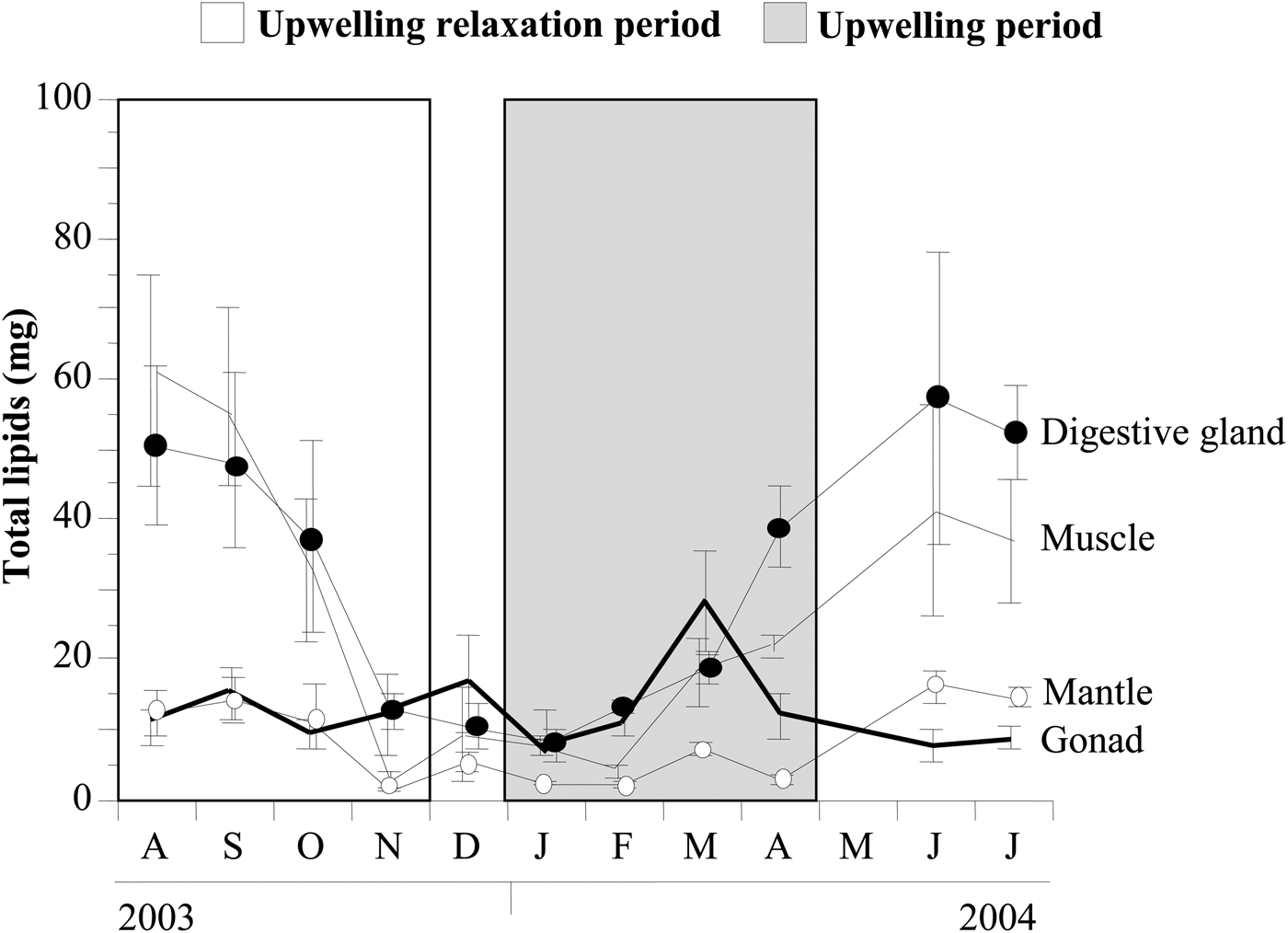
Digestive gland and muscle showed higher and more variable total lipid contents (P < 0.01) than the gonad and mantle over the annual cycle (Figure 2). Digestive gland lipid contents did not differ from those in the gonad in the period from November to February (P > 0.05). In the gonad, total lipids decreased in October 2003, January 2004 and again from April to June 2004 (coinciding with spawning: Freites et al., Reference Freites, García, Troccoli, Maeda-Martínez and Fernández-Reiriz2010). The lipid content of the digestive gland fell between September and November 2003 (during upwelling relaxation), and then rose gradually between January and June 2004 (upwelling).

Fig. 2. Total lipid contents in the gonad, muscle, mantle and digestive gland tissues of Nodipecten nodosus in the natural Chacopata bank, north-east Venezuela.
Fatty acid profile of individual tissues
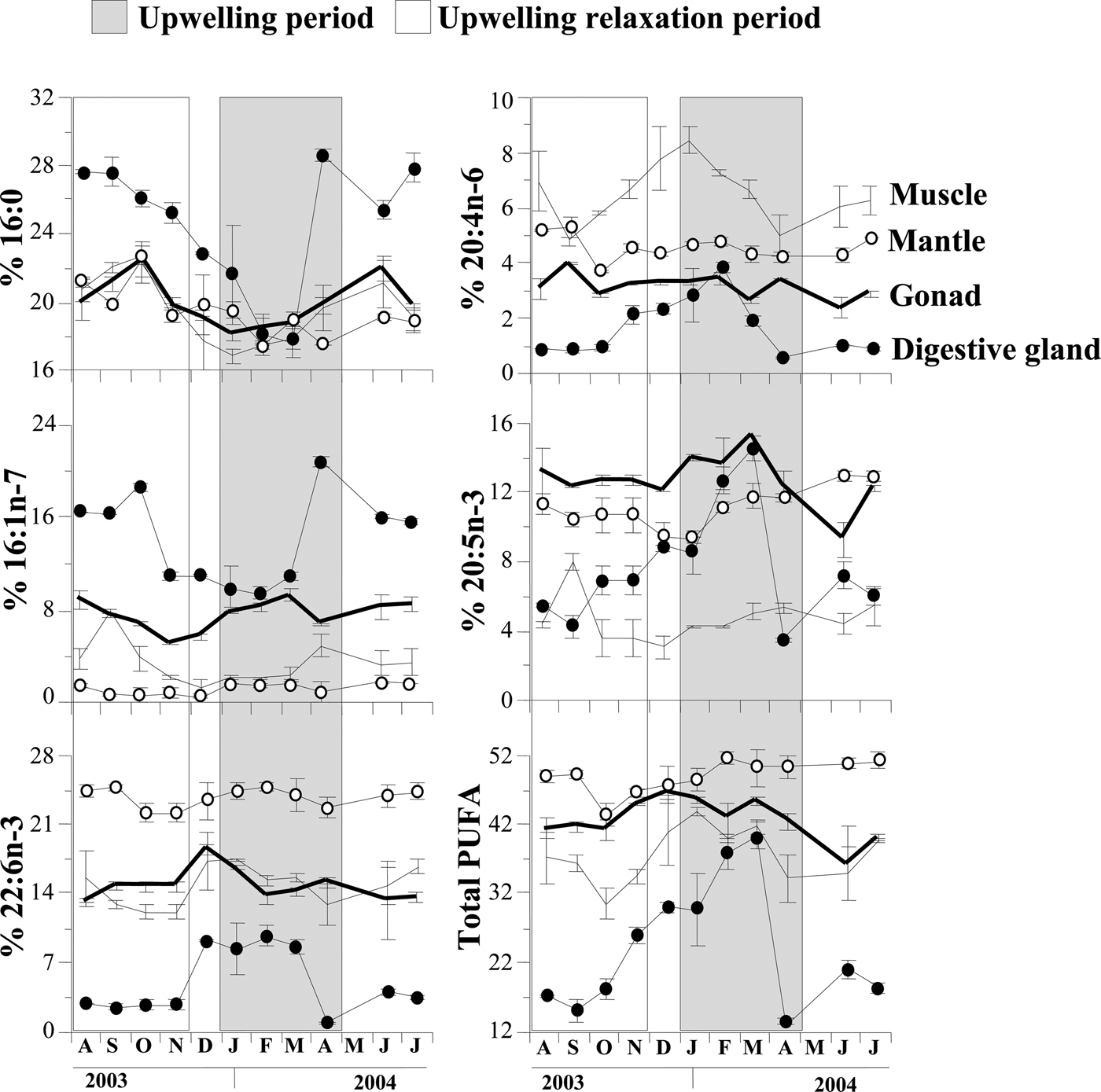
The percentages of the fatty acids, 16:0, 16:1n-7, 20:4n-6, 20:5n-3 and 22:6n-3, and total PUFA in the gonad, muscle, mantle and digestive gland varied seasonally (Figure 3). In general for all tissues, some fatty acids, such as 20:4n-6, 20:5n-3 and 22:6n-3, as well as total PUFA showed their highest levels during upwelling and lower levels during upwelling relaxation. In contrast, other fatty acids such as 16:0 and 16:1n-7, showed their highest levels during upwelling relaxation and lower levels during upwelling, except for gonad tissue.

Fig. 3. Variation of the relative percentages (%) of the fatty acids 16:0, 20:4n-6, 16:1n-7, 20:5n-3, 22:6n-3, and total polyunsaturated fatty acids (PUFA) in the different tissues of Nodipecten nodosus, during the periods of upwelling and upwelling relaxation.
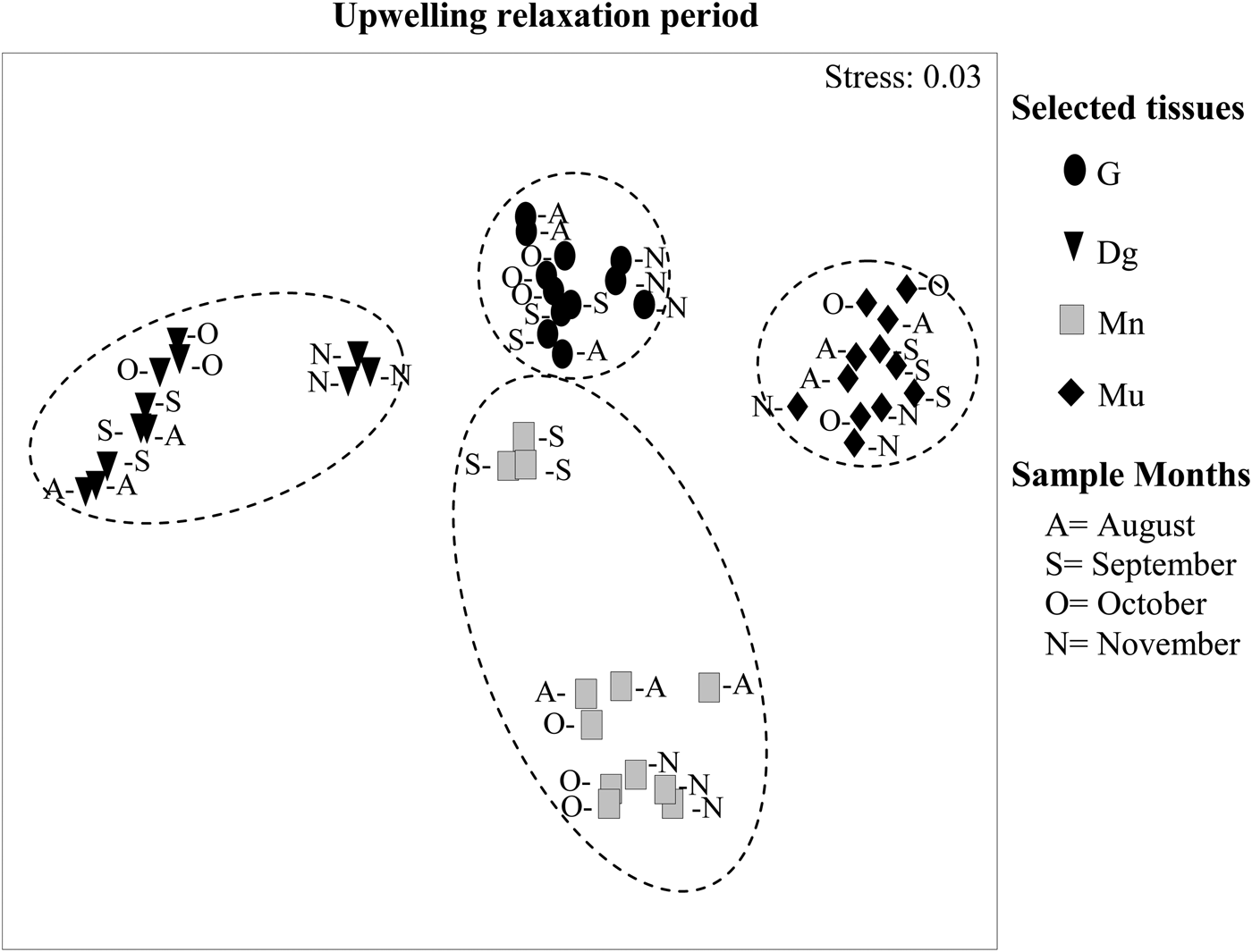
Seasonal variations of tissue FAPs
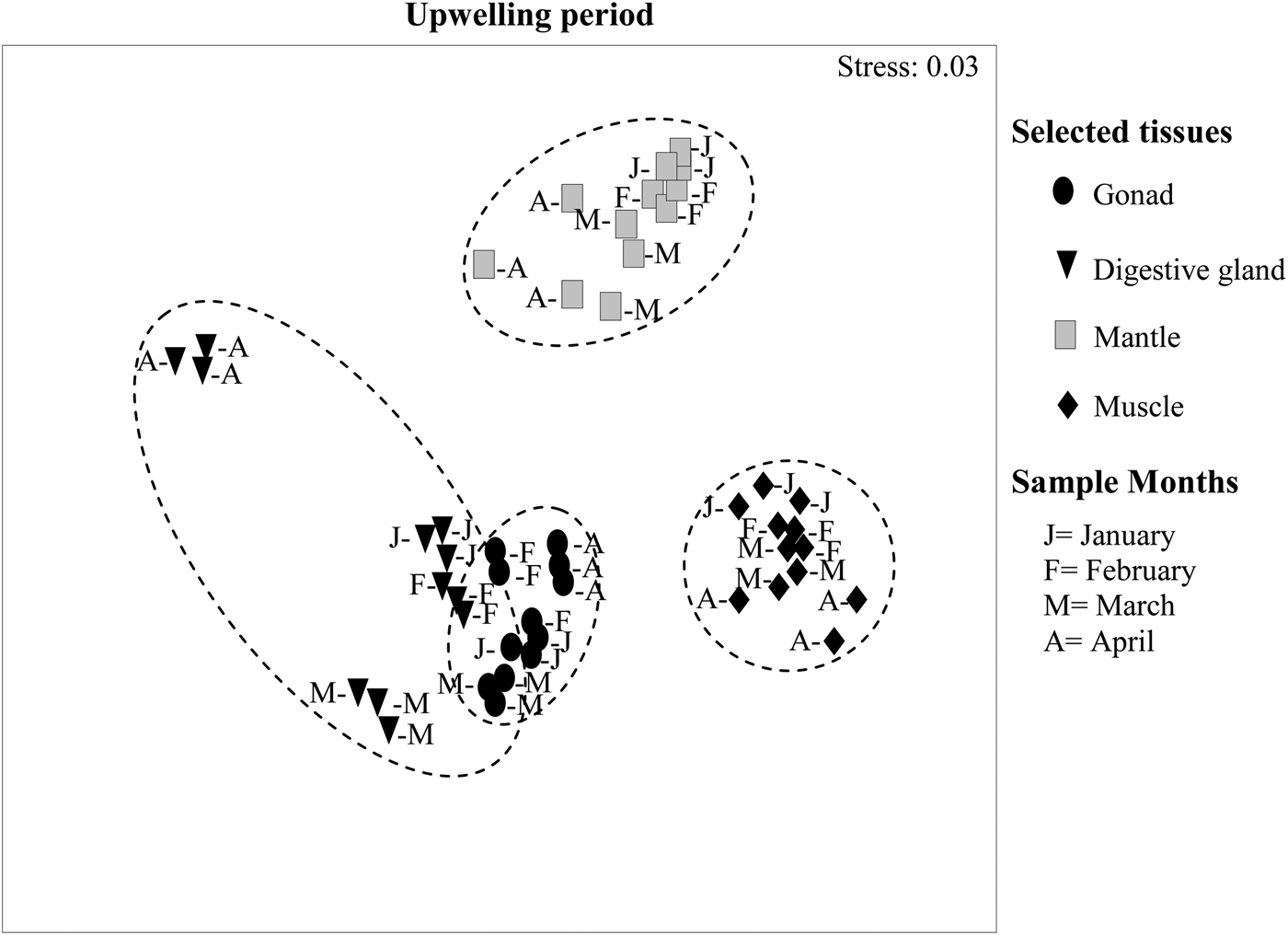
MDS-ANOSIM revealed significant differences between FAPs of the four tissues both in the upwelling (R = 0.79, P < 0.01) and upwelling relaxation periods (R = 0.95, P < 0.01). During upwelling, the FAP of the gonad, muscle and mantle formed well defined groups, whereas the FAP of digestive gland were more scattered than those of the other tissues (Figure 4). In January, February and March, the FAP of the digestive gland were very close to those of the gonad. In the upwelling relaxation period (Figure 5), FAP of gonad and muscle still show well-defined groups, while digestive gland and particularly mantle showed more scattered FAPs. Moreover, digestive gland FAP became increasingly close to gonad FAP from August to November.

Fig. 4. Multidimensional analysis (MDS-ANOSIM) comparing the variations in the fatty acid profiles (FAPs) of four tissues of Nodipecten nodosus in the upwelling period (January–April) at the study site (three similar symbols in each month corresponding to three replicate FAPs of each tissue).

Fig. 5. Multidimensional analysis (MDS-ANOSIM) comparing the variations in the fatty acid profiles (FAPs) of four tissues of Nodipecten nodosus in the upwelling relaxation period (August–November) seasons at the study site (three similar symbols in each month corresponding to three replicate FAPs of each tissue).
SIMILARITY INDEX OF FAPS OF EACH TISSUE
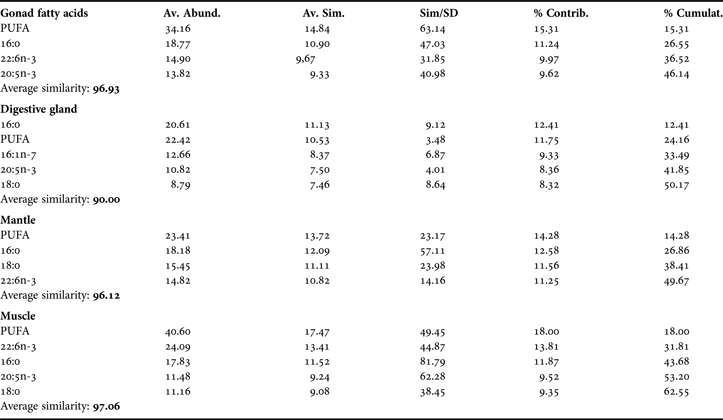
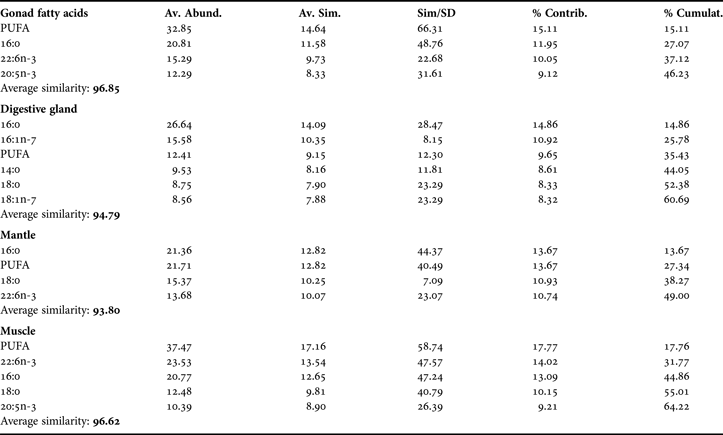
During upwelling period, SIMPER revealed a similarity index of FAP as follows: digestive gland (90.00); mantle (96.12); gonad (96.93) and muscle (97.06), showing that the FAP profiles of the latter three tissues changed less in time than those of the digestive gland (Table 1). During upwelling, 42–63% of the variance of the FAP of each tissue was explained by the fatty acids 16:0, 18:0, 16:1n-7, 20:5n-3, 22:6n-3 and total PUFA (Table 1). In the upwelling relaxation period (Table 2), SIMPER revealed similarity index of FAP within each tissue as follows: mantle (93.80); digestive gland (94.79); gonad (96.85) and muscle (96.62), showing that, in this period, gonad and muscle are most homogeneous in their FAP profiles. During upwelling relaxation, 14:0 and 18:1n-7 together with the fatty acids that vary during upwelling, explained 46 to 64% of the observed variance (Table 2).
Table 1. SIMPER results of percentage share (>8%) in the index of similarity of some fatty acids in the female gonad, digestive gland, mantle and muscle, during the upwelling season.

Table 2. SIMPER results of percentage share (>8%) in the index of similarity of some fatty acids in female gonad, digestive gland, mantle and muscle, in non-upwelling season.

DISSIMILARITY INDEX OF THE FAPS OF FOUR TISSUES
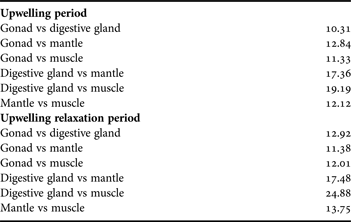
When the FAPs of four somatic tissues observed during upwelling period were compared (Table 3), gonadal FAPs showed a lower dissimilarity index with the digestive gland (10.31), than with muscle (11.33) or mantle (12.84). During upwelling relaxation, gonadal FAPs (Table 3) showed a lower dissimilarity index with the mantle (11.38), than with muscle (12.01) or digestive gland (12.92).
Table 3. SIMPER results of the index of dissimilarity of between female gonad, digestive gland, mantle and muscle, in upwelling and upwelling relaxation periods.
DISCUSSION
In marine bivalves, as in most animals, lipid quality and quantity varies with food quality and quantity as well as with physiological status. In Nodipecten nodosus as in Argopecten purpuratus, total lipids were generally higher in the digestive gland than in the gonad (this study: Caers et al., 1999a). The lowest levels of total gonadal lipids (October, January, April–June) coincided with spent periods and declines in the gonadosomatic index (Freites et al., Reference Freites, García, Troccoli, Maeda-Martínez and Fernández-Reiriz2010). These results presumably reflect the loss of lipid-rich gametes during spawning (Gabbott, Reference Gabbott and Wilbur1983). Effectively, overall lipid levels decrease markedly with spawning in the scallop Pecten maximus (Besnard et al., Reference Besnard, Lubet and Nouvelot1989; Pazos et al., Reference Pazos, Román, Acosta, Abad and Sánchez1997). Similarly, Palacios et al. (Reference Palacios, Racotta, Kraffe, Marty, Moal and Samainc2005) observed declines in MUFA proportions immediately after reproduction in N. subnodosus, while in Pecten maximus, 18:3n-3, 18:4n-3 and 20:5n-3 in neutral lipids show their lowest concentrations after the July spawning (Besnard et al., Reference Besnard, Lubet and Nouvelot1989). Digestive gland lipids showed a pronounced annual cycle, with peak values from June to August. These high total lipid reserves decline steadily until November (during gonadal ripening and period of minimal availability of phytoplankton (>1 µg l−1), see Freites et al. (Reference Freites, García, Troccoli, Maeda-Martínez and Fernández-Reiriz2010)), and to a lesser extent until January, suggesting their gradual use for the formation of reproductive tissue.
In general, saturated (SAT) and polyunsaturated fatty acids (PUFA) were the most abundant fatty acids in the four tissues of N. nodosus. This is also true for oysters Ostrea edulis (Abad et al., Reference Abad, Ruiz, Martínez, Mosquera and Sanchez1995) and Crassostrea gigas (Pazos et al., Reference Pazos, Ruiz, Garcia-Martin, Abad and Sanchez1996) and for N. subnodosus (Palacios et al., Reference Palacios, Racotta, Kraffe, Marty, Moal and Samainc2005). The FAP of bivalve molluscs reflects the influence of phytoplankton, their primary food source, which contains a high proportion of long chain polyunsaturated fatty acids (Fernández-Reiriz et al., Reference Fernández-Reiriz, Perez-Camacho, Ferreiro, Blanco, Planas, Campos and Labarta1989; Veloza et al., Reference Veloza, Chu and Tang2006).
Various environmental variables change considerably between the upwelling and upwelling relaxation periods in northeastern of Venezuela (Freites et al., Reference Freites, García, Troccoli, Maeda-Martínez and Fernández-Reiriz2010). Maximal temperatures were reached during upwelling relaxation periods (26.8°C), while minimal values were observed at beginning of upwelling (22.5°C). Temperatures remained under 25°C throughout upwelling. Relatively low concentrations of chlorophyll-a (0.5–1 µg l−1) were observed in the transitions between upwelling and upwelling relaxation periods, while the maximum median values of chlorophyll-a reached in these periods were 2.2 and 1.7 µg l−1, respectively. The organic fraction of seston showed higher concentrations during upwelling coincident with relatively high phytoplankton biomass. These patterns are consistent with the observations of Mandelli & Ferraz-Reyes (Reference Mandelli and Ferráz-Reyes1982) who showed that coastal upwelling during the first seven months of the year led to low temperatures and high production, which in some years reached values above 231 g of C m−2.
During upwelling, the MDS analysis revealed clear differences between monthly samples of the FAP of the four tissues (see Figure 4A). MDS provided insight into the similarities of the FAP composition of different tissues and their change over time. Well-defined FAP groups of mantle, muscle and gonad (>96 similarity index) were apparent. The FAP of digestive gland were more scattered which is reflected in their lower index of similarity (90%, Table 1). The MDS analysis showed a close association between gonadal FAP and FAP of digestive gland in January, February and March. This fact, together with the low index of dissimilarity (10) between gonad and digestive gland FAPs, suggests direct transfer of lipids from digestive gland to gonad and a possible opportunistic reproductive strategy (sensu Bayne, Reference Bayne and Wiley1976).
The digestive gland FAP in April was clearly separated from the other months of upwelling (see Figure 4A). This was due to strong increases in the levels of certain fatty acids, such as 16:0, 16:1n-7, 17:1n-7, 18:1n-7, whereas PUFAn-3, total PUFA (see Figure 3), the n-3/n-6 ratio as well as the levels of 18:2n-6, 20:4n-6, 18:3n-3, 18:4n-3, 20:5n-3 and 22:6n-3 decreased markedly. These changes in fatty acids with well-known origins and considerable metabolic importance in marine bivalves suggest a period of intense metabolic change (nutrition and reproductive processes). Increases in some of these monounsaturated fatty acids could be attributed to high availability of phytoplanktonic food. Effectively, some diatoms are especially rich in the fatty acids, 16:0, 16:1n-7, 18:1n-7, 18:1n-9, 18:3n-3 and 20:5n-3 (Fernández-Reiriz et al., Reference Fernández-Reiriz, Perez-Camacho, Ferreiro, Blanco, Planas, Campos and Labarta1989; Dunstan et al., Reference Dunstan, Volkman, Barret, Leroi and Jeffery1994; Napolitano et al., Reference Napolitano, Pollero, Ganoso, Macdonald and Thompson1997). The levels of these fatty acids increase when phytoplankton is readily available in the mussel M. galloprovincialis (Fernández-Reiriz et al., Reference Fernández-Reiriz, Labarta and Babarro1996) and in gonad tissue of N. subnodosus (Palacios et al., Reference Palacios, Racotta, Kraffe, Marty, Moal and Samainc2005). The high levels of 16:1n-7 in the digestive gland in April would suggest diatom ingestion. If all food-derived FA remained in the digestive gland, the n-3 FA should also be high. We interpret the decreases in PUFA-3, such as 20:5n-3, 22:6n-3, and in total PUFA as indications of their transfer to the gonad during this period of reproductive maturation (Freites et al., Reference Freites, García, Troccoli, Maeda-Martínez and Fernández-Reiriz2010). Many studies have underlined the importance of 20:4n-6, 20:5n-3, 22:6n-3 and PUFA for successful ovarian development (Utting & Millican, Reference Utting and Millican1998; Caers et al., Reference Caers, Coutteau, Cure, Morales, Gajardo and Sorgeloos1999b, Reference Caers, Coutteau, Sorgeloos and Gajardo2003). However, it is likely that an increase of PUFAs in the gonad in April was not apparent because the FAPs represent pools of 8 individuals in varied states of maturation. In April, 32% of scallops showed advanced gametogenesis, 39% were ripe and 24% had spent gonads (Freites et al., Reference Freites, García, Troccoli, Maeda-Martínez and Fernández-Reiriz2010).
During the upwelling relaxation period (see Figure 4B), FAP of female gonad and muscle tissue again formed well-defined groups, while FAPs of digestive gland and mantle were more scattered. For mantle, the FAP of September samples were closest to gonad FAP, whereas digestive gland FAP became closest to gonad FAP towards the end of the upwelling relaxation period (November), suggesting an increasing reliance on transfer of reserves from the digestive gland to gonad, and possibly a conservative strategy (sensu Bayne, Reference Bayne and Wiley1976). This conservative strategy may be the result of a low quality of phytoplankton during upwelling relaxation. Although the concentration of chlorophyll-a did not differ between upwelling and upwelling relaxation periods (Freites et al., Reference Freites, García, Troccoli, Maeda-Martínez and Fernández-Reiriz2010), it is likely that the species composition of phytoplankton differed, judging from the low levels of essential fatty acids in the digestive gland during upwelling relaxation. In this geographical area, the phytoplankton composition of the community differs between upwelling and upwelling relaxation periods (Lodeiros & Himmelman, Reference Lodeiros and Himmelman2000; Pirela-Ochoa et al., 2008): the upwelling period is dominated by diatoms and upwelling relaxation period by dinoflagellates and microflagellates. Clearly, knowledge of seasonal changes in the fatty acid composition of phytoplankton would clarify the responses of filter-feeding bivalves.
The low lipid levels in the mantle make it an unlikely source of lipids for the gonad, although transfer of protein from the mantle to the gonad has been suggested (Epp et al., 1988). Muscle FAP remained well defined and distinct from the gonad both during upwelling and upwelling relaxation, suggesting that there was no transfer of lipids between muscle and gonad. In scallops, muscle maintains high levels of glycogen and protein (Robinson et al., Reference Robinson, Wehling, Morse and Macleod1981; Epp et al., Reference Epp, Bricelj and Malouf1988; Lodeiros et al., Reference Lodeiros, Rengel, Guderley and Nusetti2001; Racotta et al., Reference Racotta, Ramirez, Ibarra, Rodríguez-Jaramillo, Carreño and Palacios2003). Thus, the significant inverse relation between the gonadosomatic index and muscle mass of N. nodosus during upwelling relaxation could reflect transfer of glycogen and protein from muscle to gonad (Garcia et al., 2007), in agreement with a conservative reproductive strategy during this period.
Our study provides clear evidence that this tropical bivalve can adapt to contrasting conditions of temperature, seston composition and phytoplankton availability (in quantitative and qualitative terms) generated by the upwelling and upwelling relaxation periods. These processes markedly influence productivity in the north-eastern regions of Venezuela (Miloslavich & Klein, Reference Miloslavich, Klein and Klein2008). We show that tropical marine bivalves are capable of adjusting to marked changes in environmental variables, adjustments that are more typical of organisms inhabiting higher latitudes (Cushing, Reference Cushing1975). During upwelling MDS-ANOSIM showed a close association between FAP of digestive gland and gonad, suggesting an almost direct or quick mobilization of fatty acids from digestive gland to the gonad, and that N. nodosus is following an opportunist strategy (sensu Bayne, Reference Bayne and Wiley1976). In contrast, during upwelling relaxation a progressive fall was observed of total lipids content in the digestive gland between August and November, which in addition to the progressive reduction in the distance in MDS plots, between FAPs of digestive gland and gonad suggests that lipid reserves from the digestive gland are gradually transferred to the gonad, which in turn suggests a conservative strategy (sensu Bayne, Reference Bayne and Wiley1976).
ACKNOWLEDGEMENTS
We acknowledge the technical assistance of M. Núñez, A. Velásquez, N. Vázquez, C. Córdoba and L. Montero and the Chacopata bank fishermen (L. Marín, R. González and J. Marcano) for their collaboration in sample gathering and in situ processing.
FINANCIAL SUPPORT
This research was partly financed by the Consejo de Investigación de la Universidad de Oriente (Project (CI-5-1802-1175/04).