Introduction
Asian Houbara Bustard Chlamydotis macqueenii is a ground-dwelling mid-sized bird of steppe, desert and semi-desert habitats distributed across a large geographical region from Central Asia to the Middle East (Allinson Reference Allinson2014). The majority of Asian Houbara populations are migrants with breeding sites in northern latitudes, especially in Kazakhstan, Turkmenistan, Uzbekistan, Mongolia and Gobi Desert in China. They take various migratory routes towards wintering areas in Turkmenistan, Pakistan, Iran, Afghanistan, India and Iraq (Judas et al. Reference Judas, Combreau, Lawrence, Saleh, Launay and Xingyi2006, Combreau et al. Reference Combreau, Riou, Judas, Lawrence and Launay2011). Breeding populations are also distributed in mid-latitudes of the Middle East in Iran, Pakistan, Afghanistan, Egypt, Iraq, Palestine, Oman, Saudi Arabia and Yemen (Allinson Reference Allinson2014). Iran provides a critical corridor in the middle of Asian Houbara migratory route (Combreau et al. Reference Combreau, Riou, Judas, Lawrence and Launay2011). Moreover, central Iranian semi-arid regions and deserts offer suitable habitats for both wintering and breeding populations (Aghanajafizadeh et al. Reference Aghanajafizadeh, Hemami and Heydari2012). Iran, in comparison to the above mentioned countries, hosts a relatively larger number of wintering populations and thousands of breeders in summer (Heydari et al. Reference Heydari, Hemami and Aghainajafizadeh2010, Allinson Reference Allinson2014). A total of 4,209 houbaras were counted at 335 census sites in Iran during a houbara census in autumn 2017 (Fakharmanesh and Hosseini Reference Fakharmanesh and Hosseini2017). However, the census did not cover all houbara habitats in Iran and hence does not represent the total number of wintering populations. The winter visitors arrive in early autumn to late winter (Combreau et al. Reference Combreau, Riou, Judas, Lawrence and Launay2011) and breeders either arrive from other areas or occur during the entire year (Aghanajafizadeh et al. Reference Aghanajafizadeh, Hemami and Heydari2012).
Asian Houbara ranks among the most valuable game species in the Middle East. Currently, this species has been faced with the threat of extinction (Riou et al. Reference Riou, Judas, Lawrence, Pole and Combreau2011) mainly because of habitat degradation resulted from anthropogenic expansion of land use activities, large-scale uncontrolled hunting in the migratory flyways, falconry hunting in Arabian countries and egg collecting during the breeding seasons (Gao et al. Reference Gao, Combreau, Qiao, Yang, Yao and Xu2009, Allinson Reference Allinson2014, Burnside et al. Reference Burnside, Collar, Koshkin and Dolman2015, Shafaeipour Reference Shafaeipour, Mousavi and Fathinia2015). The International Union for Conservation of Nature (IUCN) has included Asian Houbara as ‘Vulnerable’ under Criterion A4acd. In addition, the species is listed in Annex I of the Convention on International Trade in Endangered Species (CITES) and Annex II of Convention on Migratory Species (CMS) (Allinson Reference Allinson2014). It is therefore important to correctly outline suitable habitats of the species across its distribution range to inform conservation planning.
Species Distribution Models (SDMs) have widely been used to delineate suitable habitats of wildlife species across large geographical areas (Phillips et al. Reference Phillips, Anderson and Schapire2006, Halvorsen et al. Reference Halvorsen, Mazzoni, Dirksen, Næsset, Gobakken and Ohlson2016), to determine limiting factors (Halvorsen et al. Reference Halvorsen, Mazzoni, Dirksen, Næsset, Gobakken and Ohlson2016), to prioritise protected areas (Carvalho et al. Reference Carvalho, Brito, Crespo, Watts and Possingham2011) and to evaluate anthropogenic intrusion in natural landscapes (Franklin Reference Franklin2010). In recent decades, studies on Asian Houbara in Iran have mainly focused on assessing habitat use and evaluation in different geographic and ecological scales (Aghainajafi-Zadeh et al. 2010, Aghainajafizadeh et al. 2012, Yousefi et al. Reference Yousefi, Ahmadi, Nourani, Rezaei, Kafash, Khani, Sehhatisabet, Adibi, Goudarzi and Kaboli2017).
From a habitat selection point of view, Asian Houbara selects plain (slope < 10%) steppes and semi-desert habitats (Haghani et al. Reference Haghani, Aliabadian, Sarhangzadeh and Setoodeh2016) with vegetation cover ranging from very low (Launay et al. Reference Launay, Roshier, Loughland and Aspinall1997, Mian Reference Mian2003, Yang et al. Reference Yang, Qiao, Combreau, Gao and Zhong2003) to relatively dense shrublands (Aghainajafi-zadeh et al. 2010). To breed successfully, they rely on the dense vegetation cover of the region (Yang et al. Reference Yang, Qiao, Combreau, Gao and Zhong2003, Aghainajafi-Zadeh et al. 2010, Aghainajafizadeh et al. 2012). The species is primarily found in areas with low-level of anthropogenic disturbances, but may use agricultural lands such as alfalfa Medicago sativa and rocket Eruca sativa in winter (Aghainajafi-Zadeh et al. 2010).
Yousefi et al. (Reference Yousefi, Ahmadi, Nourani, Rezaei, Kafash, Khani, Sehhatisabet, Adibi, Goudarzi and Kaboli2017) used 130 presence points to assess the impact of climate change on habitat suitability of wintering populations of Asian Houbara in Iran using the Maximum entropy method. We collected a much larger dataset of houbara occurrence (644 points for the wintering population and 216 points for breeding populations) and used an ensemble modelling approach to predict the distribution of both wintering and breeding populations of the species. As the main goal we planned to delineate differences between seasonal distribution of wintering and breeding Asian Houbara populations in Iran. We hypothesised that wintering populations occupy a wider gradient of habitat compared to breeding ones. Accordingly, our objectives explored whether wintering populations, in comparison to breeding ones, could tolerate a broader range of climatic and topographic conditions. We also determined where the wintering and breeding habitats overlap with each other and with the existing protected areas. We identified Asian Houbara’s key wintering and breeding habitats in Iran where conservation efforts should be concentrated. Our results therefore provide significant implications for improving conservation plans and assessment of the species’ conservation status.
Material and methods
Study area
Predicting potential suitable habitats for wintering and breeding populations of Houbara was performed across the species distribution range in Iran with an area of approximately 1,620,375 km2 (Fig. 1). Iran is characterised by five ecological regions (vegetation provinces) namely Khazar (Hyrcanian), Arasbaran, Zagros, Irano-Turanian and Khalij-o-Omani. The latter two provinces, extending over about 70% of Iran, providing ideal habitats for both wintering and breeding Asian Houbara populations.
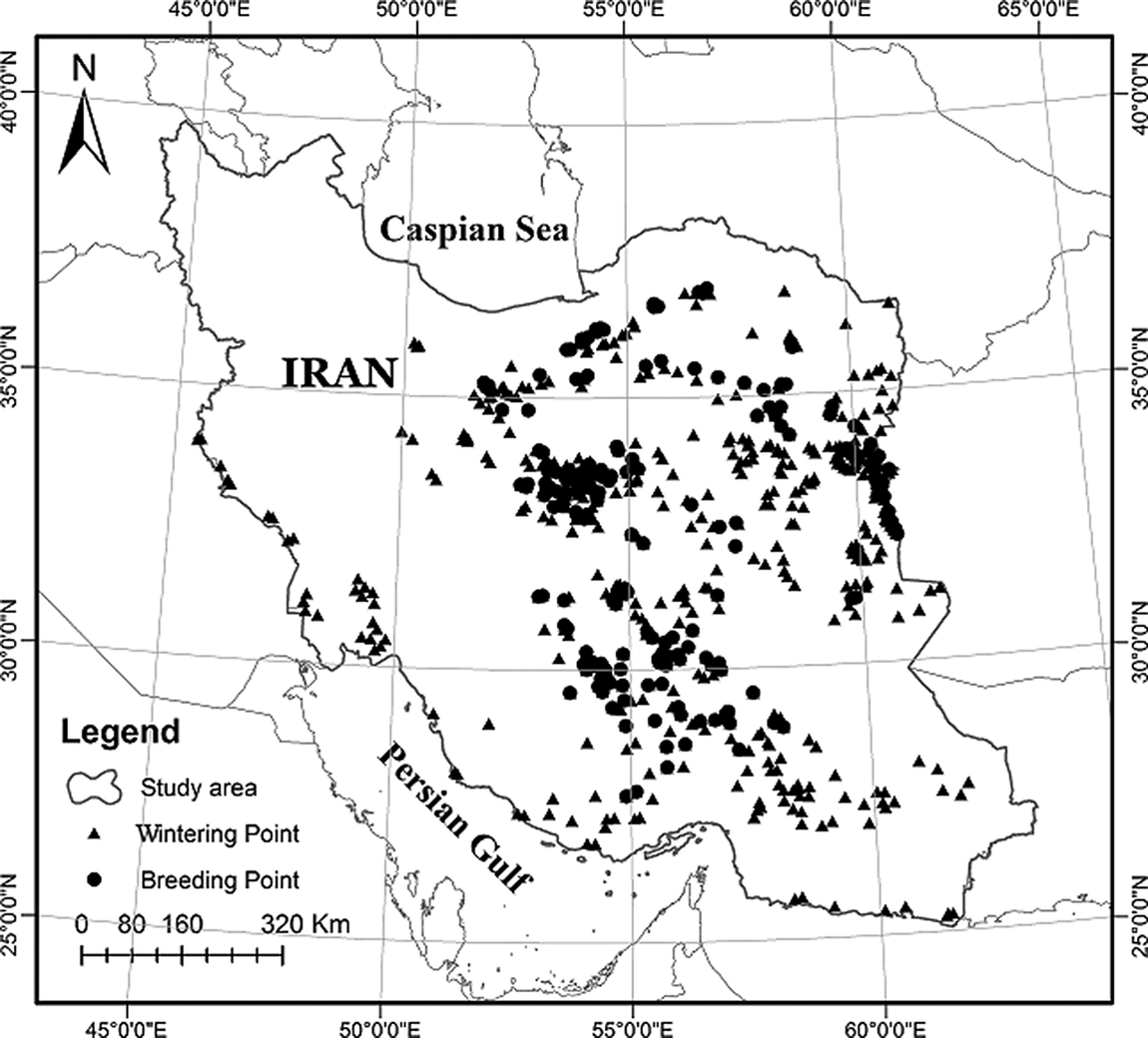
Figure 1. Map of Iran as the study area and the collected occurrence points of wintering (n=644) and breeding (n=216) Houbaras in Iran.
The Irano-Turanian ecological region has a dry climate with a mean annual precipitation of less than 200 mm (except the mountainous areas), hot summers and cold winters, and a continentality index of 30.7. This region receives much of the precipitation (at least 50%) in winter. The most frequent plant species in houbara habitat in this region are species of Haloxylon, Tamarix, Zygophyllum, Seidlitzia, Artemisia, Salsola, Anabasis, and Psammophytes. The Khalij-o-Omani ecological region has a subtropical climate with mild winters and very hot summers. The mean annual precipitation ranges from 90 mm in the east to about 413 mm in the west. The dominant bush species in this region are species of Zygophyllum, Tamarix, Astragalus, Artemisia, Salsola, Calatropis and Atriplex, Hammada salicornica, and Cleome quinquenervia, Lycium depressum, and Halocnemum strobilaceum. Mammal predators such as red fox Vulpes vulpes, Rueppell’s fox Vulpes rueppellii, caracal Caracal caracal, wildcat Felis silvestris, sand cat Felis margarita, and golden jackal Canis aureus occur in these two ecological regions.
The protected network in this study includes three categories including National Parks (NPs; IUCN Category II), Wildlife Refuges (WRs; IUCN Category IV), and Protected Areas (PAs; IUCN Category V), plus a fourth category (No-Hunting Areas; NHAs), which does not conform to the IUCN categories. No-Hunting Area is a protection category which aims to halt poaching but has no limitation on livestock grazing or development plans. A 7-km wide strip along the Iran’s border has also considered as a NHA.
Data collection
To collect occurrence data of wintering and breeding houbaras across the country, we sought assistance from the Iranian Department of Environment (DoE). We designed a questionnaire and distributed it across all the provincial DoE offices in 2015 and it was subsequently sent to the local DoEs across each province by the provincial DoEs. Experts in local DoEs recorded occurrences of houbara populations using GPS. In total, 644 occurrence points for wintering (November to February) and 216 points for breeding houbaras (March to September) were collected from 17 Iranian provinces, where there have been at least one population of houbara including Bushehr, Fars, Hormozgan, Ilam, Isfahan, Kerman, Kermanshah, South Khorasan, North Khorasan, Razavi Khorasan, Khuzestan, Markazi, Qazvin, Semnan, Sistan-o-Baluchestan, Tehran, and Yazd (Figure 1). Before performing SDMs, we applied a Global Moran’s I test to check for spatial autocorrelation of the collected data and filtered the dataset by considering the distance threshold of 5 km as the minimum distance between the points. This resulted in 571 points for wintering and 190 points for breeding populations to perform SDMs.
Habitat variables
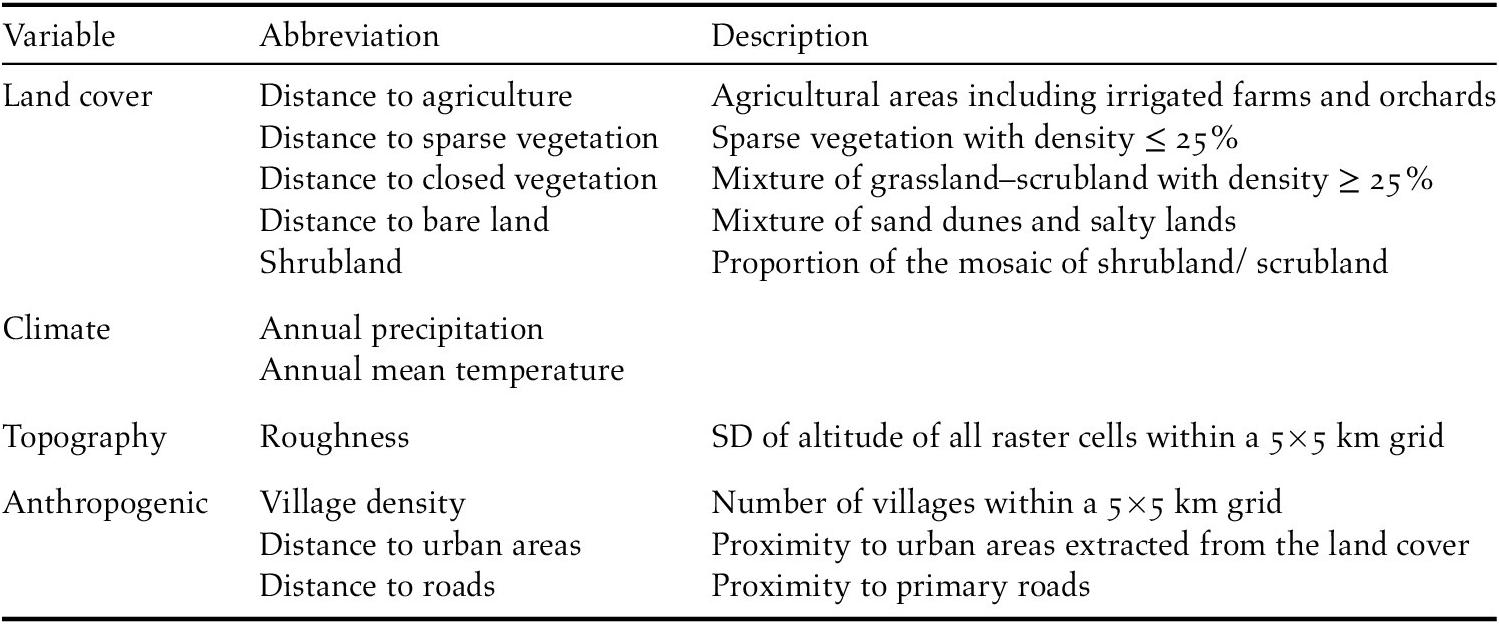
To build houbara distribution models we used 11 explanatory variables including bioclimatic, land cover, topographic and anthropogenic variables (Table 1). For climatic variables we used annual mean temperature and annual precipitation obtained from the WorldClim data set (Hijmans et al. Reference Hijmans, Cameron, Parra, Jones and Jarvis2004). We extracted five categories of land cover from the Iran’s Land use/Land cover map generated by the Iranian Forests, Rangelands and Watershed Management Organization (IFRWO), and calculated Euclidean distances to the nearest patch of each cover type in ArcMap 10.3. Using Shuttle Radar Topography Mission (SRTM) elevation model (http://srtm.csi.cgiar.org), we extracted altitude and used it to produce topographic roughness (i.e. standard deviation of altitude of all raster cells within a grid of 5 × 5 km). To incorporate anthropogenic impacts on houbara in the habitat suitability model, we generated layers of distance to roads, distance to human settlements and density of villages using topographic maps of Iranian Cartographic Organization (ICO). All explanatory variables were built in ArcMap 10.3 at spatial resolution of 1 km. Before model construction, we tested the variables for multi-collinearity by calculating pairwise Pearson correlation coefficients. Among the variables, we removed altitude because of its high correlation with annual mean temperature (r > 0.75).
Table 1. Environmental variables used for constructing Asian Houbara habitat suitability model in arid ecosystems of central Iran.
Houbara individuals are known to occur across areas with slope of less than 12%. Accordingly, we limited the extent of the study area to the range of preferred slope by the species (0–12%) to reduce the effect of this variable and avoid underestimating the importance of other variables in predicting the species distribution.
Model construction
To generate habitat suitability model of Asian Houbara we employed two generalised regression-based models (GLM), a generalised additive model (GAM), and two complex machine learning methods of generalised boosting model (GBM) and maximum entropy (MaxEnt) and combined them to a final ensemble model using biomod2 package (Thuiller et al. Reference Thuiller, Lafourcade, Engler and Araújo2009) in R 3.3.2 (R Development Core Team 2016). Combining multiple forecasts increases the accuracy and robustness of the model prediction (Clemen Reference Clemen1989). Adopting this method allowed us to combine two concepts of simplicity (suitable for extrapolation) and complexity (appropriate for interpolation) in the modelling procedure (Merow et al. Reference Merow, Smith, Edwards, Guisan, McMahon, Normand, Thuiller, Wüest, Zimmermann and Elith2014). Variable importance was evaluated using Biomod2’s randomization tool by randomizing each variable in turn and obtaining mean Pearson’s correlations between the trained model predictions and the predictions made with the same model but with permutated variables. A high correlation value implies that the relative contribution of the variable to the model is low and vice versa (Thuiller et al. Reference Thuiller, Lafourcade, Engler and Araújo2009). Since the model construction needs pseudo-absence or background data, we generated 10,000 randomly selected points across the whole study area. We used 75% of the occurrence points for model training and the remaining 25% for model testing.
We focused on the area under the ROC curve (AUC) as a threshold-independent and true skill statistic (TSS) as a threshold-dependent criterion for evaluating model performance (Allouche et al. Reference Allouche, Tsoar and Kadmon2006, Guisan et al. Reference Guisan, Thuiller and Zimmermann2017). To provide between-model comparison, we averaged these evaluation criteria among 10 replications of each model. We also calculated contribution of variables for each model and subsequently averaged the values among all implemented models.
We used Schoener’s D index to calculate the degree of spatial niche similarity between wintering and breeding populations based on the developed suitability maps using ENMTools 1.4.4 software (Warren et al. Reference Warren, Glor and Turelli2010). This index assumes that the suitability scores produced by the model are comparative to species abundance and ranges from 0 to 1 (Schoener Reference Schoener1974).
Protection coverage of houbara habitats
To calculate proportion of houbara wintering and breeding suitable habitats covered by the Iranian protected area network and to identify the protection gaps, we first extracted the suitable habitat areas by using the minimum ensemble score at species occurrence points as the threshold. The identified suitable habitat class was then further classified into three subclasses based on the mean value of ensemble model suitability scores at occurrence points (x̅): low suitability: <x̅-1SD, medium suitability: >x̅-1SD<x̅+1SD, high suitability: >x̅+1SD. The ratios of total area of houbara suitable habitats in the three subclasses of suitability to the total area of protected areas (including NPs, WRs and PAs) and or NHAs were then calculated (Table 3).
Results
The distribution maps produced by each of the different models for wintering and breeding populations were almost indistinguishable (Figure 3). Similarly, the models were consistent in their predictions of the whereabouts of low-quality habitat for houbara. The performance of all models, as assessed by an independent set of occurrence points not used to train the model, was excellent or good with regard to discrimination capacity (all AUC ˃ 0.78; Table 2) and classification accuracy (all TSS ˃ 0.43; Table 2) but MaxEnt performed best in both. For the ensemble model the performance of the model was considerably higher (wintering populations: AUC = 0.92; TSS = 0.53; breeding populations: AUC =0.95; TSS = 0.73). The four top variables determining breeding and wintering distribution of Asian Houbara, were annual mean temperature, annual precipitation, topographic roughness, and distance to sparse vegetation (Figure 2). The relationship between the probability of houbara occurrence and the gradient of important environmental variables is shown by the response curves generated from the Maxent model (the most accurate model), which imply that both wintering and breeding populations select areas with certain amounts of precipitation, temperature, roughness, and vegetation density (Figure 5). Ruggedness was identified as the most important variable in predicting suitable habitats for the houbara. The suitability of both wintering and breeding houbara populations decreased with increasing density of rural areas. However, wintering and breeding houbara populations responded differently to urban areas. Both populations kept a relatively short distance from urban areas, but then the habitat suitability decreased with increasing distance from urban areas for breeding, but not for wintering populations.
Table 2. Evaluation of five modelling algorithms predicting the distribution of Asian Houbara bustard in Iran. True skill statistic (TSS) and area under the ROC curve (AUC) were measured based on averaging validation subsets of a 10-fold data splitting on Houbara occurrences.


Figure 2. Mean importance of the environmental variables and their corresponding SD calculated over the four SDMs (For variables descriptions see Table 1).
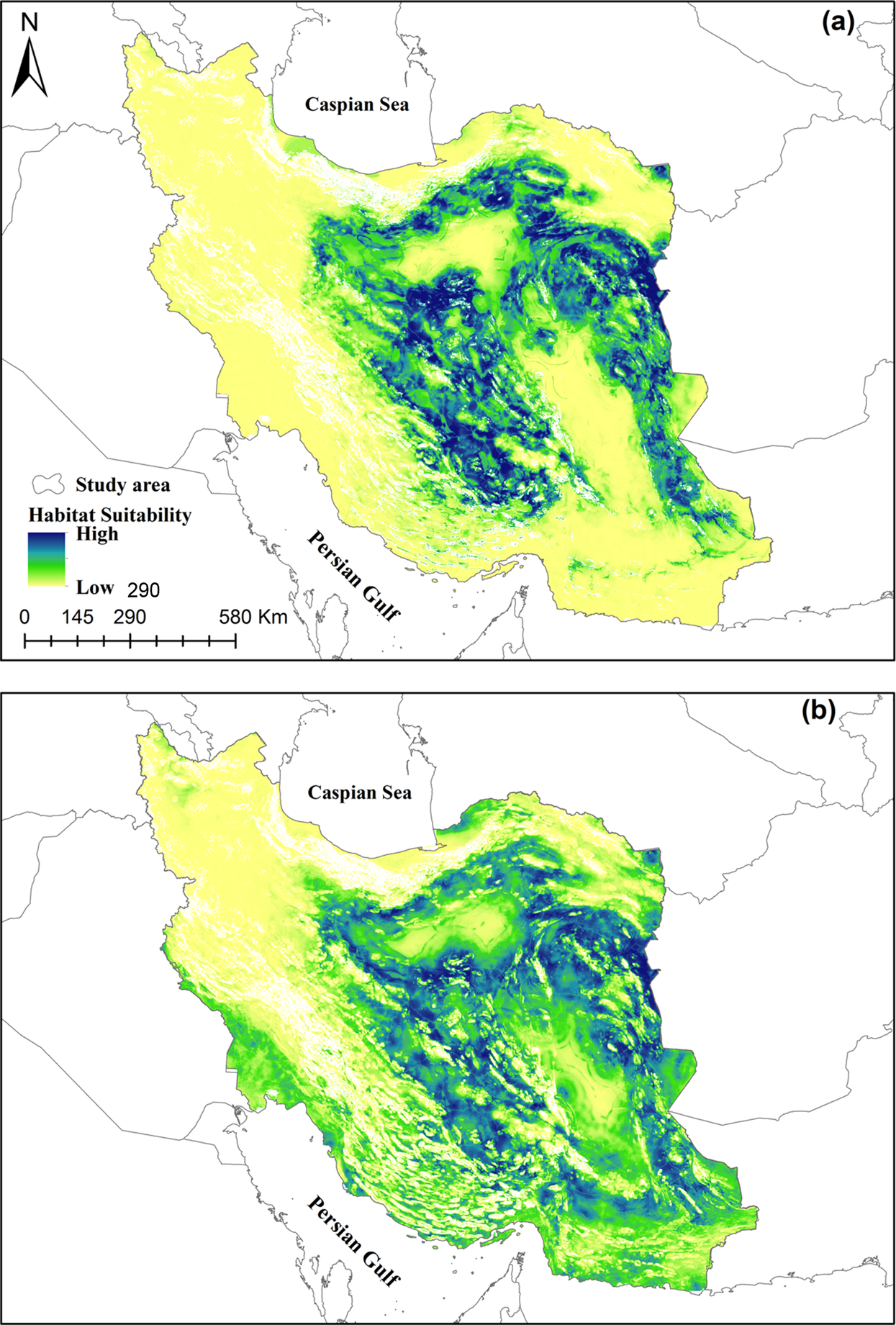
Figure 3. Prediction of the ensemble models for breeding (a) and wintering (b) populations of Asian Houbara in Iran developed based on consensus prediction across four different distribution models including GLM: generalised linear model, GAM: generalised additive model, GBM: Generalised boosting model, and MaxEnt: maximum entropy.
The distribution probability scores calculated for the wintering ensemble model ranged from 52 to 955 and for the breeding ensemble model ranged from 33 to 966. To classify the continuous suitability maps to binary suitable/unsuitable ones we set the classification threshold as the minimum suitability scores predicted for houbara occurrence (357 for breeding and 345 for wintering populations). We calculated the mean value of suitability scores of all presence points separately for wintering and breeding birds (about 670 for both populations) and set it as an additional threshold to classify suitable areas (Fig. 4). On this basis, a total area of 745,300 km2 for wintering and 451,389 km2 for breeding populations was identified as suitable (low suitable + medium suitable + high suitable) landscape for houbara, representing 45.97% and 27.84% of the study area, respectively (Figure 4 and Table 3). We calculated that about 108,000 km2 (40.6%) and 78,600 km2 (29.6%) of suitable habitat for wintering and breeding populations are already protected by PAs and NHAs (Figure 4 and Table 3). We found large suitable unprotected areas located in close proximity to the protected areas and NHAs. Spatial niche similarity between houbara wintering and breeding populations was estimated at 78% based on Schoener’s D index.
Table 3. Total suitable habitats, and area and proportion of protection granted to the habitats of wintering and breeding Houbara in Iran. Area and proportion of zones protected by conservation areas that can potentially function as both breeding and wintering habitat for Houbara are also provided. The percentages are the ratios of total area of Houbara suitable habitats in the three subclasses of suitability (low, medium, high) to the total area of protected areas (including NPs, WRs and PAs) and or No-Hunting Areas.

PAs = Protected areas; NHAs = No-hunting Areas.

Figure 4. Distribution of high, medium, and low suitable habitat areas predicted for breeding (a), and wintering (b), populations of Houbara bustard across Iran. Areas suitable for both wintering and breeding populations along with the location of Iranian protected areas and NHAs are also presented (c).

Figure 5. Responses of breeding and wintering Asian Houbaras to important habitat variables based on the output of the MaxEnt model.
Discussion
Spatial assessment of Asian Houbara distribution could be important for identifying and protecting key habitats with high potential of hosting the species. Climatic factors following by topographical variations were the most important predictive variables influencing the distribution of both wintering and breeding Asian Houbara populations in Iran. Both wintering and breeding populations responded similarly to most of the important predictive variables. However, we observed a decreasing trend in occurrence of the breeding populations with increasing temperature, whereas this trend was opposite for the wintering populations. The probability of chick survival may decrease in areas with relatively higher temperatures (Koshkin et al. 2016, Azar et al. Reference Azar, Chalah, Rautureau, Lawrence and Hingrat2018) such as the hot and dry deserts of the south and central Iran. Wintering houbaras select habitats with mild winters as such areas provide more food. Correspondingly, they do not tolerate very low temperatures as high mortality of this species has been reported from central Iran in harsh wintering conditions (Isfahan Department of Environment, unpubl. data). Both wintering and breeding populations responded similarly to the lower end of mean annual precipitation gradient (approximately 50 mm per year), but differently to the upper end of the gradient in which wintering birds occur in areas with higher amounts of precipitation up to 300 mm per year. Similarly, wintering houbaras respond differently to the upper end of the annual temperature gradient compared to breeding populations, by occurring in south central areas of Iran with higher mean annual temperatures. Having spring rainfall, although in low amounts, makes central Iran relatively suitable for breeding houbaras. In contrast, southern Iran receives a considerably higher amount of winter rainfall, resulting in increased growth and density of vegetation (Formica et al. Reference Formica, Burnside and Dolman2017) and making this region attractive to wintering houbara to fuel up for the following breeding season.
Regarding topography, the associated response curves for both wintering and breeding populations indicated a positive, but limited effect of topographic roughness on species occurrence. Slight topographic heterogeneity provides hiding opportunity for houbaras. However, this association was predicted to be negative toward more rugged areas where the topography blocks houbara’s vision and increases the risk of being predated. In central Iran, houbara nests have been found in vantage sites in low relief areas, where enable houbaras to see long distances and detect approaching predators (M. R. H. pers. obs.).
From a land cover point of view, our results showed that habitats with vegetation cover of approximately 15% to 40% are suitable for Asian Houbara. Aghainajafi-Zadeh et al. (2010) reported differences in habitat selection by Asian Houbara at local and landscape scale. At landscape level, for instance, they prefer areas with higher vegetation cover compared to local-scale (Aghainajafi-Zadeh et al. 2010). Similar studies have also found that suitable habitats for houbara incorporate low to medium vegetation cover (less than 50%) including medium-sized dense shrubs where trees are absent (Launay et al. Reference Launay, Roshier, Loughland and Aspinall1997, Osborne et al. Reference Osborne, Launay and Gliddon1997, Heezik and Seddon Reference Heezik and Seddon1999). Low vegetation areas within relatively dense vegetation communities provide enough hiding cover, but also good horizontal visibility for houbaras (Aghainajafi-Zadeh et al. 2010).
Although our large scale modelling did not show the importance of agricultural land for wintering houbara, but houbaras have reportedly been observed on farmland during autumn-winter, when natural vegetation and animal foods (e.g. invertebrates and small vertebrates; Johnsgard Reference Johnsgard1991, Tigar and Osborne Reference Tigar and Osborne2000) are scarce. Desert areas are known as historical habitats of houbara and hence it has been claimed that the current dependence of wintering houbaras on agricultural lands may primarily be due to human-induced habitat degradation (Mansouri Reference Mansouri2006, Laghai et al. Reference Laghai, Moharamnejad and Bahmanpour2012, Allinson Reference Allinson2014). However, agricultural land in desert areas are inevitably located in productive regions with sufficient water, where could naturally host houbara populations. In central Iran, wintering houbaras soon lose their dependence upon agricultural lands when the mid-winter rain causes vegetation to grow. Rural areas had a negative impact on habitat suitability of houbara as human activities such as livestock grazing are relatively high in areas with high density of villages. Similarly, we did not recognise areas in close proximity to towns as suitable houbara habitat, but breeding populations select areas not too far from small towns which are traditionally located in productive regions.
Asian Houbara appears to avoid occupying extreme deserts (flat desert plains without vegetation and with soils highly loaded by minerals), clay flats (smooth clay surfaces on the periphery of deserts), salt lakes, and saline lands. However, Mansouri (Reference Mansouri2006) reported that this species inhabits highly salinized regions near to agricultural land. The suitability of land for houbara increases as the distance to these barren areas increases. This is mainly associated with the increase in precipitation, vegetation cover, animal-food resources, and significant decrease in daily temperature variation. In addition, the suitability of land for both populations began to decrease towards the Zagros Mountains in western Iran and the Alborz Mountains in northern Iran, where precipitation and topographic roughness are significantly increased. With the arrival of autumn, wintering populations fly towards low-latitude lands and inhabit a diverse spectrum of habitats with varying environmental conditions (Combreau et al. Reference Combreau, Riou, Judas, Lawrence and Launay2011) such as desert and semi-desert habitats of Iran-o-Touranian and Khalij-o-Omani vegetation provinces. In summer, however, breeding populations are only found in Iran-o-Touranian desert and semi-desert habitats. Our findings on the distribution pattern of both breeding and wintering populations are in accordance with the results of previous studies (e.g. Mansouri Reference Mansouri2006, Allinson, Reference Allinson2014). Due to significant anthropogenic development, we did not detect any sign of houbara presence near Darab and Haji-Abad townships in Shiraz Province, where was historically recognised as one of the most iconic habitats of this species (Mansouri Reference Mansouri2006). A large part of suitable habitats found in this study differs from those found by Yousefi et al. (Reference Yousefi, Ahmadi, Nourani, Rezaei, Kafash, Khani, Sehhatisabet, Adibi, Goudarzi and Kaboli2017) as our database incorporated adequate and reliable houbara occurrence data from areas which previous studies failed to address (e.g. South Khorasan, North Khorasan, Sistan-o-Baluchestan, Yazd and eastern Isfahan Province). For instance, unlike Yousefi et al. (Reference Yousefi, Ahmadi, Nourani, Rezaei, Kafash, Khani, Sehhatisabet, Adibi, Goudarzi and Kaboli2017) we found highly suitable wintering habitats on the central Iranian plateau, which is in accordance with the results of national houbara census (Fakharmanesh and Hosseini Reference Fakharmanesh and Hosseini2017) recognising Kerman and Isfahan as provinces that incorporate the highest numbers of wintering houbaras.
Results of this study also showed that the existing protected area network of Iran covers only a small fraction of suitable habitats of both wintering and breeding populations of the species. In other words, approximately 85% of suitable houbara habitats (medium suitable + high suitable) are outside the protected area network. Although Iran embraces the most suitable habitats for wintering and breeding Asian Houbara populations (Allinson Reference Allinson2014), the corresponding network of protected areas has failed to adequately cover the suitable habitats for the species. The unprotected suitable habitats are mostly located near PAs and NHAs which have lower protection level compared to NPs and WRs. Recent analyses have shown that hunting and trapping are responsible for more than 50% of Asian Houbara mortality in Iran, the last wintering stronghold for this threatened species (Burnside et al. Reference Burnside, Collar and Dolman2018). Hunting control through law enforcement and moving towards a truly sustainably managed hunting regime are keys to ensuring the survival of Asian Houbara (Dolman et al. Reference Dolman, Collar and Burnside2018). To protect Asian Houbara in Iran, hunting regulations should be strictly enforced in suitable unprotected and No-hunting areas and the protection level of these regions should be upgraded to higher levels wherever possible. Asian Houbara is an important flagship game species for Iran. Raising the protection level of NHAs and implementing appropriate hunting regulations, could assist in conservation of Asian Houbara, and several coexisting species. At local scale and in winter, attention should be more focused on uncontrolled hunting, capturing and smuggling. It is also important to engage local communities in the protection of this vulnerable species by promoting entrepreneurship developments such as ecotourism.
Acknowledgements
We are grateful to the Iranian Department of Environment and all its subsidiary departments across the country for contributing in collecting occupancy data of Asian Houbara. We are also grateful to Robert Burnside and an anonymous reviewer for constructive comments on an earlier draft. This research received no specific grant from any funding agency, or commercial or not-for-profit sectors.










