INTRODUCTION
The introduction of species is a major driver of global change and loss of biodiversity in ecosystems (Sakai et al., Reference Sakai, Allendorf, Holt, Lodge, Molofsky, With, Baughman, Cabin, Cohen, Ellstrand, McCauley, O'Neil, Parker, Thompson and Weller2001). The number of species introduced outside their natural ranges is rapidly increasing, although a relatively small proportion of transported and introduced species become invasive (Williamson & Fitter, Reference Williamson and Fitter1996). However, the ability of ecosystem-engineering species to restructure and, hence, radically change the functioning of the recipient habitat and ecosystem process, is high (Crooks, Reference Crooks2002). Invasive species reduce the species diversity and alter the structure and functioning of the invaded ecosystems (Mack et al., Reference Mack, Simberloff, Lonsdale, Evans, Clout and Bazzaz2000; Boudouresque & Verlaque, Reference Boudouresque and Verlaque2002; MacDougall & Turkington, Reference MacDougall and Turkington2005).
The endemic fan mussel Pinna nobilis Linnaeus, 1758 is the largest Mediterranean bivalve and one of the biggest bivalves worldwide (Zavodnik et al., Reference Zavodnik, Hrs-Brenko, Legac, Boudouresque, Avon and Gravez1991), living up to 28 yr (García-March & Márquez-Aliaga, Reference Garcia-March and Márquez-Aliaga2007) and found at depths ranging from 0.5 to 60 m (Butler et al., Reference Butler, Vincente and Gaulejac1993). Its main habitats are soft sediments overgrown by seagrass meadows of Posidonia oceanica or Cymodocea nodosa (Zavodnik, Reference Zavodnik1967; Zavodnik et al., Reference Zavodnik, Hrs-Brenko, Legac, Boudouresque, Avon and Gravez1991). The anterior part of the fan mussel is buried in the seabed and usually anchored to the substratum or among rhizomes and shoots of P. oceanica by byssus threads (Richardson et al., Reference Richardson, Kennedy, Duarte, Kennedy and Proud1999; Templado, Reference Templado2004). Pinna nobilis is a filter feeder with a significant ecological role responding to habitat alteration, and can, therefore, be used as an indicator of environmental impact on seagrass meadows. The population of P. nobilis has been greatly reduced over the Mediterranean during the last decades (Vicente & Moreteau, Reference Vicente, Moreteau, Boudouresque, Avon and Garvez1991) as a result of recreational and commercial fishing, extraction for decorative purposes and incidental killing by anchoring, bottom nets and trawling (Katsanevakis, Reference Katsanevakis2006). Nowadays, P. nobilis is protected under ‘Habitats Directive’ to EU Member States, and it is also protected by the Protocol of the Barcelona Convention, to which most Mediterranean countries are signatories, and all forms of deliberate capturing or killing of them are prohibited (EEC, 1992).
Two recognized invasive seaweeds have spread rapidly through the Mediterranean: Lophocladia lallemandii (Montagne) F. Schmitz (hereafter L. lallemandii) and Caulerpa racemosa (Forsskål) J. Agardh var. cylindracea (Sonder) Verlaque, Huisman et Boudouresque (Verlaque et al., Reference Verlaque, Durand, Huisman, Boudouresque and Le Parco2003; hereafter C. racemosa). The red algae L. lallemandii is widespread throughout the tropics and subtropics and was probably introduced in the Mediterranean via the Suez Canal (Boudouresque & Verlaque, Reference Boudouresque and Verlaque2002). Lophocladia lallemandii is a filamentous algae showing defence metabolites and sexual reproduction, which give it a high invasive potential, growing on a wide range of substrata (bare bedrock, rocky seaweed bottoms, Posidonia oceanica seagrass meadows and over coralligenous communities; Ballesteros, Reference Ballesteros2006). There are few studies concerning invasion of L. lallemandii in the Mediterranean Sea, most in the western Mediterranean (Patzner, Reference Patzner1998; Cebrian & Ballesteros, Reference Cebrian and Ballesteros2010; Bedini et al., Reference Bedini, Bonechi and Piazzi2011). Lophocladia lallemandii induces an increase in mortality in P. oceanica meadows (Ballesteros et al., Reference Ballesteros, Cebrian and Alcoverro2007), grows over different benthic invertebrates (Patzner, Reference Patzner1998; Deudero et al., Reference Deudero, Blanco, Box, Mateu-Vicens, Cabanellas-Reboredo and Sureda2010) and generates a stress response in P. nobilis individuals (Box et al., Reference Box, Sureda and Deudero2009). The green algae C. racemosa has spread extensively throughout the Mediterranean during the last few decades, invading all kind of habitats (Verlaque et al., Reference Verlaque, Afonso-Carrillo, Gil-Rodriguez, Durand, Boudouresque and Le Parco2004; Piazzi et al., Reference Piazzi, Meinesz, Verlaque, Akcali, Antolič, Argyrou, Balata, Ballesteros, Calvo, Cinelli, Cirik, Cossu, D' Archino, Djellouli, Javel, Lanfranco, Mifsud, Pala, Panayotidis, Peirano, Pergent, Petrocelli, Ruitton, Žuljevič and Ceccherelli2005). The rapid expansion relies on very efficient reproductive strategies, both sexual and asexual (Panayotidis & Zŭljevič, Reference Panayotidis and Žuljevič2001). Numerous studies have dealt with its taxonomy, biology and ecology, distribution and spread, seasonality and dynamics and impacts on macrophyte assemblages (see review in Klein & Verlaque, Reference Klein and Verlaque2008). However, few studies have been undertaken to quantify the effects of C. racemosa on the native fauna communities (Vázquez-Luis et al., Reference Vázquez-Luis, Sanchez-Jerez and Bayle-Sempere2008, Reference Vázquez-Luis, Guerra-García, Sanchez-Jerez and Bayle-Sempere2009, Reference Vázquez-Luis, Sanchez-Jerez and Bayle-Sempere2010; Box et al., Reference Box, Martin and Deudero2010). Previous studies dealing with Caulerpa taxifolia detected lower densities of the bivalve Andara trapezia; moreover, the bivalves presented lower dry tissue weight when living in C. taxifolia meadows, which suggests effects on bivalve body condition (Wright et al., Reference Wright, McKenzie and Gribben2007).
In the marine protected area (MPA) of Cabrera National Park (Balearic Islands, north-western Mediterranean) the population of P. nobilis is colonized by the invasive seaweeds L. lallemandii and C. racemosa; however, the extent of this colonization over P. nobilis shells is unknown. Knowledge about colonization of invasive algae at different habitats and depths can provide information for further management of the endemic species, knowledge about the colonization degree of the algae on the bivalve and contribute to knowledge about the links among invasiveness and native and alien species interaction. There are no clear examples of MPAs providing refuge against invasions. Thus, the main objectives of the present study were: (1) to find out the extent of the invasion of L. lallemandii and Caulerpa racemosa on the P. nobilis population; and (2) to test if this invasion differed between depths.
MATERIALS AND METHODS
Study area
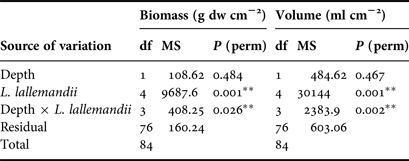
The study was carried out at the MPA of Cabrera National Park in the Balearic Islands, north-western Mediterranean (Figure 1). The MPA was established in 1991 with an area of 100.21 km2, of which 87.03 km2 are maritime. Caulerpa racemosa was recorded in the MPA in 2003 growing at 30–35 m depth, and rapidly spread to almost all benthic communities between 0 and 65 m depth. Lophocladia lallemandii was also recorded for the first time in 2003 and is currently present in nearly all habitats between 5 and 45 m depth (Cebrian et al., Reference Cebrian, Ballesteros, Linares and Tomas2011). The fieldwork was carried out within the P. oceanica seagrass meadows in the MPA, since it is the main habitat for the bivalve P. nobilis.
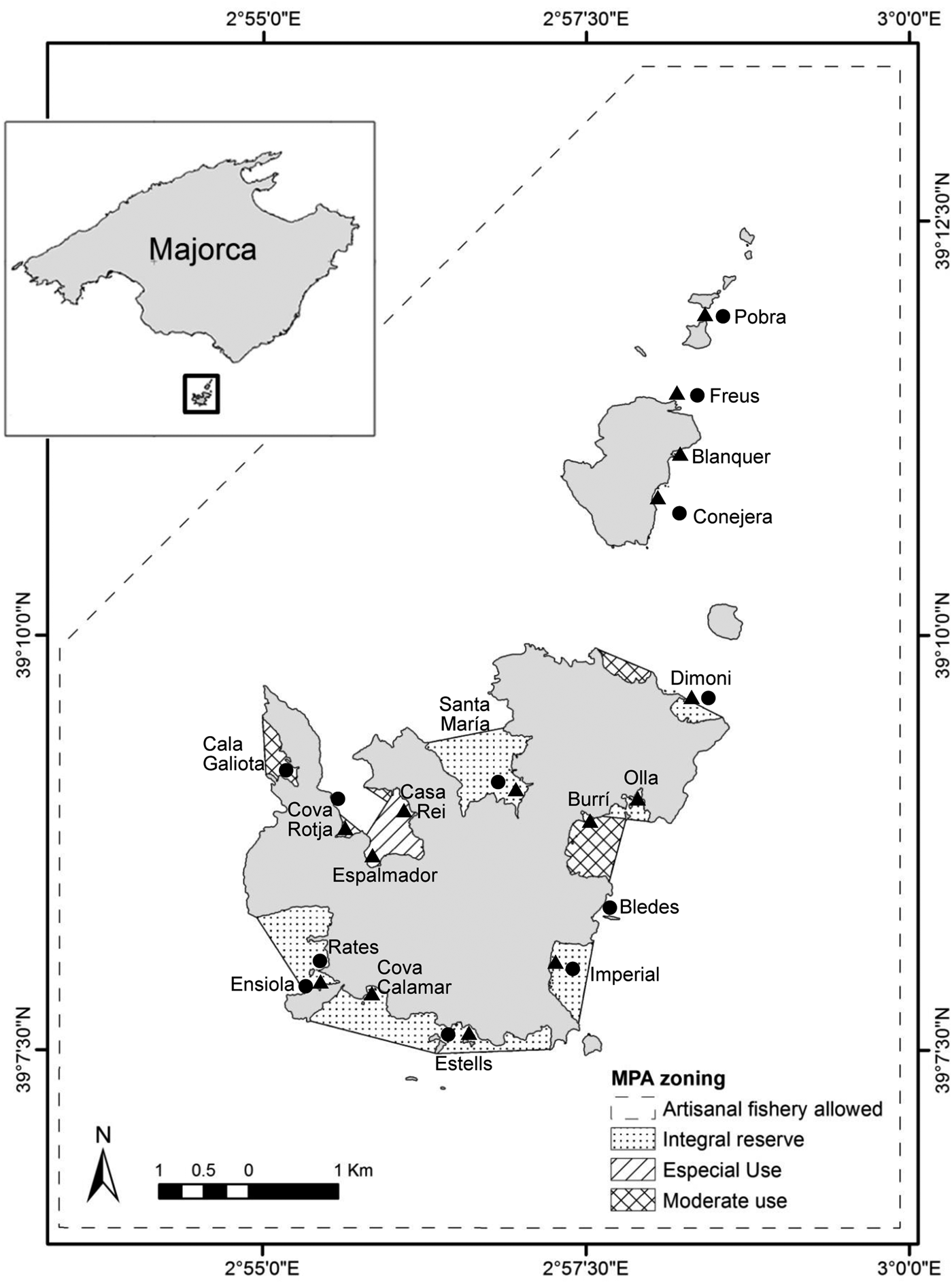
Fig. 1. Study area (ACNP) and different localities (N = 18) sampled for epiphytism of invasive seaweed Lophocladia lallemandii over endemic bivalve Pinna nobilis shells. Triangles, 10 m depth; circles, 20 m depth.
Sampling design
Pinna nobilis individuals were sampled at two different depths (10 and 20 m). Visual censuses along line transects of 30 m length and 2.5 m width (total area per transect of 75 m2) were conducted by SCUBA diving in order to survey the P. nobilis population density, size (as shell maximum width) and the presence of the two alien algae on the shells. The field survey was carried out at the end of July 2011 (average seawater temperature of 25°C), ensuring high abundances and presence of C. racemosa and L. lallemandii (Cebrian & Ballesteros, Reference Cebrian and Ballesteros2009, Reference Cebrian and Ballesteros2010). A total of 122 line transects allocated at 15 sites were established at 10 m depth, whereas 81 distributed among 12 sites were established at 20 m depth. Surveys were conducted by two divers. An index (cover index) was designed to measure the coverage of the invasive species (L. lallemandii and C. racemosa) on the two valves of each Pinna individual, by giving each invasive species a score ranking from 0 to 4: CI0 (0%), CI1 (≤25%), CI2 (25–50%), CI3 (50–75%) and CI4 (75–100%).
Individuals of P. nobilis were randomly sampled (36 individuals at 10 m depth and 48 at 20 m depth), at each depth, by scraping all seaweed epizoics on the shell surface, in order to calculate the biomass and volume of invasive species. From each sample, L. lallemandii was separated and processed to calculate volume (ml), then dried for 48 h at 60°C, and weighed to calculate biomass (g dw). Colonizable surface area of the shells (as the shell part not buried in the sediment and exposed to be colonized) was calculated from images of 35 empty shells collected during fieldwork through ImageJ software (Abramoff et al., Reference Abramoff, Magelhaes and Ram2004). A correlation between maximum width of P. nobilis shell and colonizable surface was conducted. The calculation of colonizable surface was carried out in order to standardize the results of biomass and volume. Therefore, values of volume and biomass of invasive seaweeds were expressed in ml cm−2 for volume, and g dw cm−2 for biomass.
Data analysis
Cover index of invasive seaweeds on P. nobilis individuals gathered from the visual census were analysed according to a two-factor model, where the main factors were ‘Depth’ fixed at two levels (10 m and 20 m) and ‘Site’ random (with 15 levels at 10 m and 12 levels at 20 m). Volume and biomass data from P. nobilis shells were analysed using a two-factor model with main factors ‘Depth’ fixed at two levels (10 m and 20 m) and ‘L. lallemandii’ fixed at five levels (CI0, CI1, CI2, CI3 and CI4). To investigate whether the cover index differed across depths and sites and to test differences of biomass and volume across depths and levels of L. lallemandii (CI), a permutational multivariate analysis of variance (PERMANOVA) was applied, based on the Bray–Curtis dissimilarities of untransformed data (Anderson, Reference Anderson2001; McArdle & Anderson, Reference McArdle and Anderson2001). After the permutational test, a pair-wise test was carried out to detect differences between groups. Multivariate statistical analyses were performed using PRIMER-E software (Clarke & Gorley, Reference Clarke and Gorley2006) with the add-on package PERMANOVA+ (Anderson et al., Reference Anderson, Gorley and Clarke2008).
RESULTS
A total of 872 living Pinna nobilis individuals were studied, of which 449 and 423 were found at 10 m and 20 m depths, respectively. Pinna nobilis is distributed in all seagrass meadows censused within the Cabrera MPA. The size–frequency distribution was unimodal with individuals ranging from 2.2 to 30 cm in maximum width. Densities of P. nobilis individuals at 10 m depth varied from 0 to 37.3 ind 100m−2, while at 20 m depth ranged from 0 to 29.3 ind 100m−2. In terms of mean values, densities (±standard error) of P. nobilis populations at 10 m and 20 m depth presented 4.9 ±0.5 ind 100m−2 and 6.9 ±0.7 ind 100m−2, respectively, including data from transects with values of zero.
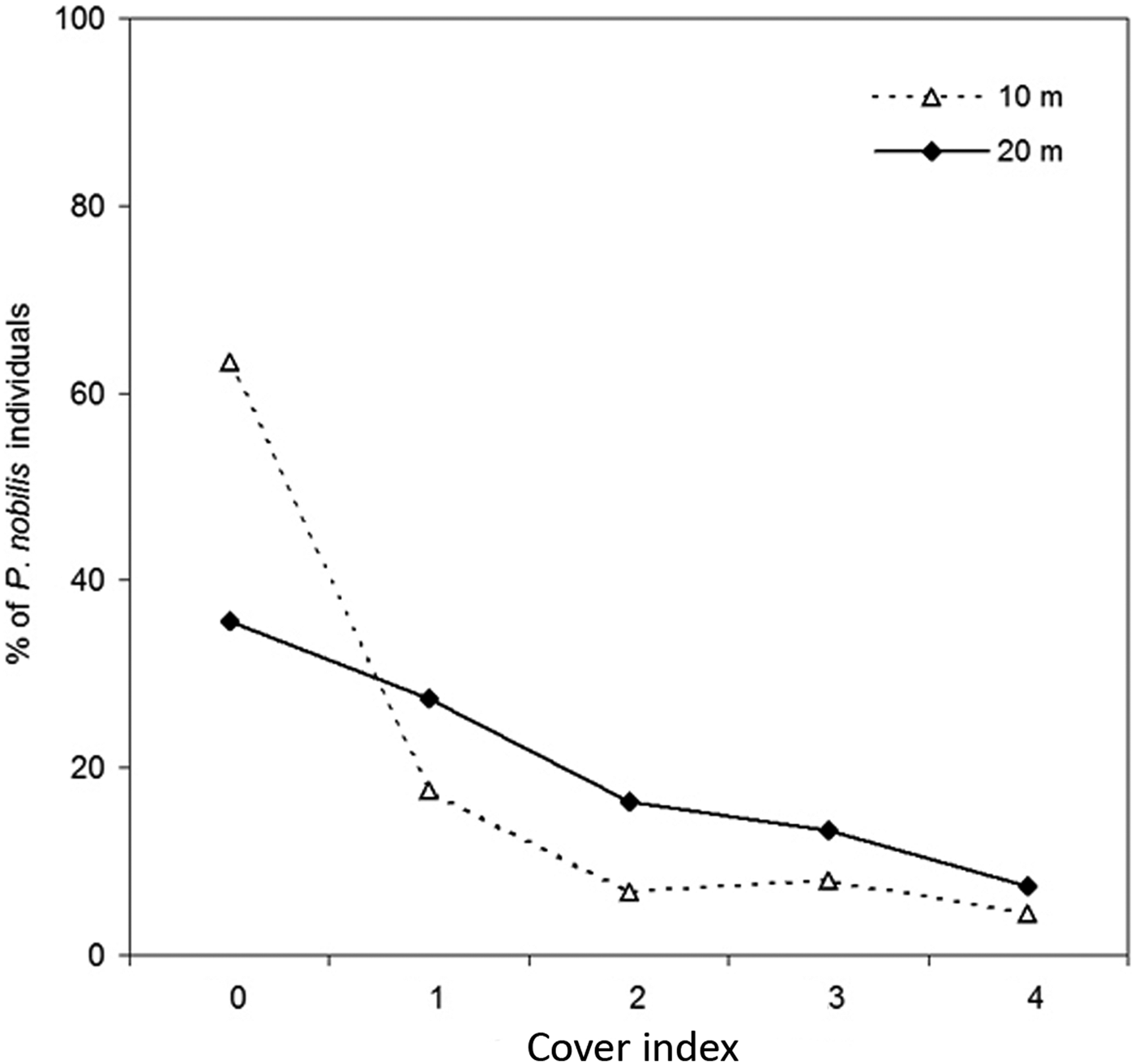
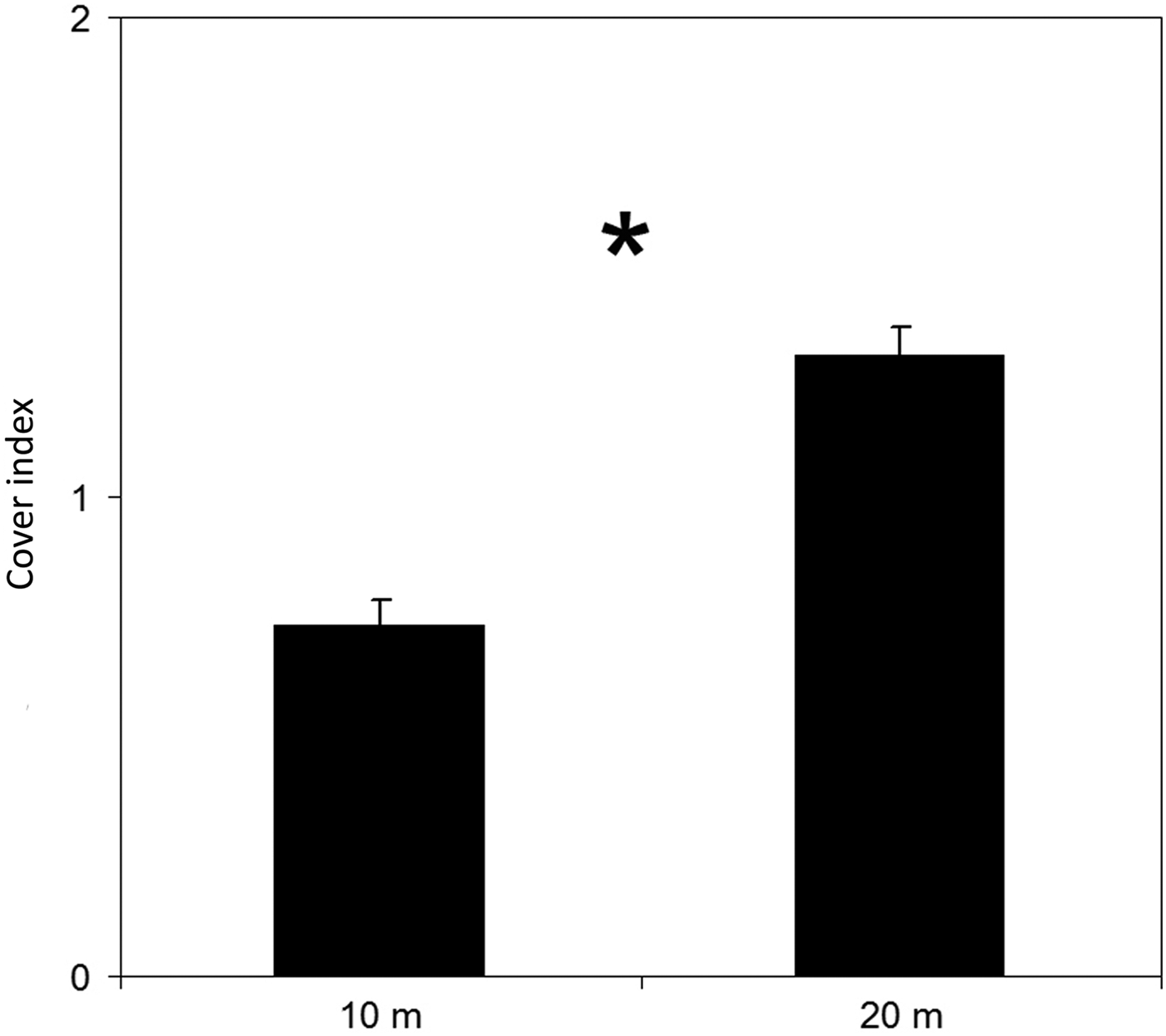
The cover index showed a high presence of Lophocladia lallemandii and a low presence of Caulerpa racemosa, being quantified in 49.37% and 1.38% of P. nobilis population, respectively. Given the low incidence of C. racemosa on P. nobilis shells, results are focused on the colonization by the invasive seaweed L. lallemandii. Most shells were non-epiphyted by invasive seaweeds at shallow waters with percentages of 63.3% at 10 m and 35.7% at 20 m depth (Figure 2). The pattern of epizoism was similar at both depths (Figure 2). In contrast, the cover index of L. lallemandii was higher in deeper areas (Figure 2). Incidence of L. lallemandii cover on P. nobilis population was significantly higher at 20 m depth (depth, PERMANOVA, P < 0.05; Table 1 and Figure 3).

Fig. 2. Percentage of Pinna nobilis individuals epiphyted at each Lophocladia lallemandii cover index at 10 and 20 m depth. See Materials and Methods section for details on codes.

Fig. 3. Mean values (±standard error) of Lophocladia lallemandii cover index on Pinna nobilis living shells at 10 and 20 m depth. *P < 0.05
Table 1. Results of two-factor multivariate PERMANOVA for Lophocladia lallemandii coverage on Pinna nobilis shells. Post–hoc test: depth: 20 m > 10 m (P = 0.012); sites: S. Maria = Casa Rei = Cova Rotja = Espalmador < other sites (P < 0.05).
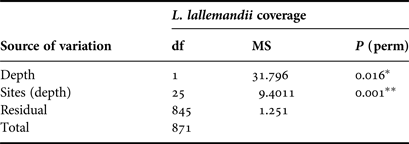
MS, mean square; p, level of significance; df, degrees of freedom; *significant at P < 0.05; **significant at P < 0.01.
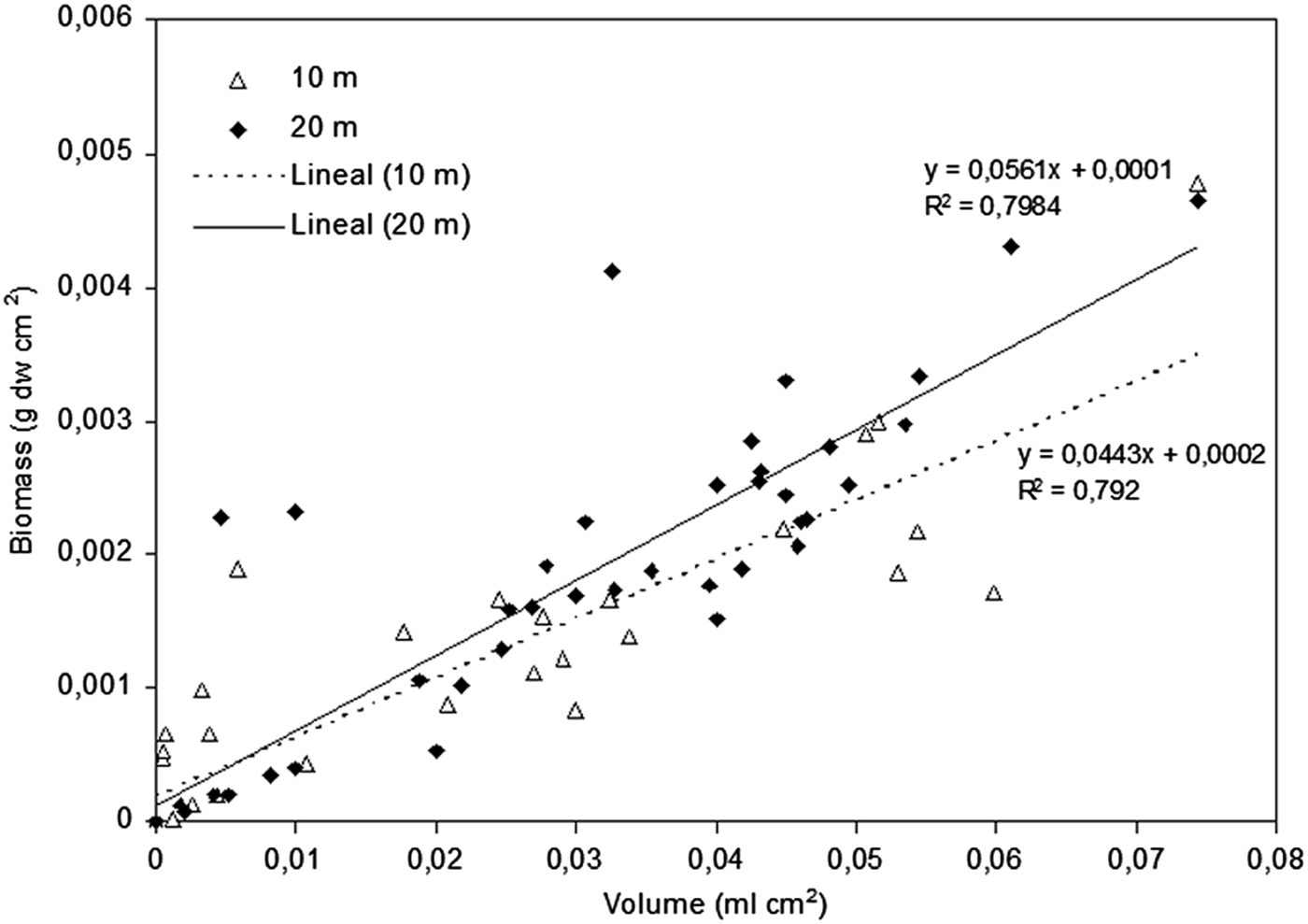
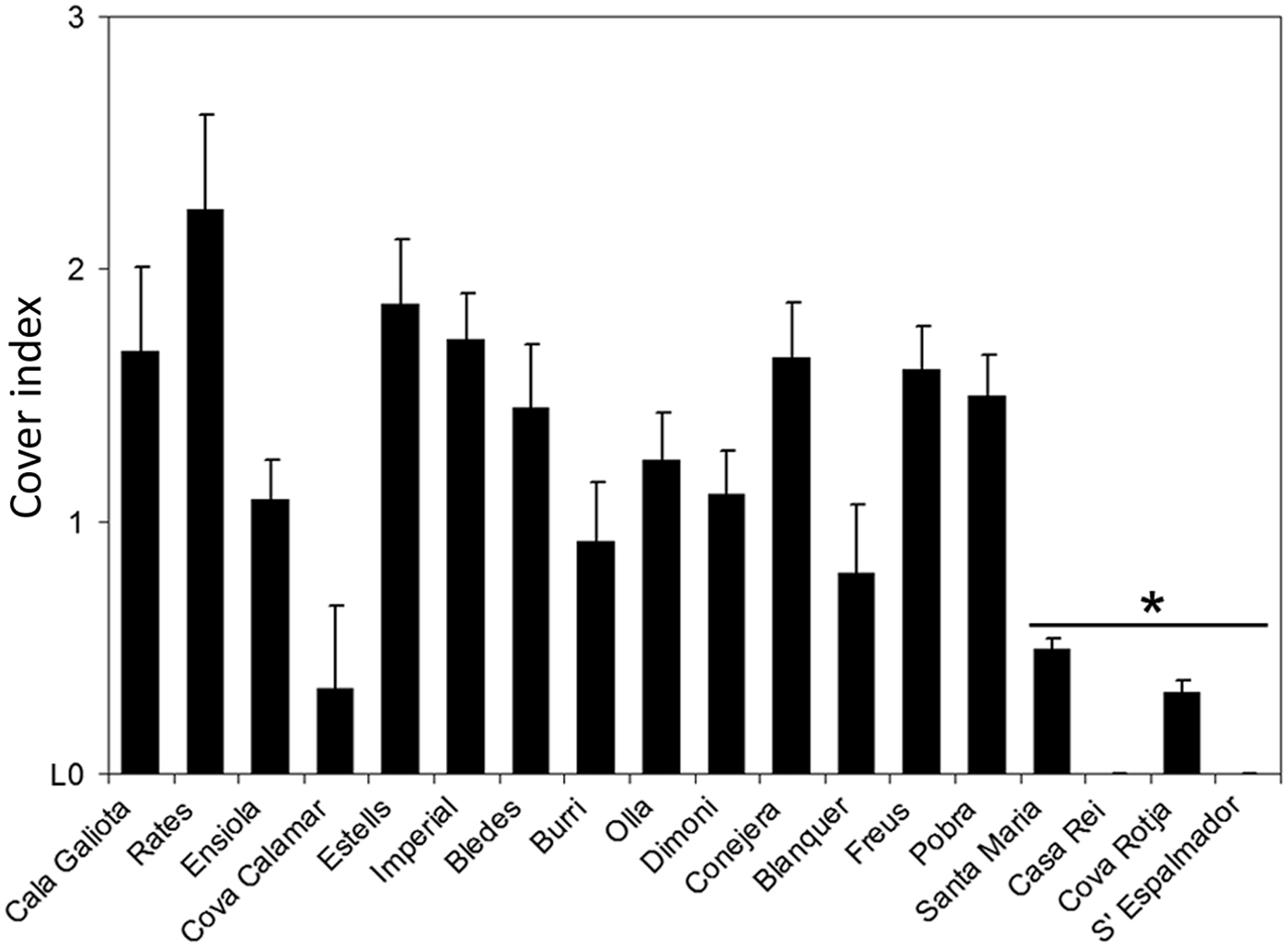
An exponential relationship between maximum shell width and colonizable surface was found (P < 0.001, Figure 4). Biomass and volume of L. lallemandii at different depths showed a similar pattern, but with different values, being both higher at 20 m depth; the R 2 values of the linear regression were similar (P < 0.001 for both depths), but the line slope differed between depths (Table 2; Figure 5). However, significant differences between depths were only found for volume at CI2, being higher at 20 m (depth × Lophocladia, PERMANOVA, P < 0.01; Table 3). Significant differences between sites were detected, with Santa Maria, Casa Rei, Cova Rotja and Espalmador being the least invaded areas (site, PERMANOVA, P < 0.01; Table 1 and Figure 6). Moreover, the variability of colonization cover index differed between sites; the least invaded areas showed low variability, whereas locations with higher values of cover index showed higher variability of the colonization level.

Fig. 4. Exponential function of the regression line of maximum shell width (cm) and colonizable surface (cm2) of Pinna nobilis (N = 35).

Fig. 5. Linear regression of volume and biomass of Lophocladia lallemandii at two depths (10 and 20 m).

Fig. 6. Mean values (+standard error) of Lophocladia lallemandii coverage on Pinna nobilis living shells at sampled sites.
Table 2. Mean values ±standard error of Lophocladia lallemandii biomass (g dw cm−2) and volume (ml cm−2) by different levels of epiphytism and depth.

N, number of samples.
Table 3. Results of two-factor multivariate PERMANOVA for Lophocladia lallemandii biomass and volume of shells of Pinna nobilis. Post–hoc test: depth × L. lallemandii: L2 volume: 20 m > 10 m (P = 0.006).

MS, mean square; P, level of significance; df, degrees of freedom; **significant at P < 0.01.
DISCUSSION
This is the first study assessing seaweed colonization on a large bivalve, in a high density Pinna nobilis population. Results showed a high level of epizoism of the alien Lophocladia lallemandii on P. nobilis shells (approximately half the population was affected). Different L. lallemandii epizoism over P. nobilis has been recorded in this study, with increasing values of L. lallemandii cover index, biomass and volume at deeper depths.
Previous studies carried out on the seaweed community in the Cabrera MPA did not find significant differences in L. lallemandii's biomass as dry weight at 10 m and 30 m depth (Cebrian & Ballesteros, Reference Cebrian and Ballesteros2010). On the contrary, Bedini et al. (Reference Bedini, Bonechi and Piazzi2011) found that in the Tuscan Archipielago the percentage cover and biomass of L. lallemandii increased with depth throughout the investigated bathymetric range. However, this study was carried out only at 3, 6 and 9 m depth. In our case all variables (cover index, biomass and volume) were higher at 20 m depth. It is likely that P. nobilis individuals living at 20 m are more exposed to invasion. One factor that could be affecting this is the lower protection offered by P. oceanica meadows at this depth. In fact, a study carried out by Marba et al. (Reference Marba, Duarte, Holmer, Martinez, Basterretxea, Orfila, Jordi and Tintore2002) found that in Santa Maria mean shoot densities were: 1000 sh m−2 at 7 m, 761.68 sh m−2 at 13 m and 461.33 sh m−2 at 20 m. Therefore, individuals living at 20 m depth are less protected by the seagrass. P. oceanica shoots are one of the preferred habitats for invasive species, thus, due to reduction of this habitat in deeper areas, L. lallemandii settles on new substratum such as P. nobilis individuals. Hydrodynamics could also affect the invasion process since L. lallemandii can be easily detached from the substratum by water movements (Cebrian & Ballesteros, Reference Cebrian and Ballesteros2010), P. nobilis being a new settlement substratum. Generally, the upper region of the exposed part of the P. nobilis shell is the first part to be colonized, and other authors suggested that vertical substrata seem to facilitate the spread of L. lallemandii when compared with horizontal substrata (Bedini et al., Reference Bedini, Bonechi and Piazzi2011). Therefore, P. nobilis could be a preferential substratum for L. lallemandii in P. oceanica meadows in deeper areas, acting as a stepping-stone and facilitating the invasion of the algae throughout seagrass meadows.
Regarding geographical variation, the sites with a lower level of L. lallemandii incidence are areas with extensive P. oceanica meadows, semi-enclosed and with little habitat heterogeneity. Healthy P. oceanica meadows have been suggested to act as a biological barrier against invasion (Ceccherelli & Cinelli, Reference Ceccherelli and Cinelli1999; Ceccherelli et al., Reference Ceccherelli, Piazzi and Cinelli2000). Occhipinti-Ambrogi & Savini (Reference Occhipinti-Ambrogi and Savini2003) suggested that a robust native ecosystem can successfully fight competitor newcomers. Therefore, a healthy pristine community could represent a natural obstacle to bioinvasion. Moreover, sites with a lower level of epizoism could have less propagule pressure since they are less exposed to currents and hydrodynamics; thus, the most epizoited areas are places more exposed to storms. However, we must take into account in the interpretation of these results the fact that some sampled sites presented only one depth.
It is important to remark that a low percentage of the P. nobilis population was epizoited to a maximum L. lallemandii cover level (L4: 4.5% at 10 m and 7.3% at 20 m). It is possible that the invasive seaweed was not found at maximum spread level, or that some mechanisms control the invasion process. Species interactions are a major driver of ecosystem dynamics (Sergio et al., Reference Sergio, Newton, Marchesi and Pedrini2006) and are important in invasion biology and ecology (Colautti et al., Reference Colautti, Ricciardi, Grigorovich and MacIsaac2004). Specialist herbivores control plants in their native range, whereas generalist herbivores provide resistance to invasion in recipient communities (Maron & Vilà, Reference Maron and Vilà2001; Parker et al., Reference Parker, Burkepile and Hay2006). For marine algae, vulnerability to native herbivores may be an important determinant of invasion success. In the case of invasive seaweeds and herbivore control, recent studies through a combined examination of sea urchin Paracentrotus lividus diets and experimental manipulations to measure interaction strength, concluded that introduced C. racemosa is likely to be affected directly by consumption, whereas direct effects on introduced L. lallemandii are unlikely, because it is avoided (Cebrian et al., Reference Cebrian, Ballesteros, Linares and Tomas2011; Tejada et al., Reference Tejada, Deudero, Box and Sureda2013). Other studies showed that L. lallemandii is also avoided by the herbivorous fish Sarpa salpa (Tomas el al., Reference Tomas, Cebrian and Ballesteros2011). However, in the enclosed areas with lower levels of occurrence of L. lallemandii on P. nobilis shells, herbivorous control by mesograzers could occur, since we detected a facilitative interaction between the two species. Positive interactions are defined as non-trophic interspecific interactions that increase the average individual fitness of one species (Callaway, Reference Callaway2007). Probably, P. nobilis enhances the coexistence of the mesograzer mollusc Haliotis tuberculata, since it has been observed that a high percentage of pen shells present H. tuberculata on their valves (personal observation). Previous studies showed that H. asinina have 72.2% of red algae ingestion (Tahil & Juinio-Menez, Reference Tahil and Juinio-Menez1999). Clavier & Chardy (Reference Clavier and Chardy1989) showed that H. tuberculata is absent in areas deeper than 8 m (northern Brittany, France), finding a correlation between abundance and biomass with reduction in water current. In our study, areas with lowest epizoism levels by L. lallemandii correspond to possible optimal areas for H. tuberculata populations, since we have observed individuals of this species on P. nobilis shells, indicating that this association could be producing herbivore control through grazing of seaweeds on P. nobilis shells. However, further studies are necessary to test if ormers could be acting as biological control of L. lallemandii spread in these areas.
Several effects of L. lallemandii invasion on P. nobilis can be envisaged, both on the community structure of associated epizoits, and on the fan mussel's physiological responses. Thus, at community level, studies carried out by Banach-Esteve (Reference Banach-Esteve2011) demonstrated shifts in the native epizoic community after seaweed colonization at the Cabrera MPA. Moreover, P. nobilis physiological status is modified, with enhanced malondialdehyde (MD) levels and thioredoxin reductase (TR) repair activity (Box et al., Reference Box, Sureda and Deudero2009). In this regard, the increased antioxidant enzyme activity is not enough to protect P. nobilis from the negative effects induced by L. lallemandii colonization (Box et al., Reference Box, Sureda and Deudero2009). At the same time, changes in P. nobilis feeding at invaded seagrass meadows occur with variable food source contributions of almost 20% of Lophocladia (Cabanellas-Reboredo et al., Reference Cabanellas-Reboredo, Blanco, Deudero and Tejada2010). Another possible effect is on the hydrodynamics of fluxes surrounding Pinna, as the three-dimensional structure of the invasive seaweed L. lallemandii can modify the particle flux and filtration rates of encrusting organisms (Deudero et al., Reference Deudero, Blanco, Box, Mateu-Vicens, Cabanellas-Reboredo and Sureda2010). The high cover of L. lallemandii over P. nobilis could reduce the availability of water flow around the sessile mollusc and its filtering ability. Bivalves are in general suspension feeders; a high seaweed coverage reduces water current velocities and, therefore, its feeding capacity (Bartoli et al., Reference Bartoli, Nizzoli, Viaroli, Turolla, Castaldelli, Fano and Rossi2001; Tyler, Reference Tyler2007). Moreover, effects of L. lallemandii on other benthic invertebrates have been detected. It is known that L. lallemandii represents an alteration to Reteporella grimaldii natural conditions by evidence of physiological responses (Deudero et al., Reference Deudero, Blanco, Box, Mateu-Vicens, Cabanellas-Reboredo and Sureda2010). These authors also found that distribution patterns of Reteporella grimaldii clearly differed in invaded and non-invaded areas, finding reduced colony abundance in invaded areas (Deudero et al., Reference Deudero, Blanco, Box, Mateu-Vicens, Cabanellas-Reboredo and Sureda2010). Similarly, all these changes could have unknown consequences for the biology, reproduction and survival of the P. nobilis population. However, L. lallemandii has a seasonal growth pattern, with high growth rates in summer and autumn and low biomass during winter and spring (Cebrian & Ballesteros, Reference Cebrian and Ballesteros2010). Therefore, the effect of L. lallemandii on P. nobilis is temporally constrained to the warmer period, and P. nobilis could recover to a healthier situation after colonization. Thus, manipulative experiments controlling the environmental conditions will be needed to show the exact effect of L. lallemandii on fan mussels.
In conclusion, high colonization rates of L. lallemandii over the endemic bivalve P. nobilis are encountered, mainly increasing with depth. This invasion might be promoting some indirect effects on the life history of the protected species, with further unknown consequences. The present results highlight the need to further address interaction across natural communities and alien-species-invaded systems before further cascade effects are driven.
ACKNOWLEDGEMENTS
We acknowledge staff of Cabrera National Park for giving permission and facilities. We are very grateful to L. Basso and F. Fuster for fieldwork assistance.
FINANCIAL SUPPORT
This work was supported by research Project ‘Estado de conservación del bivalvo amenazado Pinna nobilis en el Parque Nacional del Archipiélago de Cabrera’ (024/2010), ‘Organismo Autónomo de Parques Nacionales, Ministerio de Medio Ambiente y Medio Rural y Marino’.