INTRODUCTION
Research investigating relationships between genetic and environmental factors (G × E interaction) seeks to understand how these influences contribute to the development of cognitive phenotypes. This research is essential since descriptions of general population standards, such as age-related baseline rates of cognitive change across various domains (Craik & Salthouse, Reference Craik and Salthouse2007; Hofer & Alwin, Reference Hofer and Alwin2008; Small, Dixon, & McArdle, Reference Small, Dixon and McArdle2011), belie the fact that trajectories of cognitive functioning are influenced by moderating conditions and risk factors (Mungas et al., Reference Mungas, Beckett, Harvey, Farias, Reed, Carmichael and DeCarli2010). More importantly, individuals may be genetically predisposed to be differentially susceptible (for better or worse) to environmental influences (Belsky et al., Reference Belsky, Jonassaint, Pluess, Stanton, Brummett and Williams2009) and such vulnerability may be magnified in aging (Lindenberger et al., Reference Lindenberger, Nagel, Chicherio, Li, Heekeren and Backman2008).
G × E interactions have been used to identify factors which contribute to the heterogeneity of trajectories of cognitive decline with aging. For example, neurological and epidemiological studies have focused on the independent influences of genetic risk factors (Wisdom, Callahan, & Hawkins, Reference Wisdom, Callahan and Hawkins2011) and lifestyle activities (Small, Dixon, McArdle, & Grimm, Reference Small, Dixon, McArdle and Grimm2012) on cognitive changes with aging. Recently, studies have focused on interactions among genotype and modifiable risk factors, such as health and lifestyle (e.g., McFall et al., Reference McFall, Wiebe, Vergote, Westaway, Jhamandas and Dixon2013; Niti, Yap, Kua, Tan, & Ng, Reference Niti, Yap, Kua, Tan and Ng2008; Woodard et al., Reference Woodard, Sugarman, Nielson, Smith, Seidenberg, Durgerian and Rao2012). The results support growing evidence of genetic variation in terms of the impact of modifiable risk factors, especially those related to normative aging. Intervention strategies, including those designed to promote optimal cognitive aging, manage normative aging, or prevent cognitive decline and dementia (Daffner, Reference Daffner2010; Kraft, Reference Kraft2012), may vary in efficacy across individuals with susceptible genotypes. In the current study, we examined the influence of lifestyle activities and a key genetic polymorphism on concurrent and longitudinal changes in cognitive performance.
The current study builds upon the neurocognitive plasticity and flexibility framework proposed by Lövdén, Bäckman, Lindenberger, Schaefer, and Schmiedek (Reference Lövdén, Bäckman, Lindenberger, Schaefer and Schmiedek2010). Analogous to cognitive reserve (Stern, Reference Stern2009), plasticity refers to the potential for improvements in cognitive performance through acts of training, practice, or experience. Accordingly, deliberate efforts and everyday lifestyle activities may promote cognitive plasticity, thus playing a key role in the preservation or enhancement of various aspects of cognitive performance with aging. Notably, beneficial plastic changes only occur after sustained exposure or engagement with appropriately complex external stimuli for a sufficient period of time (Lövdén et al., Reference Lövdén, Bäckman, Lindenberger, Schaefer and Schmiedek2010). Higher levels of cognitive plasticity have been associated with more positive cognitive aging but these effects reflect and are subject to both inter-individual and intra-individual variability (Dixon et al., Reference Dixon, Lentz, Garrett, MacDonald, Strauss and Hultsch2007; Finkel & McGue, Reference Finkel and McGue2007; Ram, Gerstorf, Lindenberger, & Smith, Reference Ram, Gerstorf, Lindenberger and Smith2011). For example, the functional and structural changes in the aging brain that occur in response to complex stimuli are subject to inter-individual differences in neural integrity, enrichment effects, and clinical outcomes (Dixon et al., Reference Dixon, Lentz, Garrett, MacDonald, Strauss and Hultsch2007). Some individuals may require more or less stimulation depending on their level of cognitive flexibility, or the extent to which cognitive functions can be improved (e.g., brain or cognitive reserve; Stern, Reference Stern2009). Notably, cognitive flexibility may be influenced by genetics, especially unfavorable polymorphisms associated with exacerbated cognitive decline and dementia (see Harris & Deary, 2011). This relationship may be especially true for vulnerable populations such as older adults (Lindenberger et al., Reference Lindenberger, Nagel, Chicherio, Li, Heekeren and Backman2008) or individuals with genotypes that make them more susceptible to environmental influences (Belsky et al., Reference Belsky, Jonassaint, Pluess, Stanton, Brummett and Williams2009).
In the current study, we examined how cognitive performance in older adults is influenced by participation in two types of cognitively stimulating lifestyle activities (CSLA): (a) integrated information processing activities (CSLA-II) that require less cognitive effort, and (b) novel information processing activities (CSLA-NI) that require more cognitive effort. In addition, we examined whether allelic variants of Apolipoprotein E (APOE) made individuals differentially responsive to the effects of CSLA.
Located on Chromosome 19, APOE exists in three allelic isoforms: ɛ2, ɛ3, and ɛ4. The ɛ3 variant is most prevalent, occurring in approximately 75–80% of the general population, whereas prevalence of ɛ2 and ɛ4 are approximately 7–8% and 14–15%, respectively (Schipper, Reference Schipper2011). The ɛ4 variant has been associated with disrupting neuroplastic brain mechanisms (Teter, Reference Teter2004), and has also been implicated as a prominent risk factor for mild cognitive impairment (MCI; e.g., Brainerd, Reyna, Petersen, Smith, & Taub, Reference Brainerd, Reyna, Petersen, Smith and Taub2011; Dixon et al., Reference Dixon, DeCarlo, MacDonald, Vergote, Jhamandas and Westaway2013), sporadic Alzheimer’s disease (AD; Farrer et al., Reference Farrer, Cupples, Haines, Hyman, Kukull, Mayeux and van Duijn1997), and other changes in cognitive functioning (Farlow et al., Reference Farlow, He, Tekin, Xu, Lane and Charles2004; Packard et al., Reference Packard, Westendorp, Stott, Caslake, Murray, Shepherd and Elderly2007). Empirical research and meta-analyses revealed that APOE-ɛ4 carriers performed more poorly, compared to non-ɛ4 carriers, on multiple cognitive domains, including global cognitive function, episodic memory, and executive function (Brainerd et al., Reference Brainerd, Reyna, Petersen, Smith and Taub2011; Small, Rosnick, Fratiglioni, & Backman, Reference Small, Rosnick, Fratiglioni and Backman2004; Wisdom et al., Reference Wisdom, Callahan and Hawkins2011).
Considerable attention has focused on the potential to use lifestyle activities as natural interventions in positively influencing cognitive performance among aging adults. Reviews suggest that participation in physical activities (Kramer & Erickson, Reference Kramer and Erickson2007; Rockwood & Middleton, Reference Rockwood and Middleton2007), social activities (Fratiglioni, Paillard-Borg, & Winblad, Reference Fratiglioni, Paillard-Borg and Winblad2004), and cognitive activities (Lövdén et al., Reference Lövdén, Bäckman, Lindenberger, Schaefer and Schmiedek2010) are related to better cognitive functioning among older adults. In our own work (Small et al., Reference Small, Dixon, McArdle and Grimm2012), we examined the extent to which physical, social, and cognitive activities influenced changes in cognitive performance over a 12-year follow-up period. We reported that declines in participation in cognitively engaging lifestyle activities preceded declines in three domains of cognitive performance: processing speed, episodic memory, and semantic memory. Additionally, we found that increased frequencies of concurrent cognitive activities benefited initial level of cognitive performance, but baseline levels did not predict change in cognitive performance (Mitchell et al., Reference Mitchell, Cimino, Benitez, Brown, Gibbons, Kennison and Piccinin2012). Interestingly, change in activity level predicted change in performance. Overall, we concluded that declines in cognitively engaging lifestyle activities can be associated with concurrent deficits and may be a leading indicator of declines in cognitive functioning (see also Hultsch, Hertzog, Small, & Dixon, Reference Hultsch, Hertzog, Small and Dixon1999; Rockwood & Middleton, Reference Rockwood and Middleton2007).
Two previous studies have attempted to merge these influences on cognitive decline with conflicting results. Physical activity has been associated with a reduced risk in cognitive decline, but this benefit has been restricted to APOE-ɛ4 carriers (Niti et al., Reference Niti, Yap, Kua, Tan and Ng2008; Woodard et al., Reference Woodard, Sugarman, Nielson, Smith, Seidenberg, Durgerian and Rao2012). The influence of cognitive activities is less clear: a relatively small sample, restricted range of cognitive domains, and a short-term interval may have been responsible for the observation that cognitive performance was not associated with cognitive activities or their interaction with APOE (Woodard et al., Reference Woodard, Sugarman, Nielson, Smith, Seidenberg, Durgerian and Rao2012). Conversely, larger sample sizes indicate there is indeed a benefit for participating in “productive” cognitive activities and that this benefit differs according to APOE genotype (Niti et al., Reference Niti, Yap, Kua, Tan and Ng2008).
Using new data from the Victoria Longitudinal Study (VLS; Dixon & de Frias, Reference Dixon and de Frias2004), we apply a linear mixed model approach to determine whether participation in two types of CSLA predicted concurrent and longitudinal change in cognitive performance. Additionally, we examined whether the presence or absence of the APOE ɛ4 allele predicted interindividual differences in concurrent or longitudinal cognitive performance. We hypothesized that (a) higher frequency of baseline participation in CSLA-NI would be associated with better concurrent and longitudinal cognitive performance, and (b) APOE genotype would moderate the benefit of participation in both types of CSLA, with APOE non-ɛ4 carriers exhibiting greater responsiveness (i.e., better or worse concurrent and longitudinal cognitive performance) to the impact of CSLA.
METHODS
Study Sample
This research was conducted under full and active human ethics approval from prevailing Institutional Review Boards and all participants provided signed consent forms. This study sample included ongoing participants in the VLS; methodological details on the three main longitudinal samples of the VLS are available elsewhere (Dixon & de Frias, Reference Dixon and de Frias2004; Hultsch, Hertzog, Dixon, & Small, Reference Hultsch, Hertzog, Dixon and Small1998). The sample selected for the present study reflects a subsample of the 1014 participants of the VLS originally recruited in the late 1980s (Sample 1, original n=484) and early 1990s (Sample 2, original n=530). Specifically, inclusion criteria for this subsample were: (a) continuing as a VLS participant in one of these two longitudinal samples through the 2009–2011, (b) volunteering for the VLS genetics initiative (biofluid collection, genotyping) performed during this period, (c) community-dwelling and residing in proximity to one VLS lab, (d) showing no exclusionary signs (see below) in their immediately preceding or concurrent testing session, and (e) remaining active in the longitudinal study at the time data were collected on the activities measures used in the current study. Although the genetics initiative acquired genotyping on n=700 participants, the inclusionary criteria for this study resulted in a final study sample that included n=278 participants (n=59 originally from Sample 1 and n=219 originally from Sample 2). Participants in the current study sample are between 55 and 85 years old at baseline and displayed typical advantages in terms of age, education, and baseline cognitive performance compared to participants who were unavailable to participate due to intervening mortality, and to a lesser extent, mobility. Multivariate analyses confirmed that, on average, study participants were younger, had more years of schooling, and scored higher on all cognitive measures as compared with the substantial number of their original cohorts who did not participate.
We assembled the present study sample from roughly the same cohorts and historical periods with three full waves of data (Wave 1, 2, and 3, with each wave separated by approximately 3 years, M=3.1 and M=3.3 for the participants from Sample 1 and Sample 2, respectively). At each wave, VLS participants are tested on a battery of cognitive, neuropsychological, physical, biological, medical, sensory, health, and psychological assessments. At intake for each VLS sample, exclusionary criteria are implemented to establish relatively healthy cohorts of older adults (Dixon & de Frias, Reference Dixon and de Frias2004). These criteria include concurrent (or history of) serious health conditions that may affect mortality or baseline cognitive health (e.g., serious cardiovascular, cerebrovascular, head injury, or psychiatric conditions), with explicit exclusion of individuals with AD or other neurodegenerative conditions.
Measures
The VLS measurement battery includes the performance assessments noted above, as well as demographic background lifestyle activities (including CSLA) (Dixon & de Frias, Reference Dixon and de Frias2004; Hultsch et al., Reference Hultsch, Hertzog, Dixon and Small1998; Small et al., Reference Small, Dixon and McArdle2011). Equivalent forms for the memory tests were presented at each wave to reduce practice effects. The questionnaires and tasks were presented in the same order to all participants. The complete battery requires approximately 10–12 hr at each wave. For this study we focus on standard and well-documented verbal-based cognitive measures performed with no delay.
Verbal speed
The “lexical decision time” task (Baddeley, Logie, Nimmosmith, & Brereton, Reference Baddeley, Logie, Nimmosmith and Brereton1985) and the “semantic decision time” task (Palmer, Macleod, Hunt, & Davidson, Reference Palmer, Macleod, Hunt and Davidson1985) assessed verbal speed. In the 60-item lexical decision time task, participants must determine as quickly as possible if a 5- to 7-letter word presented on a computer screen is an English word or a nonsense word. In the 50-item semantic decision time task, participants must decide as quickly as possible whether the sentence presented on the computer screen is realistic. Mean latencies for correct responses are used for statistical analysis; longer responses indicate poorer performance.
Episodic Memory
Two immediate episodic free recall tasks were administered. Both the “word recall” and “story recall” tasks were presented in two equivalent forms at each wave, neither of which was repeated on the subsequent waves. The VLS word recall task included two categorized lists of 30 English nouns from six categories (Battig & Montague, Reference Battig and Montague1969; Howard, Reference Howard1980); participants were instructed to remember as many words possible. Participants studied the words for 2 min, and then had a 5-min period in which they wrote as many words as they could recall, in any order, on lined paper (Dixon & de Frias, Reference Dixon and de Frias2004). The number of words recalled at each 3-year follow-up assessment, averaged from both lists, is the outcome measure. For the VLS story recall test, participants are asked to recall the gist of two structurally equivalent narrative stories (Dixon et al., Reference Dixon, Wahlin, Maitland, Hultsch, Hertzog and Backman2004). The proportion of correct gist recall, averaged from both stories, is the outcome measure.
Semantic memory
A “fact recall” and “vocabulary” assessment measured semantic memory. For the fact recall test, participants were asked two sets of 40 questions which evaluated their recall of world knowledge (Nelson & Narens, Reference Nelson and Narens1980). The outcome measure was the average number of correct items from both lists. For the vocabulary measure, 54 multiple-choice (recognition) items were derived from the ETS Kit of Factor References Tests (Ekstrom & Harman, Reference Ekstrom and Harman1976). The number of vocabulary items correctly identified by the participant is the outcome measure.
Verbal fluency
An indicator of executive function, verbal fluency was measured with three tasks from the Kit of Factor Referenced Cognitive Tests (Ekstrom & Harman, Reference Ekstrom and Harman1976), including a “controlled associations test,” an “opposites task,” and a “figures of speech task.” In the controlled associations test, participants were given one of four target words (clear, dark, strong, and wild) and were instructed to write as many words as they could within 6 min that have the same or similar meaning as the target word. In the opposites task, participants were given one of four target words (calm, wrong, fair, and awkward) and were instructed to write as many words as possible within 5 min that have the opposite or nearly the opposite meaning of the target word. In the figures of speech task, participants were given one of five figures of speech (e.g., The fur was as soft as…) and were instructed to write as many words or phrases as possible that would complete the figure of speech. Scores on all three tasks were standardized and averaged to form a composite, which served as the outcome measure.
Cognitively-stimulating lifestyle activities
Assessment of participation in CSLA was measured using two subscales derived from exploratory and confirmatory factor analyses of the VLS-Activities Lifestyle Questionnaire (Hultsch, Hertzog, Small, & Dixon, Reference Hultsch, Hertzog, Small and Dixon1999): 16 CSLA-II activities and 21 CSLA-NI activities. These subscales represent common lifestyle activities that generally require a high or low degree of cognitive engagement. CSLA-II activities require less cognitive engagement, and include traveling, singing, listening to music, going to the theater, or viewing art. CSLA-NI activities require more cognitive effort, and include completing puzzles, playing chess or other knowledge/word games, watching educational television, taking a course, or using computer software. Participants rated their typical frequency of participation over the past two years on a 9-point scale (never, less than once a year, approximately once a year, 2 or 3 times a year, approximately once a month, 2 or 3 times a month, approximately once a week, 2 or 3 times a week, and daily), with higher scores indicating greater frequency of participation.
APOE genotyping
Genotyping was conducted using saliva that was collected according to standard procedures from Oragene DNA Genotek and stored at room temperature in Oragene® disks until DNA extraction. DNA was manually extracted from 0.8 ml of saliva sample mix using the manufacturer's protocol with adjusted reagent volumes. Genotyping was performed using a PCR-RFLP strategy to analyze the allele status for APOE (determined by the combination of the single nucleotide polymorphisms) (SNPs; rs429358 and rs7412).
Statistical Analyses
Before analysis, summary cognitive performance scores were converted to T-scores using the mean and standard deviation from baseline assessment for each measurement. Hardy-Weinberg equilibrium examined the allelic distribution for all variants. APOE ɛ4 zygosity was not taken into account because only 2.2% of the study sample (n=18) presented homozygous ɛ4 alleles; therefore, APOE-ɛ4 carriers were defined as individuals who presented at least one ɛ4 allele. Primary analyses were analyzed with and without participants who presented the ɛ2/ɛ4 since the presence of a single ɛ2 has been shown to protect against significant declines in executive functioning and global cognition tasks as well functional daily living activities in healthy older adults (Bonner-Jackson, Okonkwo, & Tremont, Reference Bonner-Jackson, Okonkwo and Tremont2012). No statistically significant differences were seen between these two analyses, and the presented data represents all participants with genetic data, including those with the ɛ2/ɛ4 allelic combinations.
Data analysis was conducted using SPSS 21.0 software using linear mixed effects models (Singer & Willett, Reference Singer and Willett2003), which estimate the predictive value of covariates (e.g., age, gender), predictors of interest (e.g., participation in CSLA, APOE genotype), and their interactions. Missing data for longitudinally measured variables were estimated using standard missing at random assumptions. In the current study, we examined whether participation in two types of CSLA predicts concurrent and longitudinal change in cognitive performance. Additionally, we examined whether the presence or absence of the APOE ɛ4 allele predicts interindividual differences in concurrent or longitudinal cognitive performance.
RESULTS
Demographic Characteristics
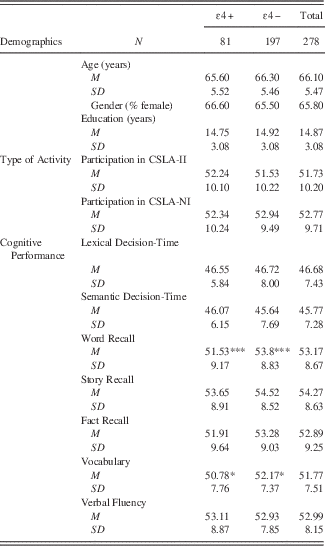
Demographic characteristics of the sample as stratified by genetic status are shown in Table 1. One-way analyses of variance (ANOVA) and Pearson χ2 analyses were used, where appropriate, to identify relationships between age, years of education, gender, and genotype. Participants were approximately 66 years at baseline, predominately female (63%), and averaged almost 15 years of education. A comparison of demographic characteristics by genotype revealed no differences between APOE-ɛ4 carriers and non-carriers. The frequency for the APOE genotype with any ɛ4 allele was .29 (n= 81), and the frequency of APOE genotype with no ɛ4 alleles was .71 (n=197). This allelic distribution is not significantly different from what would be expected in the general population, according to Hardy Weinberg equilibrium.
Table 1 Baseline demographic characteristics, activity level, and cognitive performance by APOE genotype

*p <.05, **p <.01, ***p <.001
Association of Cognitive Performance, Cognitively Stimulating Activities, and Genotype
The results for each cognitive outcome are shown in Table 2. Among the covariates that influenced cognitive performance at baseline, older age was associated with better performance on the vocabulary task, but poorer scores for fact recall and slower responses for the lexical decision-time task. Male gender was associated with better performance on the fact recall measure, but poorer performance on the tests of semantic decision time, word recall and story recall. More years of education was associated with better performance on every cognitive outcome except lexical decision time.
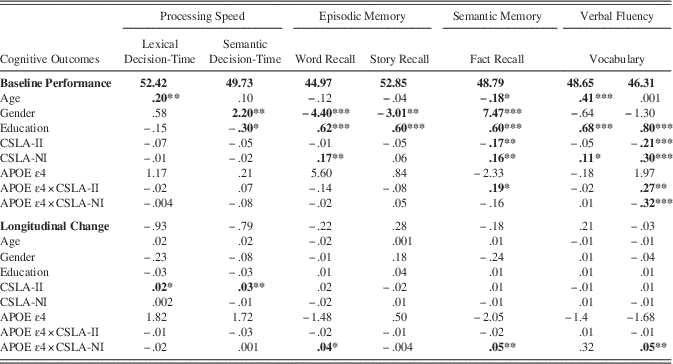
Table 2 Effects of Cognitively Stimulating Activity and Genetic Risk on Baseline Cognitive Performance and Longitudinal Change

Note Values represent estimates from the mixed effects models; * p<.05, ** p<.01, ***p<.001.
Baseline cognitive performance - Effects of CSLA and genotype
Among the predictors of cognitive performance at baseline, increased frequency of participation in CSLA-NI was associated with higher performance in measures of word recall, fact recall, vocabulary, and verbal fluency. Unexpectedly, an inverse linear relationship was found between frequency of participation in CSLA-II and fact recall and verbal fluency, with increased CSLA-II predicting poorer performance. No significant effect was seen between APOE genotype and performance on any cognitive measure.
Interactions between genotype and type of CSLA significantly predicted baseline scores in measures of semantic memory and verbal fluency. More specifically, the following interactions were found: (a) APOE × CSLA-NI had a significant effect on verbal fluency and (b) APOE × CSLA-II had a significant effect on fact recall and verbal fluency. To evaluate the effect of the presence or absence of the APOE-ɛ4 allele, the analyses were stratified by APOE genotype.
The results suggest baseline CSLA frequency significantly predicted baseline verbal fluency and fact recall scores for APOE non-ɛ4 carriers only. Non-ɛ4 carriers who reported more frequent participation in CSLA-NI tended to have higher verbal fluency and fact recall scores (b=.40; b=.33; p<.001, respectively), but those who reported higher participation in CSLA-II had lower verbal fluency and fact recall scores (b=−.24; p<.001 and b=−.15; p<.01, respectively). This effect was not seen in the APOE-ɛ4 carriers for verbal fluency (EstCSLA-NI=.03; p=.709; EstCSLA-II=.10; p=.240) or fact recall (EstCSLA-NI= .15; p=.087; EstCSLA-II=.03; p=.719).
Longitudinal cognitive performance - Effects of CSLA and genotype
Two main effects were found for longitudinal change in cognition: more frequent participation in CSLA-II was associated with poorer performance (i.e., increased response times) for both the lexical decision-time and semantic decision-time tasks (see Table 2). Interactions between genotype and type of CSLA significantly predicted longitudinal scores in measures of episodic memory, semantic memory and verbal fluency. More specifically, APOE × CSLA-NI had a significant effect on measures of word recall, fact recall, and verbal fluency. These analyses were stratified by genotype to further understand the interaction between genotype and cognitive lifestyle activity over time.
For non-ɛ4 carriers, higher participation in CSLA-NI predicted changes in word recall and fact recall (EstCSLA-NI=−.02; p=.03 and EstCSLA-NI=−.03; p=.02, respectively). Again, this relationship was not statistically significant for APOE-ɛ4 carriers. The interaction between genotype and both lifestyle activity measures did not significantly predict verbal fluency outcomes for either APOE-ɛ4 and non-ɛ4 carriers (EstCSLA-NI=.03; p=.09; EstCSLA-II=−.02; p=.294 and EstCSLA-NI=−.02; p=.09; EstCSLA-II=.01; p=.355, respectively).
DISCUSSION
Participation in CSLA and APOE genotype have been evaluated for their independent roles in influencing cognitive performance and change in aging, but it is unclear how these putatively environmental and genetic factors interact (Niti et al., Reference Niti, Yap, Kua, Tan and Ng2008; Woodard et al., Reference Woodard, Sugarman, Nielson, Smith, Seidenberg, Durgerian and Rao2012). The current study examined whether participation in two types of CSLA predicted concurrent and longitudinal change in cognitive performance, and how the presence of the APOE ɛ4 allele moderated the impact of participation in CSLA on cognitive outcomes.
In accordance with the cognitive plasticity and flexibility framework (Lövdén et al., Reference Lövdén, Bäckman, Lindenberger, Schaefer and Schmiedek2010), we first hypothesized that higher baseline participation in CSLA-NI (i.e., more complex activities) would predict significantly better concurrent and longitudinal cognitive performance. As predicted, we found positive linear relationships between participation in CSLA-NI and baseline performance on word recall, fact recall, vocabulary, and verbal fluency, although no longitudinal effects were seen. The results support the expected enrichment effects of everyday cognitive activity, but these effects are not always observed (e.g., Fratiglioni et al., Reference Fratiglioni, Paillard-Borg and Winblad2004; Hultsch et al., Reference Hultsch, Hertzog, Small and Dixon1999; Mitchell et al., Reference Mitchell, Cimino, Benitez, Brown, Gibbons, Kennison and Piccinin2012; Small et al., Reference Small, Dixon, McArdle and Grimm2012). Interestingly, we found an inverse linear relationship between participation in CSLA-II and baseline measures of fact recall and verbal fluency. These results, while unpredicted, suggest that activities requiring little cognitive effort may not provide sufficient levels of stimulation to produce positive plastic changes. Longitudinally, individuals who reported higher frequency of CSLA-II performed significantly worse over time in lexical decision-time and semantic decision-time tasks. Taken together, these results indicate that engaging in more complex cognitive activities is associated with higher cognitive performance, an effect which may persevere over time, whereas participating in less engaging cognitive activities may fail to preserve some aspects of cognitive performance.
We also hypothesized that APOE genotype would moderate the benefit of participation in both types of CSLA, with APOE non-ɛ4 carriers demonstrating greater responsiveness (i.e., significantly worse or better cognitive outcomes) to the effects of CSLA. We found that non-ɛ4 carriers who reported more frequent participation in CSLA-NI had significantly higher baseline scores in fact recall, word recall, and verbal fluency. However, more frequent CSLA-II participation for these individuals was significantly associated with decreased baseline scores in fact recall and verbal fluency. APOE-ɛ4 allele carriers experienced slightly similar benefits; however, the association between CSLA-NI and cognitive performance was only significant for outcomes in baseline word recall. Additionally, the relationship between CSLA-II and cognitive performance was not significant for ɛ4-carriers in terms of fact recall, word recall, and verbal fluency. These results suggest that non-ɛ4 carriers may experience significantly better cognitive outcomes, but this effect is linked primarily to engagement in the cluster of complex cognitive activities, whereas more passive activity participation is linked to poorer cognitive performance.
Although the current study identified concurrent effects of type of CSLA and APOE genotype, few longitudinal effects were found. This result appears contrary to some genetic research identifying the APOE ɛ4 as a key allelic variation that influences normal age-related cognitive decline (De Jager et al., Reference De Jager, Shulman, Chibnik, Keenan, Raj, Wilson and Evans2012; Niti et al., Reference Niti, Yap, Kua, Tan and Ng2008) or the development of AD or dementia (Ferrari et al., Reference Ferrari, Xu, Wang, Winblad, Sorbi, Qiu and Fratiglioni2013). For example, Niti et al. (Reference Niti, Yap, Kua, Tan and Ng2008) found that participating in at least one physical, social or productive activity (e.g., reading) significantly reduced the risk of cognitive decline for non-ɛ4 carriers, whereas this protective relationship was only seen for productive activities in ɛ4-carriers. In a separate longitudinal study, high frequency of participation in one or two lifestyle activities (i.e., social, cognitive, or physical) significantly reduced the risk of dementia or AD for non-ɛ4 carriers, but ɛ4-carriers had to report high frequency of participation in all three activities to experience similar risk reduction (Ferrari et al., Reference Ferrari, Xu, Wang, Winblad, Sorbi, Qiu and Fratiglioni2013).
Some dissimilarities of results across studies can be attributed to differences in study population (e.g., healthy vs. demented), assessment of cognitive function (e.g., measures of global function compared to specific domains), definition of cognitive decline (e.g., change over time vs. clinical diagnosis), measurement of lifestyle engagement (single items vs. scales), classification of lifestyle activities (e.g., general vs. specific), and as we show in this study, functional differences within domains (active vs. passive cognitive activities). Additionally, the effect of a single gene has been demonstrated to be very small (Small et al., Reference Small, Rosnick, Fratiglioni and Backman2004) and may prove difficult to detect in heterogeneous samples. Limitations in the replication of genetic effects on cognitive phenotypes may be an indication that genes make individuals more susceptible to positive and negative environmental influences as opposed to having a direct effect on behavior (Belsky et al., Reference Belsky, Jonassaint, Pluess, Stanton, Brummett and Williams2009). When a genotype is classified strictly as a risk factor, the resulting outcomes are interpreted as either the presence or absence of risk (i.e., the presence or absence of negative outcomes). This perspective presents a skewed interpretation of results because the possibility for beneficial outcomes as a function of other influences or interactions among influences is ignored (Fotuhi et al., Reference Fotuhi, Mohassel and Yaffe2009). Future research must consider this possibility and interpret G × E results in terms of potential benefits (as well as potential risks).
The current study has several strengths and limitations. Among the strengths is the novelty of examining participation in two different types of CSLA. In addition, we examined the moderating influence of APOE genotype on changes in cognitive performance. Previous cross sectional and longitudinal research generally focused on the direct effects of genotype or lifestyle activities, rather than the interaction between the two. Additionally, previous research has grouped cognitive activities into an undifferentiated or universal category without acknowledging the potential difference between activities that require complex cognitive processing (e.g., playing chess) compared to more passive activities (e.g., listening to music).
However, this study also had several limitations. First, it is not possible to determine the directional effects of the relationships between CSLA and cognitive performance. Contrary to the cognitive plasticity/flexibility model, it is possible that individuals with higher levels of cognitive functioning are simply more likely to engage in CSLA-NI. In an earlier VLS study, Hultsch et al. (Reference Hultsch, Hertzog, Small and Dixon1999) applied an alternative analytical model and found evidence that general cognitive decline predicted declines in CSLA-NI. They concluded that while these results do not invalidate theories which advocate the protective relationship between cognitive engagement and declines in cognitive function, it is essential to consider alternative explanations when evaluating the relationship between lifestyle activities and cognitive performance (Hultsch et al., Reference Hultsch, Hertzog, Small and Dixon1999; Small et al., Reference Small, Dixon, McArdle and Grimm2012). Second, the results are limited by the use of self-reported participation in various lifestyle activities. While previous research has identified the VLS activity measure as valid and reliable longitudinal assessment of self-reported activity (Hultsch et al., Reference Hultsch, Hertzog, Small and Dixon1999; Small et al., Reference Small, Dixon, McArdle and Grimm2012), the current study is not immune to the inherent biases associated with self-reported behavior patterns. Third, it is important to note the sample represents relatively well-educated, mostly white, healthy adults. Furthermore, longer follow-up periods (or older study samples) may be required to detect genetic effects on cognitive performance since the heritability of mental functions (including cognition) increases significantly with age (Deary, Johnson, & Houlihan, Reference Deary, Johnson and Houlihan2009). Since the influence of the APOE gene is more evidenced later in life (Deary et al., Reference Deary, Whiteman, Pattie, Starr, Hayward, Wright and Whalley2002), extensive periods of follow-up (i.e., 6 to 12 years) may be required to detect the influence of the APOE gene on cognitive outcomes, particularly for groups of highly educated individuals (Bretsky, Guralnik, Launer, Albert, & Seeman, Reference Bretsky, Guralnik, Launer, Albert and Seeman2003; Dik et al., Reference Dik, Jonker, Comijs, Bouter, Twisk, van Kamp and Deeg2001).
As noted, the educational attainment of this sample may be relevant given recent evidence indicating eight or more years of education reduces the risk of dementia and AD for individuals with and without the ɛ4 allele, but this effect appears to be strongest in non-ɛ4 carriers (Ferrari et al., Reference Ferrari, Xu, Wang, Winblad, Sorbi, Qiu and Fratiglioni2013; Wang et al., Reference Wang, Gustafson, Kivipelto, Pedersen, Skoog, Windblad and Fratiglioni2012). Educational attainment may also represent the effect of prolonged exposure to cognitively enriched environments, which may make highly educated individuals less likely to demonstrate significant gains in cognitive function. We note that a recent VLS study showed the benefits of education in normal aging appeared exclusively in the initial level of performance and not in rate of change (Zahodne et al., Reference Zahodne, Glymour, Sparks, Bontempo, Dixon, MacDonald and Manly2011). Nevertheless, the sensitivity of cognitive tests is limited at higher ability levels, undermining the detection of early cognitive changes (Reiman et al., Reference Reiman, Brinton, Katz, Petersen, Negash, Mungas and Aisen2012).
In summary, our results support the idea that participating in complex cognitive activities is preferential to passive activities in terms of improving or maintaining cognitive abilities, but these effects are moderated by the plastic influence of APOE genotype. Future research would benefit from examining the relationship between of lower levels of education and APOE genotype, as well as longer follow-up periods to gauge the longitudinal effects of APOE on cognitive performance. Additionally, the replication of the current study using samples with MCI, AD, or other neurocognitive disorders could provide additional information about the extent of participation in lifestyle activities and the plasticity effect of APOE.
ACKNOWLEDGMENTS
The authors report no conflicts of interest. The information in this manuscript and the manuscript itself has never been published either electronically or in print. This research was supported by grants from (a) the National Institutes of Health/National Institute on Aging (R01 AG008235) to Roger A. Dixon, (b) Alberta Health Services and University Hospital Foundation (to Roger Dixon, Jack Jhamandas, David Westaway), (c) the Canada Research Chairs Program, (d) the University of South Florida (doctoral fellowship to Shannon K. Runge), and (e) Alberta Innovates--Health Solutions (doctoral fellowship to Peggy G. McFall). In addition, we appreciate the genotyping expertise of David Vergote and the important contributions of the VLS participants and staff. Correspondence concerning the Victoria Longitudinal Study should be addressed to Roger A. Dixon, Department of Psychology, P-217 Biological Sciences Bldg., Edmonton, Alberta, Canada T6G 2E9; email: rdixon@ualberta.ca.