Introduction
The Nile crocodile, Crocodylus niloticus Laurenti, 1768 (Crocodylia: Crocodylidae), is the largest semi-aquatic freshwater predator in Africa (Junker et al. Reference Junker, Boomker, Govender and Mutafchiev2019; Ross Reference Ross1989). Historically, Nile crocodiles were managed as a singles species (van Asch et al. Reference van Asch, Versfeld, Hull, Leslie, Matheus, Beytell, du Preez, Slabbert and Rhode2019), but morphological differences and nucleotide evidence suggest two species (Hekkala et al. Reference Hekkala, Shirley, Amato, Austin, Charter, Thorbjarnarson, Vliet, Houck, Desalle and Blum2011; Meredith et al. Reference Meredith, Hekkala, Amato and Gatesy2011; Oaks Reference Oaks2011; Schmitz et al. Reference Schmitz, Mansfeld, Hekkala, Shine, Nickel, Amato and Böhme2003; van Asch et al. Reference van Asch, Versfeld, Hull, Leslie, Matheus, Beytell, du Preez, Slabbert and Rhode2019). Hekkala et al. (Reference Hekkala, Shirley, Amato, Austin, Charter, Thorbjarnarson, Vliet, Houck, Desalle and Blum2011) accepted the west Africa crocodile, Crocodylus suchus Geoffroy, 1807 (Crocodylia: Crocodylidae), for crocodiles ranging in the Niger and Congo River Basins, where they overlap with C. niloticus that ranges throughout western and southern Africa (Hekkala et al. Reference Hekkala, Aardema, Narechania, Amato, Ikram, Shirley, Vliet, Cunningham, Gilbert and Smith2020; Hekkala et al. Reference Hekkala, Shirley, Amato, Austin, Charter, Thorbjarnarson, Vliet, Houck, Desalle and Blum2011; Meredith et al. Reference Meredith, Hekkala, Amato and Gatesy2011; Oaks Reference Oaks2011). Nile crocodiles eat fish, reptiles, aquatic birds, mammals, amphibians, insects, and molluscs (Utete Reference Utete2021). Nile crocodiles host a diversity of metazoan parasites (Tellez Reference Tellez2013), but they are difficult to obtain (Dutton et al. Reference Dutton, Jacobs, Beytell, Netherlands, DuPreez and Bullardin press), and perhaps partly related to that, their parasites remain under-sampled and relatively infrequently reported in the literature.
Odhner (Reference Odhner1902) erected Stephanoprora Odhner, Reference Odhner1902 (Echinosotomatoidea: Echinochasmidae Odhner, Reference Odhner and Jägerskiöld1910) for a new species, Stephanoprora ornata Odhner, Reference Odhner1902 (type species), infecting Nile crocodiles in Sudan. Since then, the complex taxonomic history of Stephanoprora spp. includes numerous synonymies and a contentious status for both Mesorchis Dietz, Reference Dietz1909 and Monilifer Dietz, Reference Dietz1909, which were synonymized with Stephanoprora by Odhner (Reference Odhner and Jägerskiöld1910). Authors working on these genera dismissed the number and arrangement of collar spines to differentiate Stephanoprora (type species has 26 collar spines) from Mesorchis (type species has 22 collar spines) (Beaver Reference Beaver1936; Bhalerao Reference Bhalerao1926; Dawes Reference Dawes1946; Gupta Reference Gupta1963; Kostadinova Reference Kostadinova, Jones, Bray and Gibson2005; Mendheim Reference Mendheim1943; Skrjabin Reference Skrjabin1956; Sutton et al. Reference Sutton, Lunaschi and Topa1982; among others). Kostadinova (Reference Kostadinova, Jones, Bray and Gibson2005; p 14) summarized the systematic and nomenclature history of Echinochasmidae Odhner, Reference Odhner and Jägerskiöld1910, including Dissurus Verma, Reference Verma1936; Stephanoprora; Mehrastomum Saksena, 1959; Microparyphium Dietz, Reference Dietz1909; Pulchrosomoides Freitas & Lent, 1937; Echinochasmus Dietz, Reference Dietz1909; and Saakotrema Skrjabin & Bashkirova, Reference Skrjabin1956 (see Tkach et al. [Reference Tkach, Kudlai and Kostadinova2016] for the most recent phylogenetic hypothesis of Echinostomatoidea). In doing so, Kostadinova (Reference Kostadinova, Jones, Bray and Gibson2005) concluded that Mesorchis and Monilifer should remain junior subjective synonyms of Stephanoprora. She then proposed the two subgenera Stephanoprora (Stephanoprora) with S. (S.) ornata as the ‘type species’ and Stephanoprora (Monilifer) with S. (Mo.) spinulosa as the ‘type species’ (Kostadinova Reference Kostadinova, Jones, Bray and Gibson2005; p 15, 44). There are 44 nominal species of Stephanoprora that have 20, 22, or 24, but the type species, S. ornata, is the only congener with 26 collar spines. Recent nucleotide-based phylogenetic analyses of the Echinochasmidae recovered two clades from sequences ascribed to species with different number of collar spines (Islas-Ortega et al. Reference Islas-Ortega, Aldama-Prieto, Sereno-Uribe and García-Varela2024; Kalinina et al. Reference Kalinina, Besprozvannykh, Tatonova and Shchelkanov2023; Tatonova et al. Reference Tatonova, Izrailskaia and Besprozvannykh2020; Tkach et al. Reference Tkach, Kudlai and Kostadinova2016). In the absence of taxonomic resolution from morphology, previous authors have highlighted the significance of obtaining nucleotide information for S. ornata to test monophyly of Mesorchis and Monilifer (Islas-Ortega et al. Reference Islas-Ortega, Aldama-Prieto, Sereno-Uribe and García-Varela2024; Tkach et al. Reference Tkach, Kudlai and Kostadinova2016).
We herein provide a supplementary morphological description of S. ornata collected from a Nile crocodile from the Kavango River, Namibia. We emend Mesorchis and discuss the validity of several species previously assigned to Stephanoprora. We also provide the first 28S and ITS2 nucleotide information for the species as well as furnish Maximum likelihood (ML) and Bayesian inference (BI) phylogenetic analysis.
Materials and methods
Specimen collection, preparation, and deposition
We examined a large male adult Nile crocodile (500–650 kg; 4,100 mm total length, estimated to be between 75 and 100 years of age) from a site (18°08’24.5"S, 21°40’58.4"E) in the Kavango River (northeastern Namibia) on 10 February 2024. The intestine was excised, longitudinally cut to open its lumen, and exposed to 60 C freshwater. The contents of the intestine were then placed into an acrylic settling column and allowed to settle while the mucosa of the intestine was examined using a Zeiss Stemi 305 stereo-dissecting microscope (Carl Zeiss Microscopy, White Plaines, NY). The fluid in the column was decanted, and the sediment added to glass petri dish and examined using a stereo-dissecting microscope. Heat-killed trematode specimens from the petri dish were then pipetted into 10% neutral buffered formalin (n.b.f.) for morphology or into 95% non-denatured ethanol (EtOH) for DNA extraction. Fixed specimens were rinsed in water and then stained overnight in Van Cleave’s hematoxylin with several drops of Ehrlich’s hematoxylin, dehydrated with a graded EtOH series, made basic at 70% EtOH with lithium carbonate and n-butylamine, dehydrated in absolute EtOH, cleared with clove oil, and permanently mounted on glass slides and coverslip using Canada balsam. These specimens were drawn using an Olympus BX51 compound microscope (Olympus, Tokyo, Japan) equipped with differential interference contrast optical components and a drawing tube. Measurements were obtained using a Jenotipik Gryphax camera (Jenotipik AG, Jena, Germany) and are reported in micrometers (μm), unless otherwise stated, as the range followed by the mean and sample size in parentheses (Table 1). Only spines in near optimal or optimal lateral or dorso-ventral view were measured (Table 1). Nomenclature follows Odhner (Reference Odhner1902), Odhner (Reference Odhner and Jägerskiöld1910), Kostadinova (Reference Kostadinova, Jones, Bray and Gibson2005), and Tkach et al. (Reference Tkach, Kudlai and Kostadinova2016). Morphological terms and specific terminology for collar spines follow Kanev et al. (Reference Kanev, Fried and Radev2009) and Cajiao-Mora et al. (Reference Cajiao-Mora, Brule, Dutton and Bullar2024). Terminology of the oesophagus follows Truong et al. (Reference Truong, Warren, Ksepka, Curran and Bullard2021; oesophagus length comprises the oesophagus from the posterior end of the oral sucker to the oesophageal bifurcation). Three voucher specimens were deposited in the National Museum of Natural History’s Invertebrate Zoology Collection (USNM, Smithsonian Institution, Washington, DC).
Table 1. Measurements of specimens of Stephanoprora ornata Odhner, Reference Odhner1902 and Mesorchis pseudoechinatus (Olsson, 1875) Dietz, 1910

* Estimated from published drawing.
DNA extraction
One EtOH-preserved specimen was used for DNA extraction and sequencing. Extraction was made using the DNeasyTM Blood and Tissue kit (Qiagen) following the manufacturer’s protocol, except that the proteinase-K incubation period was extended overnight. Once extracted, DNA concentration was measured using a NanoDrop-One Microvolume Spectrophotometer (Thermo Fisher Scientific), diluted to 50 ng/μL, and stored at −20°C. The partial 28S gene was amplified using primers U178 (5’-GCACCGCTAAYTTAAG-3’) and L1642 (5’-CCAGCGCCATCCATTTTCA-3’) (Lockyer et al. 2003), the ITS2 region using the primers GA1 (5’-AGAACATCGACATCTTGAAC-3’) (Anderson and Barker 1998) and ITS2.2 (5’-CCTGGTTAGTTTCTTTTCCTCCGC-3’) (Cribb et al. 1998). Additionally, the sequencing primers 300F (5’-CAAGTACCGTGAGGGAAAGTTG-3’) and 1200R (5’-GCATAGTTCACCATCTTCGG-3’) were used to improve sequencing coverage on the 28S gene (Lockyer et al. 2003). PCR reactions for the 28S and ITS2 were performed with the following thermocycling parameters: initial denaturation step of 94 C for 4 min; followed by 40 cycles of 94 C for 40 sec, 60 C for 30 sec, 72 C for 2 min; with a final extension step of 72 C for 5 min. DNA amplification was verified with a 1% agarose gel stained with ethidium bromide. PCR products were purified using the QIAquick PCR Purification Kit (Qiagen) according to the manufacturer’s protocol, except that the last elution step was performed with autoclaved nanopure water. DNA sequencing was performed by Genewiz (South Plainfield, New Jersey). Sequence assembly and analysis of chromatograms were performed with Geneious prime version 2023.2.1. Forward and reverse sequences were aligned using MAFFT tool (Katoh and Standley Reference Katoh and Standley2013), and low-quality read-ends were trimmed resulting in sequences of 1527 base pairs (bp) for the 28S gene and 661 bp for the ITS2. All sequence data were deposited in GenBank under accession numbers XXXXX (28S), XXXXX (ITS2).
Phylogenetic analysis
The ingroup taxa comprised our newly generated sequence and those ascribed to Echinochasmidae (Table 2) but excluded identical (duplicated) sequences. The 28S sequences ascribed to Echinochasmus pseudobeleocephalus Kalanina, Besprozvannykh, Tatonova, & Shchelkanov, Reference Kalinina, Besprozvannykh, Tatonova and Shchelkanov2023 were too short to be used in the present phylogenetic analysis (GenBank accession no. OR076694–5; 701 bp). The outgroup taxa include sequences of Psilostomidae and Himasthlidae (Table 2) based on the topology of the 28S phylogenetic tree of the Echinostomatoidea published by Tkach et al. (Reference Tkach, Kudlai and Kostadinova2016). Sequences were aligned using MAFFT tool. For the 28S analysis, the alignment was trimmed to the shortest sequence (1035 bp); for the ITS2 analysis, the alignment was trimmed to the ITS2 region (454 bp). Maximum likelihood (ML) and Bayesian inference (BI) analyses were conducted. ML phylogeny was inferred using IQTREE v.1.16.12 (Nguyen et al. Reference Nguyen, Schmidt, von Haeseler and Minh2015). Substitution model testing was done with ModelFinder (Kalyaanamoorthy et al. Reference Kalyaanamoorthy, Minh, Wong, von Haeseler and Jermiin2017) as implemented in IQTREE. After model testing, tree inference was done using best-fitting substitution models (Chernomor et al. Reference Chernomor, von Haeseler and Minh2016). These were TIM3+F+G4 for the 28S alignment and TPM3u+F+G4 for the ITS2 alignment. Default tree search parameters were used, except perturbation strength was set to 0.2 and 500 iterations had to be unsuccessful to stop the tree search. Tree inference was done 20 times with only the tree with the best log-likelihood score reported. Support for relationships was measured with 1,000 ultrafast bootstrap replicates (UFBoot) (Hoang et al. Reference Hoang, Chernomor, von Haeseler, Minh and Vinh2018). To perform BI analysis, the aligned sequences were reformatted (from .fasta to .nexus) using the web application ALTER (Glez-Peña et al. Reference Glez-Peña, Gómez-Blanco, Reboiro-Jato, Fdez-Riverola and Posada2010). BI was run using MrBayes version 3.2.7a (Ronquist and Huelsenbeck Reference Ronquist and Huelsenbeck2003) using substitution model averaging (nst-mixed) and a gamma distribution to model rate-heterogeneity. Defaults were used in all other parameters. Three independent runs with 4 Metropolis-coupled chains were run for 5,000,000 generations, sampling the posterior distribution every 1,000 generations. Convergence was checked using Tracer v1.6.1 and the ‘sump’ command in MrBayes: all runs appeared to reach convergence after discarding the first 25% of generations as burn-in. A majority-rule consensus tree of the post-burn posterior distribution was generated with the ‘sumt’ command in MrBayes. The inferred phylogenetic trees were visualized using FigTree v1.4.4 (Rambaut et al. Reference Rambaut, Suchard, Xie and Drummond2018) and further edited for visualization purposes with Adobe Illustrator (Adobe Systems) (Figure 4).
Table 2. ITS and 28S sequences used herein. New sequence in bold. Type species indicated by asterisk (*)
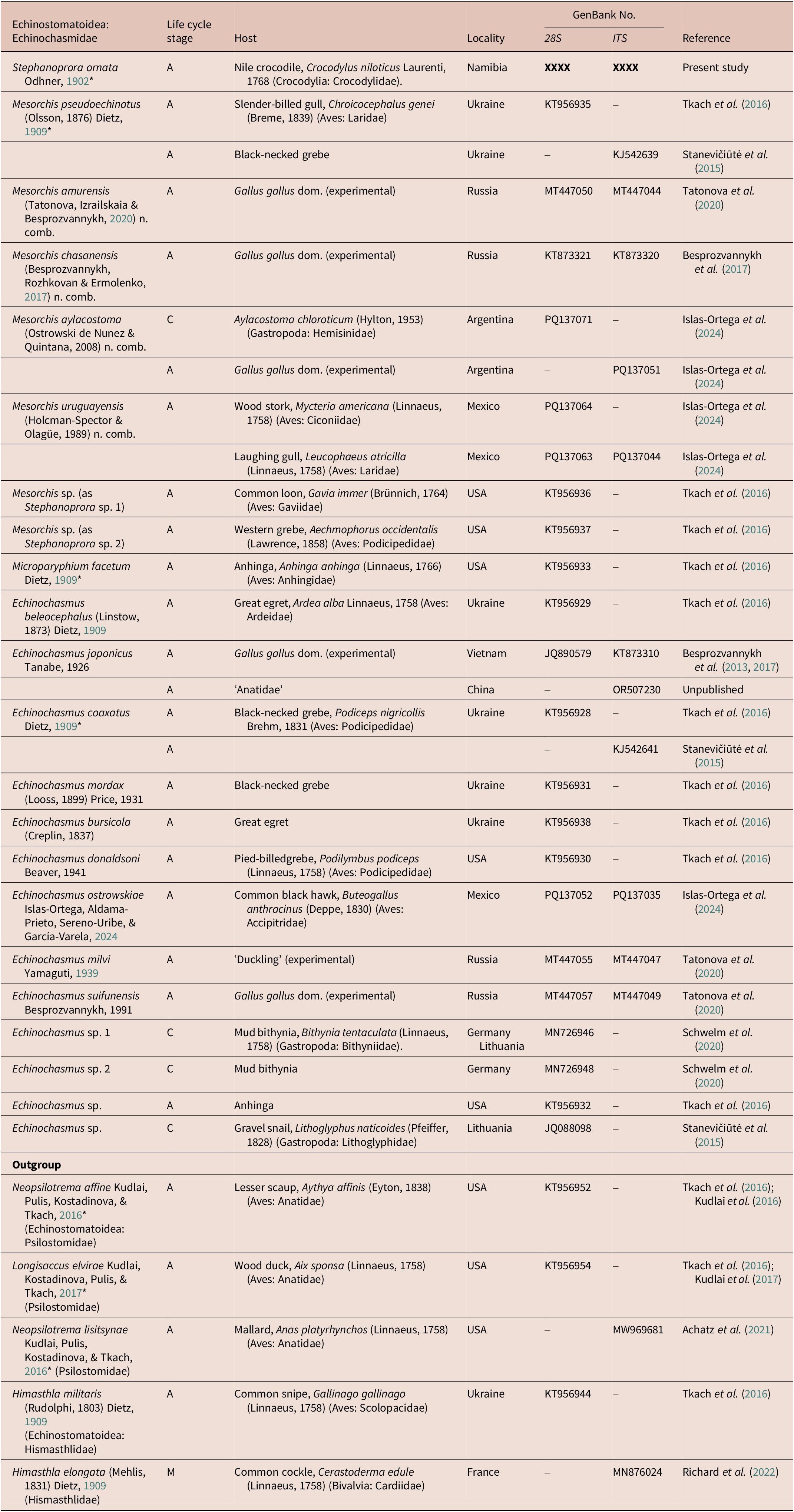
Abbreviations: Adult (A); Cercaria (C); Metacercaria (M)
Results
Digenea
Echinostomatoidea
Echinochasmidae
Stephanoprora ornata Odhner, Reference Odhner1902
Description (Figures 1–3a; measurements in Table 1)

Figure 1. Stephanoprora ornata Odhner, 1909 (Digenea: Echinochasmidae) (voucher USNM XXXXX) infecting the intestine of a Nile crocodile, Crocodylus niloticus Laurenti, 1768 (Crocodylia: Crocodylidae) from the Kavango River, Namibia. Scale value beside bar. Ventral view. Whole body. Abbreviations: anterior testis (at); cecum terminalia (ct); collar (c); common genital pore (cgp); dextral cecum (dc); dextral vitelline duct (dvd); oesophagus (oe); oesophageal bifurcation (oeb); ovary (ov); pharynx (p); ventral sucker (vs); vitellarium (v).
Light microscopy of 3 adult specimens; USNM collection nos. XXXXX, XXXXX, XXXX: Body elongate, 13× longer than wide, maximum width at level of testis (Figure 1). Forebody representing 16−26% (20%; 3) of total body length. Forebody: hindbody length ratio 1:2.2. Tegument thick, containing body surface spines heavily damaged or lost, remnants of their location visible at level of forebody as a crenulated area in the tegument (Figure 2a). Ventral sucker in first quarter of body, rounded in outline (Figure 1), protruding from body (Figure 2a), 3−4× (3; 2) longer than oral sucker, 3−4× (3; 2) wider than oral sucker. Collar small, constricted from body ventrally and laterally, having collar spines, lacking a ventral ridge, with a protuberance containing the oral sucker (Figure 2a). Collar spines 26 in total, bullet shaped, comprising dorsal spines, lateral spines, and corner spines (Figure 3a). Collar dorsal spines 2 in number, medial, directing dorsally or posteriad. Collar lateral spines 6 in number per side of collar (12 total), directing laterad. Dorsal and lateral spines in a single interrupted dorsally row. Collar corner spines comprising 3 pairs per side of ventral lappet (12 in total), each pair containing 1 oral and 1 aboral spine, each pair separated from each other. Oral sucker terminal, ovoid, protruding from collar (Figures 1, 2a, 3a), containing mouth opening, continued by pre-pharyngeal portion of oesophagus (Figure 3a), 1× (1; 2) longer than wide. Nerve commissure dorsal to pre-pharyngeal oesophagus (Figure 3a); nerve chords extending from nerve commissure anteriad and posteriad at sides of body (Figure 3a). Oesophagus in midline of body, extending from posterior margin of oral sucker to slightly anterior to common genital pore, representing 13−21% (17%; 3) of total body length (Figures 1, 2a). Pre-pharyngeal oesophagus representing 13−26% (18%; 3) of oesophagus length (Figures 1, 2a). Pharynx ovoid, in anterior portion of oesophagus, anterior margin at level of last pair of corner spines, mainly posteriad to collar (Figures 1, 2a), representing 9−15% (12%; 3) of oesophagus length. Pos-pharyngeal oesophagus very long, thick walled, representing 59−78% (70%; 3) of oesophagus length, representing 13–21% (17%; 3) of total body length; intestinal bifurcation anterior to ventral sucker and common genital pore (Figure 1). Caeca slender, blind, extending posteriad at sides of body, nearly reaching posterior end of body (Figure 1); posterior half of dextral and sinistral caeca enveloped by vitellarium (Figure 1).

Figure 2. Stephanoprora ornata Odhner, 1909 (Digenea: Echinochasmidae) infecting the intestine of a Nile crocodile, Crocodylus niloticus Laurenti, 1768 (Crocodylia: Crocodylidae) from the Kavango River, Namibia. Scale value beside bar. (a) Anterior third of body including male genitalia. Lateral view of voucher USNM XXXXX. (b) Second third of body including female genitalia. Ventral view of voucher USNM XXXXX. Abbreviations: anterior testis (at); cirrus (ci); cirrus sac (cs); collar corner spines (ccs); common genital pore (cgp); dextral vitelline duct (dvd); egg (eg); Laurer’s canal (Lc); Mehlis’ gland (Mg); oesophagus (oe); oötype (oö); oral sucker (os); ovary (ov); pharynx (p); pre-pharyngeal oesophagus (poe); prostatic cells (pc); seminal vesicle (sv); uterine seminal receptacle (usr); ventral sucker (vs).

Figure 3. Anterior end of body of echinochasmids with 26, 24, 22, and 20 collar spines. (a) Stephanoprora ornata Odhner, 1909 (Digenea: Echinochasmidae; 26 collar spines) infecting the intestine of a Nile crocodile, Crocodylus niloticus Laurenti, 1768 (Crocodylia: Crocodylidae) from the Kavango River, Namibia (USNM XXXXX). (b) Museum voucher of Mesorchis pseudoechinatus (Olsson, 1876) Dietz, Reference Dietz1909 (Digenea: Echinochasmidae; 22 collar spines) infecting the slender-billed gull Chroicocephalus genei (Brème, 1839) (Aves: Laridae) from the Kherson region, Ukraine (HWML101865). (c) Museum voucher of Echinochasmus coaxatus Dietz, Reference Dietz1909 (Digenea: Echinochasmidae; 24 collar spines) infecting the eared grebe Podiceps nigricollis Brehm, 1831 (Aves: Podicipedidade) from the Kherson region, Ukraine (HWML 101859). (d) Museum voucher of Echinochasmus donaldsoni Beaver, 1941 (Digenea: Echinochasmidae; 20 collar spines) infecting the pied-billed grebe Podilymbus podiceps (Linnaeus, 1758) (Aves: Podicipedidade) from North Dakota, USA (HWML 101861). Abbreviations: collar corner spines (ccs); collar dorsal spines (cds); collar lateral spines (cls); nerve commissure (nc); pharynx (p); pre-pharyngeal oesophagus (poe).
Testes 2 in number, asymmetrical, elongate ovoid, irregular in outline, in tandem, spaced apart (Figure 1); posterior testis 1× longer than anterior testis; post-testicular space 37−41% (38%; 3) of total body length. Cirrus sac thin walled, dorsal or anteriad and dorsal to ventral sucker (Figures 1, 2a), containing bipartite seminal vesicle, pars prostatica, prostatic cells, and aspinose cirrus (Figures 1, 2a); seminal vesicle bipartite, filled with seminal material; proximal seminal vesicle chamber being bigger than distal one (Figure 2a); pars prostatica short; prostatic cells arranged in a compact mass surrounding pars prostatica and distal seminal vesicle; cirrus short, medial, aspinose; genital pore ventral, in midline of body, posterior to oesophageal bifurcation, anterior (Figure 1) or dorsal (Figure 2a) to ventral sucker.
Ovary transversely ovoid in outline, in midline of body, dorsal to distal portion of uterine seminal receptacle (Figure 1, 2b), pre-testicular, equatorial (Figure 1); pre-ovarian space representing 34–46% (39%; 3) of total body length; post-ovarian space representing 57–64% (61%; 3) of total body length. Oviduct emerging from posterior margin of ovary (Figures 1, 2b), tubular, sinuous. Laurer’s canal short, emanating from proximal portion of oviduct, sinistral, having dorsal opening (Figure 2b). Oötype ovoid in shape, post-ovarian, 2× longer than wide, surrounded in anterior portion by Mehlis’ gland, continued by uterine seminal receptacle (Figure 2b). Mehlis’ gland compact, ranging from middle ovary to anterior portion of oötype (Figure 2b). Uterus coiling anteriad between caeca, overlapping with caeca, ranging from oötype to common genital pore, having numerous eggs (Figure 1), occupying 17−23% (19%; 3) of total body length; proximal portion containing sperm, serving as uterine seminal receptacle (Figures 1, 2b). Eggs ovoid in outline. Vitellarium distributing in 2 bilaterally symmetrical fields of follicles, ranging from anterior end of posterior testis to nearly reaching posterior end of body, enveloping caeca, with each respective vitelline field becoming wider posteriad, occupying 41−43% (42%; 3) of total body length (Figure 1); vitelline ducts extending from posterior end of posterior testis to vitelline reservoir, conjugating into vitelline reservoir (Figure 1); vitelline reservoir dorsal and sinistral to oötype, dorsal to uterine seminal receptacle, connecting with oviduct (Figure 2b).
Excretory vesicle Y shaped, anterior end not observed. Excretory pore sub-terminal.
Taxonomic summary
Type and only known host: Nile crocodile, Crocodylus niloticus Laurenti, 1768 (Crocodylia: Crocodylidae).
Type locality: ‘Sudan’.
New locality reported herein: Kavango River (18°9’3.44"S; 21°41’40.34"E), Namibia.
Site of infection: Intestine.
Prevalence of infection: The Nile crocodile examined herein was infected with 4 specimens of S. ornata.
Specimens deposited: Voucher accession No. USNM XXXXXXX.
Representative DNA sequences: 28S GenBank accession No. XXXXXXXX; ITS2 GenBank accession No. XXXXXXXX.
Stephanoprora Odhner, Reference Odhner1902
Emended generic diagnosis (based on Kostadinova Reference Kostadinova, Jones, Bray and Gibson2005): Body elongate, widest at level of testes; forebody long; hindbody longer than forebody. Tegument armed with scale-like spines, restricted to anterior body half. Tegumental spines may be present on anterior margin of oral sucker. Ventral sucker round, in first quarter of body, intercaecal. Collar reniform, small, narrower than maximum body width, having collar spines, lacking a ventral ridge. Collar spines 26 in number, comprising dorsal spines, lateral spines, and corner spines; dorsal spines 2 in number; lateral spines 6 in number per side of collar (12 total); dorsal spines and lateral spines forming a single dorsally interrupted row; corner spines comprising 3 pairs (6 corner spines) in each lappet (12 total), directing ventrally and posteriad. Oral sucker smaller than ventral sucker, ovoid. Pre-pharyngeal oesophagus longer than pharynx, extending posteriad to posterior-most pair of corner spines; pharynx ovoid, posterior to corner spines; oesophagus long. Intestinal bifurcation just anterior to ventral sucker. Caeca 2 in number, simple, blind, extending posteriad in parallel with respective body margin, posterior half enveloped by vitellarium, nearly reaching posterior end of body. Testes 2 in number, irregular in outline, in tandem, in third quarter of body, equatorial or post-equatorial. Cirrus sac anteriad to ventral sucker, extending to middle portion or last portion of ventral sucker, containing seminal vesicle, pars prostatica, prostatic cells, and cirrus. Seminal vesicle bipartite; pars prostatica tubular; prostatic cells comprising a dense mass surrounding pars prostatica. Cirrus short, slender, aspinose. Genital pore ventral, medial, posterior or ventral to oesophageal bifurcation. Ovary rounded or ovoid, in second quarter of body, pre-testicular. Oviduct emerging from posterior margin of ovary, slightly sinuous, dorsal to uterine seminal receptacle. Laurer’s canal short, emanating from proximal portion of oviduct, opening on dorsal surface of body. Oötype ovoid, surrounded by Mehlis’ gland, ventral to vitelline reservoir. Uterus comprising short proximal portion and compacted and convoluted distal portion; proximal portion comprising uterine seminal receptacle, filled with sperm, ventral to ovary, oviduct, and vitellin reservoir, extending from oötype, curving anteriad to anterior border of ovary to form the distal portion; distal portion lacking sperm, having typical lumen filled with eggs, coiling anteriad between caeca; metraterm not observed. Vitellarium comprising 2 bilateral fields of vitelline follicles, enveloping caeca, distributing anteriad to anterior portion of posterior testis, widening and becoming nearly confluent posterior to testes; vitelline ducts long, slender, from end of vitellarium to vitelline reservoir at oötype level; vitelline reservoir dorsal to uterine seminal receptacle and oötype, connecting with oviduct. Excretory pore terminal. Excretory vesicle Y shaped.
Differential diagnosis: Body elongate. Collar having 26 spines (2 dorsal spines, 12 lateral spines, 12 corner spines); dorsal and lateral spines forming a single interrupted dorsally row; corner spines in 3 pairs per side of collar. Pre-pharyngeal oesophagus long, extending posteriad to posterior-most pair of corner spines. Pharynx posterior to collar. Testes irregular in outline. Vitellarium extending from posterior end of body to anterior border of posterior testes.
Type and only species: Stephanoprora ornata Odhner, Reference Odhner1902 infecting the Nile crocodile, Crocodylus niloticus Laurenti, 1768 (Crocodylia: Crocodylidae).
Mesorchis Dietz, Reference Dietz1909
Emended generic diagnosis (based on Dietz [Reference Dietz1909; 1910] and Kostadinova [Reference Kostadinova, Jones, Bray and Gibson2005]): Body elongate, widest at level of ventral sucker; forebody short; hindbody much longer than forebody. Tegument armed with small scale-like spines, extending to testis or posteriorly. Tegumental spines may be present on anterior margin of oral sucker. Ventral sucker strongly muscular, in first quarter of body, intercaecal. Collar reniform, having collar spines, lacking a ventral ridge. Collar spines 22 in number, comprising dorsal spines, lateral spines, and corner spines; dorsal spines 2 in number; lateral spines 6 in number per side of collar (12 total); dorsal spines and lateral spines forming a single dorsally interrupted row; corner spines comprising 2 pairs (4 corner spines) in each lappet (8 total), directing ventrally and posteriad. Oral sucker smaller ovoid or rounded in shape. Pre-pharyngeal oesophagus short, shorter than pharynx; pharynx ovoid, at level of corner spines; oesophagus long, reaching just anterior or half-way between collar and ventral sucker. Intestinal bifurcation anterior to ventral sucker. Caeca 2 in number, simple, blind, or having a uroproct (M. aylacostoma; M. polycestus), extending posteriad in parallel with body margins, posterior half enveloped by vitellarium, nearly reaching posterior end of body. Testes 2 in number, ovoid or rounded in shape, having smooth margins, rarely elongated and slightly sinuous (M. nattereri), or smaller than ovary (M. microtestius), in tandem, pre-equatorial, equatorial, or pos-equatorial. Cirrus sac anteriad to ventral sucker, extending to middle portion or last portion of ventral sucker, containing seminal vesicle, pars prostatica, prostatic cells, and cirrus. Seminal vesicle bipartite; pars prostatica tubular; prostatic cells surrounding pars prostatica. Cirrus short, slender, aspinose. Genital pore ventral, in midline of body, posterior to oesophageal bifurcation. Ovary round, pre-testicular. Uterus comprising a proximal portion and a distal portion; proximal portion comprising uterine seminal receptacle, filled with sperm; distal portion lacking sperm having typical lumen filled with eggs, coiling anteriad between caeca; eggs same size as ovary or smaller than ovary; metraterm not observed. Vitellarium comprising 2 bilateral fields of vitelline follicles, enveloping caeca, distributing anteriad to posterior portion of posterior testes or ovary, widening and becoming nearly confluent posterior to testes; vitelline ducts long, slender, from end of vitellarium to vitelline reservoir; vitelline reservoir connecting with oviduct. Excretory pore terminal.
In intestine of a wide range of fish-eating birds, mammals, and reptiles. Cercariae lacking collar spines, in prosobranch and pulmonated gastropods; metacercariae having collar spines, on gills or inner surface of oesophagus of freshwater, euryhaline and marine teleost.
Differential diagnosis: Body elongate. Collar having 22 spines (2 dorsal spines, 12 lateral spines, 8 corner spines); dorsal and lateral spines forming a single interrupted dorsally row; corner spines in 2 pairs per side of collar. Pre-pharyngeal oesophagus short, not extending posteriad to posterior-most pair of corner spines. Pharynx anterior to or at level of corner spines. Testes rounded, ovoid or oblong in shape, having smooth margins; pre-equatorial, equatorial, or pos-equatorial. Vitellarium extending from a point close to posterior body end to any level between posterior margin of posterior testis and ovary.
Type species: Mesorchis pseudoechinatus (Olsson, 1876) Dietz, Reference Dietz1909; type by original designation.
Synomys. Pseudoechinostomum Schupakov, 1936 nec Odhner, Reference Odhner and Jägerskiöld1910; Aequistoma Beaver, Reference Beaver1942; Beaverostomum Gupta, Reference Gupta1963.
Included species: Mesorchis denticulatus (Rudolphi, 1802) Dietz, Reference Dietz1909; Mesorchis pendulus (Looss, 1899) Dietz, Reference Dietz1909; Mesorchis conciliatus Dietz, Reference Dietz1909; Mesorchis polycestus Dietz, Reference Dietz1909; Mesorchis microtestius Kurova, 1927; Mesorchis pennati (Verma, Reference Verma1936) Cajiao-Mora & Bullard, n. comb.; Mesorchis jacaretinga (Teixeira de Freitas & Lent, Reference Teixeira de Freitas and Lent1938) Cajiao-Mora & Bullard, n. comb.; Mesorchis fusca (Lal, Reference Lal1939) Cajiao-Mora & Bullard, n. comb.; Mesorchis magniovatus (Yamaguti, Reference Yamaguti1939) Cajiao-Mora & Bullard, n. comb.; Mesorchis gracilis (Mendheim, 1940) Cajiao-Mora & Bullard, n. comb.; Mesorchis pseudodenticulatus (Mendheim, 1940) Cajiao-Mora & Bullard, n. comb.; Mesorchis skrjabini (Dotsenko in Skrjabin & Baschkirova, Reference Skrjabin1956) Cajiao-Mora & Bullard, n. comb.; Mesorchis gigantica (Gupta, Reference Gupta1963) Cajiao-Mora & Bullard, n. comb.; Mesorchis nigerica (Gupta, Reference Gupta1963) Cajiao-Mora & Bullard, n. comb.; Mesorchis pandei (Nath, 1973) Cajiao-Mora & Bullard, n. comb.; Mesorchis acridotherei (Wang, 1976) Cajiao-Mora & Bullard, n. comb.; Mesorchis tringae (Wang, 1976) Cajiao-Mora & Bullard, n. comb.; Mesorchis elongatus (Gupta & Jahan, 1979) Cajiao-Mora & Bullard, n. comb.; Mesorchis paradenticulatus (Nasir & Rodriguez, 1969) Cajiao-Mora & Bullard, n. comb.; Mesorchis dogieli (Holman-Spector & Olague, 1989) Cajiao-Mora & Bullard, n. comb.; Mesorchis uruguayensis (Holman-Spector & Olague, 1989) Cajiao-Mora & Bullard, n. comb.; Mesorchis nattereri (Ostrowski de Núñez, 2003) Cajiao-Mora & Bullard, n. comb.; Mesorchis aylacostoma (Ostrowski de Núñez & Quintana, Reference Ostrowski de Núñez and Quintana2008) Cajiao-Mora & Bullard, n. comb.; Mesorchis chasanensis (Besprozvannykh, Rozhkovan & Ermolenko, 2016) Cajiao-Mora & Bullard, n. comb.; Mesorchis amurensis (Tatonova, Izrailskaia & Besprozvannykh, Reference Tatonova, Izrailskaia and Besprozvannykh2020) Cajiao-Mora & Bullard, n. comb.
Taxonomic remarks
We resurrect Mesorchis and retain monotypic Stephanoprora as emended. Mesorchis differs from Stephanoprora by the number of collar spines (22 vs. 26), by the anterior position of the pharynx (vs. being posterior to the collar), and by the shape of the testis (being ovoid, atypically elongated vs. being irregular in outline). Regarding Monilifer, we retain it as a synonym of Echinochasmus because it has a compact, elongate oval body shape with a short forebody, truncate uterus, short post-testicular field, and 22 collar spines. Further, the type species of Monilifer [Echinochasmus spinulosus (Rudolphi, 1809) Baschkirova, 1947, as Monilifer spinulosus (Rudolphi, 1809) Dietz, Reference Dietz1909] was transferred to Echinochasmus by Baschkirova, 1947 by the combination of characters above described. However, we acknowledge the necessity of reconsidering the paraphyletic Echinochasmus, which currently comprises species that have 20−24 collar spines. This matter is out of the scope of the present paper and will require the examination and redescription of several species and the elucidation of their life cycles. This could lead to the resurrection of Monilifer and perhaps the proposal of new echinochasmid genera.
Our specimens resemble those of S. ornata Odhner (Reference Odhner1902; 1910) (see Table 1) by the following combination of morphological features: the number and arrangement of collar spines (26; 14 dorsal and lateral spines in a single row interrupted dorsally, corner spines arranged as 3 pairs with each pair having 1 oral and 1 aboral spine), a long pre-pharyngeal oesophagus (pharynx posterior to collar), an oesophageal bifurcation anterior to the ventral sucker, a cirrus sac not extending posteriad to the ventral sucker, and a vitellarium extending from the anterior border of the posterior testis to the posterior body end. Our specimens differ from the original description of S. ornata by Odhner (Reference Odhner1902, Reference Odhner and Jägerskiöld1910) by having a larger collar, a cirrus sac posterior to the oesophageal bifurcation (vs. cirrus sac ventral to oesophageal bifurcation), and a medial ovary (vs. dextral ovary) as well as by lacking tegumental spines about the oral sucker (our specimens evidently lost all of the body surface spines during specimen processing). We consider these differences intraspecific variation.
Stephanoprora ornata differs from all echinochasmids infecting reptiles by the number of collar spines (26 vs. 24 in S. odhneri inc. sed.; 22 in Me. jacaretinga and Me. nattereri). It differs from the echinochasmids infecting birds by the number of collar spines, except S. larkanensis Saeed, Das, Bushra & Ghazi, Reference Saeed, Das, Bushra and Ghazi2018 infecting the red-wattled lapwing, Vanellus indicus Boddaert, 1783 (Aves: Charadriiformes) that reportedly has 26 collar spines. However, the systematic affiliation of S. larkanensis is doubtful due to the size of the ventral sucker, a vitellarium reaching the ventral sucker, the oesophageal bifurcation dorsal to the ventral sucker, and the testes being smaller than the ovary. Further, the illustrations and photomicrographs of the collar suggest that some spines are missing; thereby making the number of collar spines indeterminate (Saeed et al. Reference Saeed, Das, Bushra and Ghazi2018; Figure A, B, and E). Moreover, the species description contains inconsistencies in the measurements as they do not agree with the illustrations. No molecular voucher exists for this species. Until an examination and redescription of the type specimens of S. larkanensis can be completed, we consider it as a species inquirenda.
We regard several species previously assigned to Stephanoprora and that have incomplete or dubious descriptions [S. anomala Travassos, Reference Travassos1922; S. graciosa (Suarikov, 1950) Yamaguti, Reference Yamaguti1958; S. minutus Bhutta & Khan, Reference Bhutta and Khan1975; S. argentinensis Sutton, Lunaschi & Topa, Reference Sutton, Lunaschi and Topa1982] as species inquirendae. Stephanoprora anomala was described by Travassos (Reference Travassos1922) infecting the neotropical cormorant, Nannopterum brasilianum (Gmelin, 1789) (Aves: Phalacrocoracidae) [as Carbo vigua (Vieillot, 1817)] from Brazil. Travassos (Reference Travassos1922) stated that the specimens were in poor condition (‘posterior extremity damaged’ Travassos Reference Travassos1938; p 462) and that the collar was distinctive with 5 or 6 spines barely visible. However, other characters were described as the vitellarium extending to the posterior end of posterior testis. The poor material does not allow for a correct identification since the requisite taxonomic characters such as the collar and its spines were absent or indistinct. Stephanoprora graciosa (Sudarikov, Reference Sudarikov1950) (originally Sobolevistoma [see Sudarikov Reference Sudarikov1950]) was originally described from the osprey, Pandion haliaetus (Linnaeus, 1758) (Aves: Pandionidae) in Russia. Sudarikov (Reference Sudarikov1950) described the collar (‘proadoral disk’) as highly muscular and heart-shaped with minute, tooth-like spines along its margin. The drawings show a vitellarium extending from the midline of the anterior testis to posterior body end. The unique collar of this specimen indicates that it probably belongs to another genus, perhaps Sobolevistoma as originally assigned by Sudarikov (Reference Sudarikov1950). However, the brief description and drawings do not allow a confident genus assignment. Until a redescription of the type specimens or until the collection of new material from the type host and type locality is completed, we consider S. graciosa as a species inquirenda. Stephanoprora minutus was described by Bhutta and Khan (Reference Bhutta and Khan1975) infecting the gharial, Gavialis gangeticus (Gmelin, 1789) (Crocodylia: Gavialidae) from the Sutlej River, India. The description was based upon four immature specimens having 24 collar spines; no vitellarium nor egg were illustrated nor described, and the testes were not yet developed (Bhutta and Khan Reference Bhutta and Khan1975; p 85–86, Figure 39); suggesting that the trematode specimens described were juveniles and without a differential diagnostic feature that distinguishes it from congeners. As a result, we consider S. minutus as a species inquirenda. Stephanoprora argentinensis was described by Sutton et al. (Reference Sutton, Lunaschi and Topa1982) infecting the great grebe, Podiceps major (Boddaert, 1783) (Aves: Podicipedidae) and the white-tufted grebe, Rollandia rolland (Quoy & Gaimard, 1824) (Aves: Podicipedidae) from Provincia de Río Negro, Argentina. They described the collar as having a single row of 20–21 spines. Species of Echinochasmidae are characterized by having an even number of collar spines, which casts doubt on the count. Further, the collar spines count cannot be confirmed by the published drawings since they show broken and missing spines (Sutton et al. Reference Sutton, Lunaschi and Topa1982; Figures 1, 3). Until re-examination of the type specimens (1 holotype and 20 paratypes deposited in the Museo de La Plata, Argentina), we considered it a species inquirenda.
Some species previously assigned to Stephanoprora and that have 24 collar spines [S. mahendrai (Garg, Reference Garg1965), S. iliensis (Gvozdev, Reference Gvozdev1962), and S. odhneri Yamaguti, Reference Yamaguti1971; S. kasachi (Baschkirova, 1941) Yamaguti, Reference Yamaguti1958; S. ozakii (Asada, 1927) Beaver, 1937] are herein regarded as incertae sedis. Stephanoprora mahendrai was described infecting the Ganges River dolphin, Platanista gangetica (Lebeck, 1801) (Mammalia: Cetacea: Platanistidae) from India. A combination of characters (i.e., collar spines count [24 interrupted dorsally], oral sucker larger than ventral sucker, an elongate rather than ovoid body, and a vitellarium extending from the ovary to the posterior body end) do not allow for a correct classification within the current genera belonging to Echinochasmidae. Stephanoprora iliensis was described as Mesorchis iliensis (Gvozdev, Reference Gvozdev1962) infecting the little bittern Ixobrychus minutus (Linnaeus, 1766) (Plecaniformes: Ardeidae) from Kazakhstan. Collar spines count (24), the elongate shape of body, plus an incomplete description (Gvozdev Reference Gvozdev1962 do not described any aspect of male terminal genitalia) do not allow for a correct classification within the current genera belonging to Echinochasmidae. Stephanoprora odhneri was originally described by Odhner (Reference Odhner and Jägerskiöld1910) [as Allechinostomum crocodili (Poirier, Reference Poirier1886)] infecting a Nile crocodile from Sudan. Yamaguti (Reference Yamaguti1971) noted the inconsistency between the accounts of A. crocodili from Odhner (Reference Odhner and Jägerskiöld1910) vs. that of Poirier (Reference Poirier1886) since the latter described the presence of a cirrus sac extending far posteriad to the ventral sucker and reaching the level of ovary. Yamaguti (Reference Yamaguti1971) proposed a replacement name (S. odhneri) for A. crocodrili described by Odhner (Reference Odhner and Jägerskiöld1910). This species has 24 collar spines interrupted dorsally, an elongate body (rather than oval as in Echinochasmus), and a vitellarium that extends anteriad to the ventral sucker. Having these features, it cannot be assigned to any currently included echinochasmid genera. Stephanoprora advena (Shchupakov, 1936) Eslami & KIani, Reference Eslami and Kiani2009 infecting the Caspian seal, Pusa caspica (Gmelin,1788) (Mammalia: Phocidae), is also considered herein as incertae sedis. Shchupakov (1936) described S. advena without collar or collar spines. He also proposed Pseudoechinostomum Shchupakov, 1936 for S. advena differentiating the new genus from Echinostoma by lacking collar and collar spines. Beaver (Reference Beaver1942) proposed a replacement name, Aequistoma Beaver, Reference Beaver1942, since Pseudoechinostomum Odhner, 1911 was preoccupied. Later, Aequistoma was synonymized with Stephanoprora without justification, what its clearly an error since the species lack a collar or collar spines (Skrjabin Reference Skrjabin1966; Eslami & Kiani Reference Eslami and Kiani2009). The taxonomic affiliation of S. advena therefore its indeterminate.
We consider Mesorchis camponica (Nasir & Diaz, Reference Nasir and Diaz1971; as S. camponica) a junior subjective synonym of Me. jacaretinga; both originally reported from the South American spectacled caiman, Caiman crocodilus (Linnaeus, 1758) (Crocodylia: Alligatoridae) in Brazil. Nasir & Diaz (Reference Nasir and Diaz1971) differentiated Me. camponica from Me. jacaretinga because the latter has a ‘relative greater ratio of the suckers, the oesophagus almost extends to the anterior margin of the ventral sucker, lobed testes, and larger eggs, collar spines, and pharynx’ (Nasir & Diaz Reference Nasir and Diaz1971; p 242). However, both descriptions were based upon evidently strongly contracted specimens (Me. jacaretinga) or osmotically damaged specimens (Me. camponica) as suggested by the illustrations (Figure 1 of Teixeira de Freitas & Lent Reference Teixeira de Freitas and Lent1938; Figure 5 of Nasir & Diaz Reference Nasir and Diaz1971). We confirmed this with the holotype of Me. camponica (USNM 1367696) and with a voucher specimen of Me. jacaretinga (USNM 1377925). We think that the slight measurement differences relied upon by Nasir & Diaz (Reference Nasir and Diaz1971) to differentiate these species are inadequate. Both species have the same number and arrangement of collar spines and both have a vitellarium that extends from the level of the ovary to the posterior body end. We also follow Ostrowski de Nuñez et al. (2004) in considering Mesorchis podicipei (Etchegoin & Martorelli, 1997; as S. podicipei) as a junior subjective synonym of Me. uruguayense because, as shown by Ostrowski de Nuñez et al. (2004), the features used to separate these species (i.e., measurements of the body, collar, testes, oesophagus, and uterus) overlap with measurements of Me. uruguayense (Ostrowski de Nuñez et al. 2004). We agree with Beaver (Reference Beaver1936) in considering Stephanoprora merulae (Yamaguti, Reference Yamaguti1933) a junior subjective synonym of Me. polycestus because they differ only slightly in the ratios of the suckers, cirrus sac position, and egg size.
Regarding other species assigned to Stephanoprora, Stephanoprora pitangi (Lutz, Reference Lutz1924; as Monilifer pitangi) was transferred to Echinochasmus by Beaver (Reference Beaver1936) without explanation. We agree with the decision because of the body shape (elongate ovoid), vitellarium distribution (extending to the oesophageal bifurcation), and the short post-testicular region (see Figure 14 in Lutz Reference Lutz1924). Stephanoprora singularis (Lutz, Reference Lutz1924; as Me. singularis) was redescribed by Kohn & Fernandes (Reference Kohn and Fernandes1976) and transferred to Echinoparyphium Dietz, Reference Dietz1909 because they proved that the description by Lutz (Reference Lutz1924) miscounted the collar spines (as 22 collar spines), which actually number 33 (Kohn & Fernandes Reference Kohn and Fernandes1976). Stephanoprora macroovarium (Chertkova & Kosupko, 1966; as Echinochasmus macroovarium) was transferred to Monilifer later synonymized with Stephanoprora. As stated above, we consider Monilifer a synonym of Echinochasmus.
Phylogenetic results
Our 28S and ITS2 sequences representing S. ornata comprised 1527 nucleotides (GenBank accession no. XXXX) and 661 nucleotides (GenBank accession no. XXXX), respectively. They were most similar (94.8% similarity, 79 bp different [28S]; 83.8% similarity; 48 bp different [ITS2]) to those of Echinochasmus japonicus Tanabe, 1926 (Echinochasmidae) (GenBank accession no. OR532444 [28S]; OR507230 [ITS]; from a human in Vietnam [no morphological identification or museum voucher provided]). Our 28S sequence of S. ornata was 93.2–94.3% similar (69–78 bp different) to all other sequences ascribed to Mesorchis. All those sequences ascribed to Mesorchis and used in our phylogenetic analysis exhibited 99.4–99.9% similarity (1–6 bp difference).
The 28S ML analysis (Figure 4a) recovered our sequence of S. ornata sister to a clade comprising all other echinochasmid sequences (3 of 9 genera; 15 of >120 nominal species) (except that of E. pseudobeleocephalus, which was not used herein and did not have ITS2 nucleotide information [GenBank accession no. OR076694–5]). Our 28S tree topology (Figure 4a) resembles that of Tkach et al. (Reference Tkach, Kudlai and Kostadinova2016), Tatonova et al. (Reference Tatonova, Izrailskaia and Besprozvannykh2020), Schwelm et al. (Reference Schwelm, Kudlai, Smit, Selbach and Sures2020), Kalinina et al. (Reference Kalinina, Besprozvannykh, Tatonova and Shchelkanov2023), and Islas-Ortega et al. (Reference Islas-Ortega, Aldama-Prieto, Sereno-Uribe and García-Varela2024). Two clades were recovered: one containing a sequence identified as Echinochasmus coaxatus Dietz, Reference Dietz1909 (Echinochasmus type species; GenBank accession no. KT956928; Figure 3c) and several Echinochasmus spp. having 24 collar spines and a short-tailed cercariae. The second clade comprises a sequence identified as Me. pseudoechinatus (Mesorchis type species; GenBank accession no. KT956935; as S. pseudoechinata; Figure 3b) and several other sequences identified as Mesorchis spp. (as Stephanoprora) and Echinochasmus spp.; all species in this clade reportedly have 20–22 collar spines. We recovered the sequences identified as Echinochasmus donaldsoni Beaver, 1941 (GenBank accession no. KT956930, Figure 3d) and Echinochasmus ostrowskiae Islas-Ortega, Aldama-Prieto, Sereno-Uribe, & García-Varela, Reference Islas-Ortega, Aldama-Prieto, Sereno-Uribe and García-Varela2024 (GenBank accession no. PQ137052) in a well-supported clade. These comprise the only echinochasmid sequences of species having 20 collar spines. All other sequences in the clade represent species that have 22 collar spines and a short-tailed cercaria. Our ITS2 tree topology (Figure 4b) is similar to that of the 28S tree by having our S. ornata sequence sister to a clade with all the other echinochasmid sequences, and the analysis recovered the same clades according to the number of collar spines (see Figure 4b).

Figure 4. Maximum likelihood phylogenetic trees. Values aside nodes are posterior probability obtained with BI and bootstrap percentage obtained with ML, respectively. Scalebar is in substitutions per site. GenBank accession numbers are in parenthesis following each taxon. Type species are indicated by asterisk (*). The newly generated sequence of Stephanoprora ornata Odhner, 1909 (Digenea: Echinochasmidae) is highlighted in bold. Labels along vertical bars represent the number of collar spines (cs). (a) Large subunit ribosomal (28S) DNA phylogeny. (b) Internal Transcribed Spacer region 2 (ITS2) phylogeny.
Discussion
Dietz (Reference Dietz1909) proposed Mesorchis (Me. pseudoechinatus, type species) for bird-infecting species previously assigned to Echinostoma Rudolphi, 1809 [Echinostoma pseudoechinatus (Olsson, 1876), Echinostoma pendulum (Looss, 1899), Echinostoma denticulatum (Rudolphi, 1802)] and two new species (Me. polycestus and Me. conciliatus). He differentiated Mesorchis from Echinostoma by having a small body, 22 collar spines interrupted dorsally, medial testes, and a vitellarium not extending anteriad beyond the testes. Dietz (Reference Dietz1909) proposed Monilifer for Echinochasmus spinulosus (as Mo. spinulosus) and differentiated it from Mesorchis by its ‘compact body shape, the transversally elongated shape of testes, as well as the extreme shortness of the uterus’ (Dietz 1909; p 183). Odhner (Reference Odhner and Jägerskiöld1910) did not consider the collar spines count nor the spines at level of the oral sucker to be of sufficient value to differentiate these genera. He did not consider body shape, testes shape, and pharynx position and thereby synonymized both Mesorchis and Monilifer with Stephanoprora (Odhner Reference Odhner and Jägerskiöld1910; p 142, 162). He provided a brief generic diagnosis that included 22 to 26 collar spines, cirrus sac well-developed, and a minute cirrus (Odhner Reference Odhner and Jägerskiöld1910; p 162). After Odhner’s (Reference Odhner and Jägerskiöld1910) synonymy, several authors discussed it (Beaver Reference Beaver1936; Bhalerao Reference Bhalerao1926; Dawes Reference Dawes1946; Gupta Reference Gupta1963; Mendheim Reference Mendheim1943; Skrjabin Reference Skrjabin1956; Sutton et al. Reference Sutton, Lunaschi and Topa1982; Verma Reference Verma1936). The main argument was the significance of the extension of the vitellarium as a character of generic value while ignoring the collar spines count and other character states described above. Travassos (Reference Travassos1922), Bhalerao (Reference Bhalerao1926), Yamaguti (Reference Yamaguti1933, Reference Yamaguti1939), Beaver (Reference Beaver1936), Lal (Reference Lal1939), Mendheim (Reference Mendheim1943), Dawes (Reference Dawes1946), Gupta (Reference Gupta1963), and Sutton et al. (Reference Sutton, Lunaschi and Topa1982) followed Odhner (Reference Odhner and Jägerskiöld1910) in considering Mesorchis and Monilifer a synonym of Stephanoprora. Lutz (Reference Lutz1924), Linton (Reference Linton1928), and Verma (Reference Verma1936) retained Mesorchis and Monilifer. Skrjabin (Reference Skrjabin1956) accepted Mesorchis, considered Monilifer a synonym of Echinochasmus, and accepted Stephanoprora as a monotypic genus. Recent literature follows Odhner’s (Reference Odhner and Jägerskiöld1910) system (Ostrowski de Núñez Reference Ostrowski de Núñez2002, Reference Ostrowski de Núñez2007; Ostrowski de Núñez et al. Reference Ostrowski de Núñez, Flores, Viozzi and Kreiter2004; Ostrowski de Núñez & Quintana Reference Ostrowski de Núñez and Quintana2008; Stanevičiūtė et al. Reference Stanevičiūtė, Stunžėnas and Petkevičiūtė2015). Kostadinova (Reference Kostadinova, Jones, Bray and Gibson2005; p 15, 44) agrees with Odhner’s (Reference Odhner and Jägerskiöld1910) synonyms and proposed two subgenera: Stephanoprora (Stephanoprora) with S. (S.) ornata as the ‘type species’ and Stephanoprora (Monilifer) with S. (Mo.) spinulosa as the ‘type species’.
Nucleotide-based phylogenetic analyses indicate that the two clades recovered from sequences of echinochasmids contain species with different number of collar spines (Kalinina et al. Reference Kalinina, Besprozvannykh, Tatonova and Shchelkanov2023; Islas-Ortega et al. Reference Islas-Ortega, Aldama-Prieto, Sereno-Uribe and García-Varela2024; Tatonova et al. 2020; Tkach et al. Reference Tkach, Kudlai and Kostadinova2016) (Figures 4a, b). In a 28S phylogenetic analysis of Echinostomatoidea, Tkach et al. (Reference Tkach, Kudlai and Kostadinova2016) recovered two clades with polyphyletic Echinochasmus. They suggested that the clade containing E. coaxatus (type species) represented Echinochasmus and that the second clade containing Mesorchis (as Stephanoprora) plus Echinochasmus represented the two subgenera proposed by Kostadinova (Reference Kostadinova, Jones, Bray and Gibson2005). None of those subgenera were accompanied by a list of inclusive members; therefore, those sub-genera were not adopted by subsequent authors (Ostrowski de Núñez & Quintana Reference Ostrowski de Núñez and Quintana2008; Tatonova et al. Reference Tatonova, Izrailskaia and Besprozvannykh2020). Tatonova et al. (Reference Tatonova, Izrailskaia and Besprozvannykh2020) recovered the same clades but indicated that ‘cluster 2’ (see Figure 2 in Tatonova et al. Reference Tatonova, Izrailskaia and Besprozvannykh2020) represented ‘Stephanoprora-like’ species.
Our results indicate that the species assigned to Stephanoprora in the ‘Stephanoprora-like’ clade and that have 22 collar spines should be assigned to Mesorchis. Much wider sampling combining morphology and nucleotide evidence is necessary to resolve the complex problems in the paraphyletic Echinochasmus and Mesorchis. Stephanoprora ornata is the only echinochasmid infecting a reptile that has been sequenced to date. Our phylogenetical analyses indicate that it shares a common ancestor with all other echinochasmids infecting birds and having available sequences.
Acknowledgements
We thank Gail Barton (Department of Resource Sharing, Auburn University) for assisting with inter-library loan; Amanda Robinson and Anna Phillips (Department of Invertebrate Zoology, National Museum of Natural History, Smithsonian Institution, Washington, DC), Scott Gardner and Gabor Racz (Harold W. Manter Laboratory of Parasitology, University of Nebraska-Lincoln, Lincoln, NE); and Jesus Hernandez-Orts (Department of Life Sciences, Parasitic Worms Collection, Natural History Museum, London, UK) for loaning Echinochasmidae specimens to us for study. We thank Anna Phillips, Chad Walter, Kathryn Ahlfeld, and William Moser (all Department of Invertebrate Zoology, National Museum of Natural History, Smithsonian Institution, Washington, DC) for curating our museum specimens. We also thank field staff from the Ministry of Environment, Forestry and Tourism from the Mahango Core area as well as staff from the Ministry of Fisheries and Marine Resources from Kamutjonga Inland Fisheries Institute.
Financial support
This study was supported by the Southeastern Cooperative Fish Parasite and Disease Project (Auburn University), U.S. Fish and Wildlife Service (Department of Interior), United States Department of Agriculture (National Institute of Food and Agriculture), and the Alabama Agricultural Experiment Station (Auburn University, College of Agriculture). Fieldwork was funded by the Oak Foundation through the Kwando Carnivore Trust of Namibia.
Competing interest
The authors declare that they have no conflict of interest.
Ethical standard
All applicable institutional, national, and international guidelines for the care and use of animals were followed.








