Introduction
Despite warnings of marijuana's dangers in pregnancy by many authorities, use has increased, especially in younger women (Brown et al., Reference Brown, Sarvet, Shmulewitz, Martins, Wall and Hasin2017; Volkow et al., Reference Volkow, Compton and Wargo2017; American College of Obstetricians and Gynecologists, 2018). Many women are using at conception, and many continue to use as a natural remedy for morning sickness, depression, and stress and anxiety (Roberson et al., Reference Roberson, Patrick and Hurwitz2014; Brown et al., Reference Brown, Sarvet, Shmulewitz, Martins, Wall and Hasin2017). Intrauterine effects can include altered blood flow, neurological problems, and growth restriction (El Marroun et al., Reference El Marroun, Tiemeier, Steegers, Jaddoe, Hofman, Verhulst, van den Brink and Huizink2009, Reference El Marroun, Tiemeier, Steegers, Roos-Hesselink, Jaddoe, Hofman, Verhulst, van den Brink and Huizink2010; Metz et al., Reference Metz, Allshouse, Hogue, Goldenberg, Dudley, Varner, Conway, Saade and Sliver2017). Late first trimester is a vulnerable exposure period when fetal brain cerebro-cortical laminae develop (Richardson et al., Reference Richardson, Day and Goldschmidt1995; Huang et al., Reference Huang, Xue, Zhang, Ren, Richards, Yarowsky, Miller and Mori2009). Subsequent effects occur on childhood brain development, behavior, and cognition (Fried et al., Reference Fried, Watkinson and Gray2003; Goldschmidt et al., Reference Goldschmidt, Richardson, Willford, Severtson and Day2012; El Marroun et al., Reference El Marroun, Tiemeier, Franken, Jaddoe, van der Lugt, Verhulst, Lahey and White2016). Today's marijuana cigarettes with up to 10-fold more tetrahydrocannabinol (THC) than in 1990 may be more detrimental (Cabrera, Reference Cabrera2016). No current prenatal interventions prevent THC's effects (Calvigioni et al., Reference Calvigioni, Hurd, Harkany and Keimpema2014). The only advice is to promote ‘good parenting skills…to compensate for a less than optimal prenatal environment’ (Huizink, Reference Huizink2015).
Ten weeks gestation begins a critical period when cerebro-cortical laminae are forming but maternal choline levels are lowest (Huang et al., Reference Huang, Xue, Zhang, Ren, Richards, Yarowsky, Miller and Mori2009; Orczyk-Pawilowicz et al., Reference Orczyk-Pawilowicz, Jawien, Deja, Hirnle, Zabek and Mlynarz2016). Although choline has several functions in development, the mechanism of choline's effects on cerebro-cortical inhibition involves direct activation of α7-nicotinic cholinergic receptors responsible for maturation of inhibitory and excitatory neurotransmission (Alkondon et al., Reference Alkondon, Pereira, Cortes, Maelicke and Albuquerque1997; Liu et al., Reference Liu, Neff and Berg2006). Null mouse mutants of its gene CHRNA7 block this effect (Stevens et al., Reference Stevens, Choo, Stitzel, Marks and Adams2014). Corroborating evidence is CHRNA7 pharmacogenomic effects in clinical trials of phosphatidylcholine supplements (Ross et al., Reference Ross, Hunter, McCarthy, Beuler, Hutchison, Wagner, Leonard, Stevens and Freedman2013, Reference Ross, Hunter, Hoffman, McCarthy, Chambers, Law, Leonard, Zerbe and Freedman2016). α7-Nicotinic acetylcholine receptors are expressed in early gestation at levels nearly 10-fold higher than in newborns and adults, but they do not receive acetylcholine synapses until just before birth (Court et al., Reference Court, Lloyd, Johnson, Griffiths, Birdsall, Piggott, Oakley, Ince, Perry and Perry1997; Descarries et al., Reference Descarries, Aznavour and Hamel2008). Millimolar concentrations of choline in amniotic fluid are sufficient for it to be an agonist at fetal α7-nicotinic receptors (Alkondon et al., Reference Alkondon, Pereira, Cortes, Maelicke and Albuquerque1997; Ilcol et al., Reference Ilcol, Uncu and Ulus2002). The expression of CB1 receptors on the same interneurons as CHRNA7 suggests a specific mechanism for the mitigating effects of higher choline levels on fetal brain development for mothers who use marijuana.
Newborns' auditory P50 sensory gating paradigm assesses the development of cerebral inhibition and is thus an early biomarker of the competing effects of THC and choline on the child's fetal interneuron development. The initial stimulus activates a response P50S1 and also activates collateral inhibitory interneurons. Strength of the inhibition is tested by the decrease in response P50S2 after a second stimulus (Adler et al., Reference Adler, Pachtman, Franks, Pecevich, Waldo and Freedman1982). Inhibition of the P50S2 response is the critical variable that reflects the activity of the interneurons that are the site of the convergence of effects of choline and THC (Miller and Freedman, Reference Miller and Freedman1995). Lower P50 inhibition in newborns predicts childhood behavior problems in attention and social withdrawal associated with ADHD and other mental illnesses (Ross et al., Reference Ross, Hunter, Hoffman, McCarthy, Chambers, Law, Leonard, Zerbe and Freedman2016).
The hypothesis of this prospective study is that higher levels of maternal choline in early gestation might mitigate marijuana's effects on fetal brain development as measured by the newborn's P50 inhibition and the child's behavior at 3 months of age. Higher choline levels improved fetal brain development and early childhood behavior in studies that found positive behavioral effects through 4 years of age (Wu et al., Reference Wu, Dyer, King, Richardson and Innis2012; Ross et al., Reference Ross, Hunter, McCarthy, Beuler, Hutchison, Wagner, Leonard, Stevens and Freedman2013, Reference Ross, Hunter, Hoffman, McCarthy, Chambers, Law, Leonard, Zerbe and Freedman2016; Caudill et al., Reference Caudill, Strupp, Muscalu, Nevins and Canfield2018; Jacobson et al., Reference Jacobson, Carter, Molteno, Stanton, Herbert, Lindinger, Lewis, Dodge, Hoyme, Zeisel, Meintjes, Duggan and Jacobson2018; Freedman et al., Reference Freedman, Hunter, Law, Wagner, D'Allesandro, Christians, Noonan, Wywra and Hoffman2019). Healthy women and women with mental illness, infection, and alcoholism benefitted. However, no study has examined the relationship of maternal choline levels to marijuana exposure in pregnancy. A model for the use of a prenatal nutrient to protect fetal development is folic acid prevention of spina bifida, which is effective in a wide range of maternal risks (Beaudin and Stover, Reference Beaudin and Stover2009).
Participants and methods
Maternal assessment and recruitment
Women were enrolled from a public safety-net prenatal clinic at 14–16 weeks gestation from July 2013 until July 2016. Gestational age was timed from the last menstrual period and by ultrasound. Exclusions were fetal anomaly and major maternal medical morbidity. The Colorado Multiple Institution Review Board approved the study; all participants gave informed consent. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Women were asked to participate in a prospective study of stress in pregnancy, including drug use, on their child's development. The women were informed that drug use would be assessed by interviewers and by urine toxicology and these results were not reportable to authorities under Colorado law.
Psychiatric diagnoses were made using the Structured Clinical Interview for DSM-IV Axis I Disorders with DSM-5 criteria. Self-ratings on Center for Epidemiological Studies of Depression-R (CESD-R), State-Trait Anxiety Inventory-State Version (STAI-S), and the Perceived Stress Scale (PSS) were performed. Maternal sociodemographics and health, including infections and BMI, were assessed. Labor, delivery, and neonatal parameters were recorded from the medical record. Investigators were blinded to drug choline levels during assessments.
Assessment of maternal substance use
Mothers had structured interviews at 16 weeks gestation to assess use at conception, including substance types and frequency, and use during subsequent weeks, anchored by calendar dates and gestational milestones including pregnancy tests and prenatal visits. The interview was repeated every 6 weeks through term. Urine toxicology (Alere iCup Dx14, Waltham MA) was obtained at 16 weeks. Recent research finds that self-report and urine toxicology, although not always concordant, both indicate the timing of past use equally well (Smith et al., Reference Smith, Alden, Herrold, Roberts, Stern, Jones, Barnes, O'Connor, Huestis and Breiter2018b).
Choline measurements
Maternal plasma choline and its metabolite betaine at 16 weeks gestation were assayed by the Colorado Translational Research Center Metabolomics Laboratory using mass spectroscopy. Blood samples were obtained at least two hours after breakfast. Plasma was quickly separated by refrigerated centrifugation to prevent platelet phosphatidylcholine release.
Stable isotope standards for betaine (N,N,N-trimethylglycine, cat no D-3352) and choline (cat no D-2464) were purchased from CDN Isotopes. Serum samples were thawed on ice, then 20 µL was extracted with 480 µL of ice-cold extraction buffer (5:3:2 MeOH:MeCN:H2O) containing 0.1 µM each of N,N,N-trimethylglycine-D9 (betaine) and [1,1,2,2-D4]choline. Extraction was performed by vigorous agitation at 4 °C for 30 min followed by centrifugation at 12 000 rpm, 4° C for 10 min. A 100 µL aliquot of supernatant was transferred to a glass vial, dried under N2 flow, and resuspended in an equal volume of water containing 0.1% (v/v) formic acid. Aqueous extracts were analyzed by ultra-high pressure liquid chromatography-mass spectrometry (UHPLC-MS) on a Thermo Vanquish UHPLC (San Jose, CA) coupled to a Thermo Q Exactive mass spectrometer (Bremen, Germany) via positive electrospray ionization. Solvents were water (phase A) and acetonitrile (phase B) supplemented with formic acid (0.1%) and the flow rate was 0.25 mL/min. Metabolites were separated using a Kinetex C18 (Phenomenex, Torrance, CA) column (2.1 × 150 mm, 1.7 µm) with a 6 min gradient of 0–2 min 2% B; 2–2.5 min increase to 25% B; 2.5–4 min hold at 25% B; 4–4.01 min decrease to 2% B; and 4.01–6 min hold at 2% B. The Q Exactive mass spectrometer was operated in a full scan mode over the range of 65–950 m/z. Samples were randomized and a quality control sample was injected every 10 runs. The coefficient of variation was <10%. Data analysis was performed using Maven Metabolomic Analysis and Visualization Engine (Princeton University) following file conversion by MassMatrix (Case Western Reserve University). Absolute concentrations were obtained using the equation: [light choline] = (peakarealight/peakareaheavy)[heavy choline]*DF, where DF = dilution factor, in this case, 25 (i.e. 20 µ of serum in a total 500 µ volume).
Mothers received information on diets higher in choline, but dietary intake was not estimated because of the low relationship of self-reported intake to maternal choline levels in pregnant women, r = 0.2 (Wu et al., Reference Wu, Dyer, King, Richardson and Innis2012). The placental choline transporter CLT1 produces amniotic fluid levels approximately twice maternal plasma levels (Ilcol et al., Reference Ilcol, Yilmaz and Ulus2003; Baumgartner et al., Reference Baumgartner, Trinder, Galimanis, Post, Phang, Ross and Winn2015). Uptake is proportional to maternal plasma concentration, which suggests that higher peak levels may be important determinants of amniotic fluid levels (Iwao et al., Reference Iwao, Yara, Hara, Kawai, Yamanaka, Nishihara, Inoue and Inazu2016). Maternal levels obtained in non-fasting conditions, as in the present study, can be elevated, but only after high-choline meals that exceed the recommended daily intake (Zeisel et al., Reference Zeisel, Growden, Wurtman, Magil and Logue1980; Holm et al., Reference Holm, Ueland, Kvalheim and Lien2003; Abratte et al., Reference Abratte, Wang, Li, Axume, Moriarty and Caudill2009). Prenatal vitamin use did not affect choline levels. Only choline activates α7-nicotinic receptors (Alkondon et al., Reference Alkondon, Pereira, Cortes, Maelicke and Albuquerque1997). We found no effects of its metabolite betaine.
Neonatal physiological recording of cerebral inhibition
Newborns were studied at 1 month (44 weeks) after birth adjusted for gestational age. Vertex electroencephalogram, electro-oculogram, submental electromyogram, and respiration were continuously recorded while infants napped (Kisley et al., Reference Kisley, Polk, Ross, Levisohn and Freedman2003; Hunter et al., Reference Hunter, Kisley, McCarthy, Freedman and Ross2011). Recording of the cerebral auditory evoked potential P50, a positive EEG wave 50 ms post-stimulus, occurred in the second active sleep episode, the developmental precursor of REM sleep, identified by low-voltage desynchronized vertex activity with the absence of K-complexes, change in respiration, and large eye movements with submental atonia (Anders et al., Reference Anders, Emde and Parmelee1971). The second active sleep episode was reached ~45 min after sleep onset. In adults, P50 inhibition in REM and waking are equivalent (Griffith and Freedman, Reference Griffith and Freedman1995).
Two identical auditory stimuli are delivered 500 ms apart to elicit P50S1 and P50S2. P50 inhibition is often assessed as amplitude ratios P50S2/P50S1 or (P50S1−P50S2)/P50S1 (Adler et al., Reference Adler, Pachtman, Franks, Pecevich, Waldo and Freedman1982). However, the skew inherent in ratios limits their power for correlation with risk factors. P50S2 amplitude, covaried for P50S1, which is normally distributed, has therefore also been used (Smith et al., Reference Smith, Boutros and Schwarzkopf1994). Lower P50S2 amplitudes indicate increased inhibition. The assumption is that P50S1 variance is small, compared to P50S2 variance. In 151 newborns, effect sizes for P50S1 differences between newborns whose mothers had no known risk v. women with depression or schizophrenia ranged from 0–0.16. Effect sizes for a decrease in P50S2 amplitude were 0.21–0.50 (Hunter et al., Reference Hunter, Kisley, McCarthy, Freedman and Ross2011). The effect of maternal schizotypy on newborn P50 inhibition has been replicated by another group, who also found increased P50S2 amplitudes (Smith et al., Reference Smith, Crawford, Thomas and Reid2018a). Intraclass correlation between two newborn recordings 1 week apart is r ICC = 0.84. Other technical aspects of recordings have been published (Hunter et al., Reference Hunter, Corral, Ponicsan and Ross2008; Reference Hunter, Gillow and Ross2015).
Childhood behavioral assessments
Parents completed the Infant Behavior Questionnaire-Revised Short Form (IBQ-R) when the infant was 3 months of age (Gartstein and Rothbart, Reference Gartstein and Rothbart2003; Putnam et al., Reference Putnam, Helbig, Gartstein, Rothbart and Leerkes2014). The Parental Distress Subscale of the Parenting Stress Index was also completed as a possible covariate for parental bias (Abidin, Reference Abidin2012). The 91-item IBQ-R Short Form, commonly used to study behavior in children at this age, rates 14 aspects of child behavior, which the IBQ-R developers clustered into 3 indices by factor analysis. Surgency summarizes the child's level of activity and positive effect. Negativity summarizes fearfulness and anxiety. Regulation summarizes duration of attention, responsiveness to parents, and enjoyment of quiet play. Two components of Regulation are also in Surgency, smiling and soothability, and the two indices are highly correlated in the present sample (r = 0.51, p < 0.001). Covariation with Surgency isolates elements of Regulation that are more specific to the early development of attention and less attributable to the child's general psychomotor activation. A similar covariance between Regulation and Surgency (0.80) has been documented by another group, who have also proposed revisions to the factor structure (Bosquet-Enlow et al., Reference Bosquet-Enlow, White, Hails, Cabrera and Wright2016).
Statistical analyses
Neonatal P50S2 inhibition and childhood IBQ-R Regulation were the two principal outcomes, based on a previous work that found P50 inhibition was a biomarker of choline's effect and that regulatory behaviors were the most affected outcome (Ross et al., Reference Ross, Hunter, Hoffman, McCarthy, Chambers, Law, Leonard, Zerbe and Freedman2016). Kolmogorov–Smirnov tests for each outcome did not find a significant deviation from normal distributions. One-way ANOVAs with Tukey's post-hoc contrasts or χ2 with Fisher's exact test analyzed differences between the four marijuana exposure categories. Generalized linear models analyzed the four maternal marijuana-use groups as a categorical effect and the choline level as a continuous effect, with infant sex as a covariate. Factor analysis of the other maternal sociodemographic, gestational, labor and delivery, and neonatal covariates by principal components analysis with Varimax rotation and Kaiser normalization identified three factors termed Socio-economic, Neonatal Status, and Maternal Health (online Supplementary Table A1).
Choline's effect size on P50 inhibition in a previous study was Cohen's d’ = 0.7 (Cheatham et al., Reference Cheatham, Goldman, Fischer, da Costa, Reznick and Zeisel2012). We expected 20% of the women would have adequate choline levels and 30% attrition (Cheatham et al., Reference Cheatham, Goldman, Fischer, da Costa, Reznick and Zeisel2012; Ross et al., Reference Ross, Hunter, Hoffman, McCarthy, Chambers, Law, Leonard, Zerbe and Freedman2016). Therefore, we enrolled 200 women to have power 1-β > 0.95, α = 0.05, 1-tail to observe an overall choline effect. For the women who continued marijuana usage 10 weeks or later, the post hoc power for the observed effect of marijuana was for P50 inhibition d’ = 0.55, 1-β = 0.79, 1-tail α = 0.05 and for IBQ-R Regulation d’ = 0.79, 1-β = 0.93, 1-tail α = 0.05; the power for the observed effect for the maternal choline level was for P50 inhibition |ρ| = 0.40, 1-β = 0.68, 1-tail α = 0.05 and for IBQ-R Regulation |ρ| = 0.53, 1-β = 0.85, 1-tail α = 0.05.
Attrition from prenatal enrollment to postnatal assessments is inevitable. Most were due to the mother's leaving the area to return to her own mother's house to raise her child. The percentage of women at each time point who used marijuana for at least 10 weeks gestation was no different, indicating no differential dropout based on marijuana use (Fig. 1).
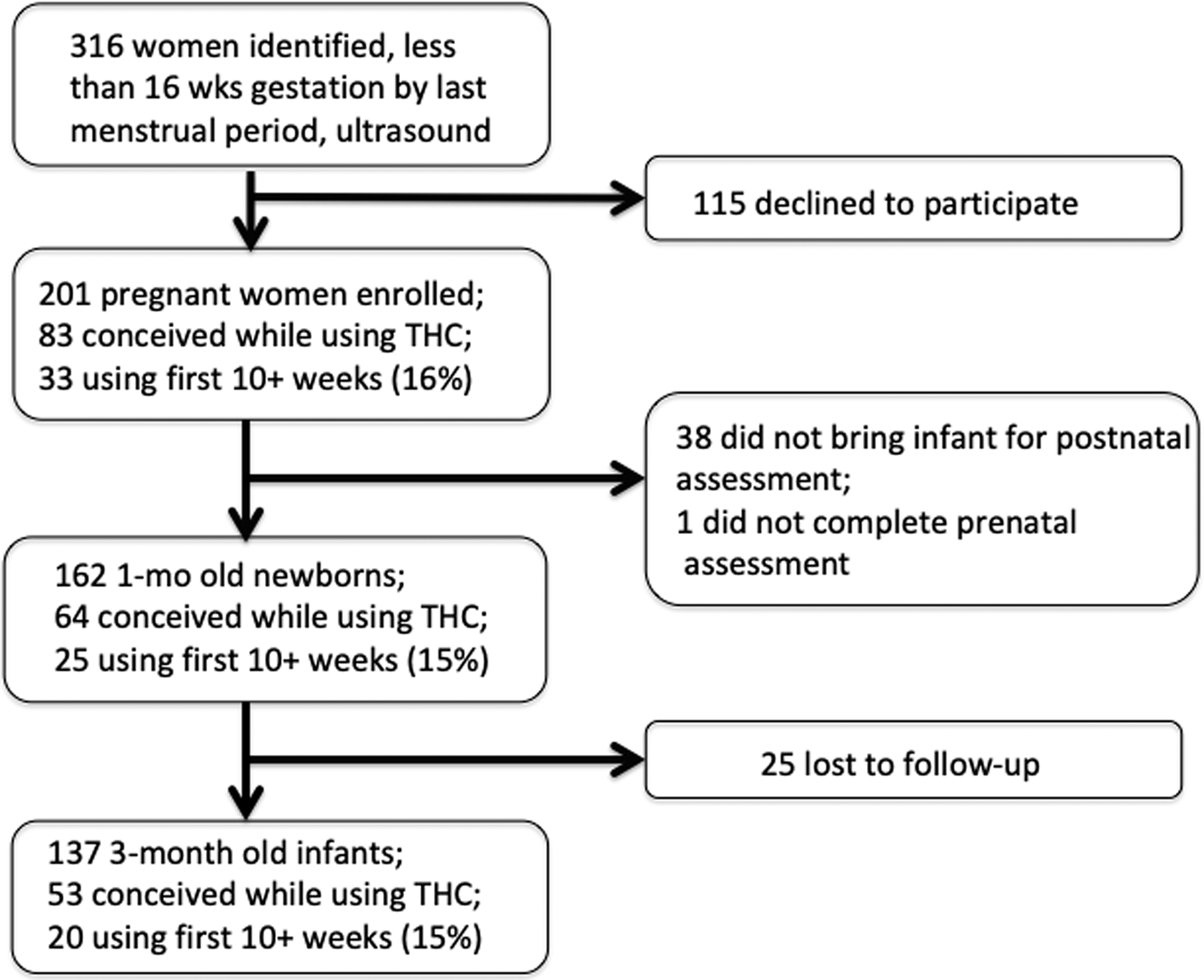
Fig. 1. Enrollment of subjects from initial prenatal visit through the child's first year. The proportion of mothers who were using marijuana at 10 weeks gestation did not change at each stage.
Results
Marijuana use during gestation
Of the 201 women who enrolled in the trial, 162 women brought their newborns for P50 evoked potential recording at 1 month of age, adjusted for gestational age at birth; 98 (60%) did not use marijuana, 26 (16%) reported using it only at the time of conception, 13 (8%) continued during the first weeks of gestation but had discontinued by 10 weeks gestation, and 25 (15%) continued use from conception to 10 weeks gestation, and then with variable frequency until term. The four categories of mothers based on their gestation marijuana use – none, conception, 4 weeks gestation, and 10 weeks or more – were selected because each successive time period had a significantly different proportion of mothers using marijuana compared to the previous period (online Supplementary Fig. A1). Most women smoked marijuana 1–3 times per week.
All women were informed by both their treating clinicians and research personnel about the risks to the fetus of substance use, including marijuana. Mothers who continued to use marijuana during pregnancy were using it at conception. The most common reasons for continuing marijuana were the mothers' belief that it was safer and more effective than pharmaceuticals for morning sickness, depression, and stress.
Urine toxicology was assessed at 14–16 weeks gestation for 91 mothers, of whom 12 were positive for marijuana. Urine samples were not collected from the other mothers because of a protocol error. Over half the mothers who said they used marijuana tested negative, a rate found in other studies (El Marroun et al., Reference El Marroun, Tiemeier, Steegers, Roos-Hesselink, Jaddoe, Hofman, Verhulst, van den Brink and Huizink2010, Reference El Marroun, Tiemeier, Jaddoe, Hofman, Verhulst, Van Den Brink, van den Brink and Huizink2011). Only 1 mother who denied marijuana use tested positive.
Women who used marijuana were younger and less educated (Table 1). Differences in depression, anxiety and stress were expected based on the mothers' self-medication of these symptoms with marijuana. Smoking and other drug use were rare and not related to marijuana use. Alcohol use was related to marijuana use with 22 (88%) women reporting marijuana use also using alcohol at 10 weeks gestation. BMI was increased in mothers who used marijuana. Preterm labor was more common and vaginal birth less common among marijuana users. Neonatal complications were not different in those who used marijuana.
Table 1. Maternal demographics, gestation, labor, and delivery

a Different from mothers who did not use marijuana, Tukey p < 0.05.
b Different from mothers who did not use marijuana, Fisher's exact test p < 0.05.
Levels of choline and its metabolite betaine were not different in women who used marijuana, compared to those who did not (Table 2). Nor were they affected by the mother's mental or medical status or socio-demographic differences or prenatal vitamin use. Choline levels were not related to any labor, delivery, or neonatal issues.
Table 2. Maternal metabolomics and newborn and child outcomes

a Different from children of mothers who did not use marijuana, Tukey p < 0.05
Gestational development of cerebral inhibition
Gestational development of central nervous system inhibition was assessed at 44 weeks gestational age, 1 month after full term birth and later for premature births, as the P50S2 evoked potential amplitude to the second of two paired auditory stimuli (S1, S2). P50S2 amplitude was significantly different between the children of the different groups of marijuana users (F 3,158 = 3.65, p = 0.024; Table 2). Newborns of mothers who used marijuana at 10 weeks gestation or longer had less inhibition with greater P50S2 amplitudes, 1.11 ± 0.86 µV, than newborns whose mothers did not use 0.71 ± 0.56 µV, Tukey's p < 0.05, d’ = 0.55.
Higher maternal choline levels decreased newborn P50S2 amplitude for all women β = −0.49 [95% CI −0.77 to −0.20] p = 0.001. Effects of marijuana use and choline level on P50 inhibition had significant interaction (Wald χ2df3 = 9.31, p = 0.027; Fig. 2, online Supplementary Table A2). Effects of the socio-economic, neonatal status, and maternal health factors were not significant (online Supplementary Tables A1, A2). The effect of the choline level was greatest for newborns whose mothers used marijuana at 10 weeks of gestation or longer (β = −0.40 [95% CI −0.75 to −0.01], p = 0.034; r s = −0.50, p = 0.011, Fig. 2 inset). Effects of choline were not significant in mothers who did not use marijuana during gestation [β = −0.10 (95% CI −0.23 to 0.03) p > 0.1]; their P50 inhibition was already at the level observed in healthy women previously (Hunter et al., Reference Hunter, Kisley, McCarthy, Freedman and Ross2011).

Fig. 2. Effects of gestational exposure to marijuana and maternal choline levels on newborn cerebral auditory evoked potential (P50) inhibition. Newborns whose mothers used at conception only are grouped with those who used <10 weeks gestation, because there was little difference in the outcome. Inset (left): Newborn P50S1 and P50S2 auditory evoked responses: Mother used marijuana through week 16, P50S2 amplitude 2.97 µV, choline level 3.54 µM; Mother did not use marijuana, P50S2 amplitude 0.05 µV, choline level 8.81 µM. Vertical axes −2.5 to 2.5 µV; horizontal axes −50 to 125 ms. Inset (right): Individual subject data for mothers who used marijuana 10+ weeks, r s = −0.50, p = 0.011.
Child sex and maternal BMI and alcohol use did not affect the newborns’ P50 inhibition (online Supplementary Tables A3–A5). Six women used marijuana during lactation, and eight were using at 1 year (Table 1). Their newborns' outcomes were similar to others in their gestational use groups.
Infant behavior at 3 months of age
At 3 months of age, IBQ-R ratings of Regulation, adjusted for Surgency, were significantly different between the children of the different maternal groups of marijuana users (F 3,132 = 5.21, p = 0.002). Children of mothers who used marijuana at 10 weeks gestation or longer had lower ratings, 3.97 ± 0.70, than the children of mothers who did not use, 4.47 ± 0.55, Tukey's p < 0.05, d’ = 0.79. Children of mothers who stopped using at conception or before 10 weeks had intermediate values (Table 2).
Higher maternal choline levels increased Regulation in 3-month-olds for all women β = 0.17 [95% CI 0.02–0.32] p = 0.022. Effects of marijuana use and choline level on Regulation had significant interaction (Wald χ2df3 = 9.60, p = 0.022; Fig. 3, online Supplementary Table A6). The effect of the choline level was greatest for children whose mothers used marijuana at 10 weeks of gestation or longer (β = 0.53 [95% CI 0.08–0.98] p = 0.02; r s = 0.54, p = 0.013). Duration of attention (β = 0.69 [95% CI 0.04–1.35] p = 0.04), enjoyment of play with toys (β = 0.59 [95% CI 0.15–1.03] p = 0.01), and cuddliness and bonding with parents (β = 0.54 [95% CI 0.01–1.08] p = 0.03) were the most affected symptom ratings; all were in the Regulation index.

Fig. 3. Effects of gestational exposure to marijuana and maternal choline levels on the 3-month-old child's self-regulation behavior. Inset: Subject data for mothers who used marijuana 10+ weeks, r s = 0.54, p = 0.013.
Higher P50S2 amplitude reduced IBQ-R Regulation β = −0.421, but the effect was not significant. The Parental Distress subscale of the Parenting Stress Index was not different between the marijuana-using and non-using groups and had no effect on IBQ-R ratings.
Comment
Effects of maternal marijuana use on inhibition of the newborn's P50 auditory evoked potential provide evidence soon after birth of adverse effects on fetal brain development, as suspected from studies of older children and adolescents exposed to marijuana prenatally (Fried and Watkinson, Reference Fried and Watkinson2001; Goldschmidt et al., Reference Goldschmidt, Richardson, Willford, Severtson and Day2012). This study is the first to detect central nervous system effects in newborns before most mothers have resumed postnatal marijuana use, and it identifies a vulnerable gestational period for effects of marijuana on fetal brain development. Mothers reported relatively moderate levels of marijuana use and most stopped marijuana before delivery, but use for the first 10 gestational weeks was sufficient to adversely affect fetal brain development. By 3 months of age, adverse effects on the child's self-regulation appeared. Higher maternal choline levels mitigated these effects.
α7-Nicotinic acetylcholine receptors are expressed on the same hippocampal inhibitory interneurons as Cannabinoid 1 (CB1) receptors (Morales et al., Reference Morales, Hein and Vogel2008). Animal models demonstrate that prenatal THC interferes with endogenous cannabinoid signaling through CB1 receptors that normally promote the development of these interneurons by transactivating the TrkB receptor, whereas choline activation of α7-receptors promotes induction of the potassium membrane pumps that support their inhibitory neurotransmission (Liu et al., Reference Liu, Neff and Berg2006; de Salas-Quiroga et al., Reference de Salas-Quiroga, Díaz-Alonso, García-Rincón, Remmers, Vega, Gómez-Cañas, Lutz, Guzman and Galve-Roperh2015). Cholinergic activation enhances and THC decreases long-term potentiation, a mechanism of learning and memory (de Salas-Quiroga et al., Reference de Salas-Quiroga, Díaz-Alonso, García-Rincón, Remmers, Vega, Gómez-Cañas, Lutz, Guzman and Galve-Roperh2015; Freund et al., Reference Freund, Graw, Choo, Stevens, Leonard and Dell'Acqua2016). Impairment of interneuron development by prenatal marijuana diminishes normal social and executive behavior (Vargish et al., Reference Vargish, Pelkey, Yuan, Chittajallu, Collins, Fang and McBain2017). In humans CB1 receptors and mRNA are already expressed at the earliest time observed, 14–20 weeks of gestation, especially in the developing limbic system (Biegon and Kerman, Reference Biegon and Kerman2001; Mato et al., Reference Mato, Del Olmo and Pazos2003; Wang et al., Reference Wang, Dow-Edwards, Keller and Hurd2003). CHRNA7 mRNA and α7-nicotinic receptors are also expressed beginning at 8–9 weeks for gestation, including in the limbic system (Court et al., Reference Court, Lloyd, Johnson, Griffiths, Birdsall, Piggott, Oakley, Ince, Perry and Perry1997; Agulhon et al., Reference Agulhon, Abitbol, Bertrand and Malafosse1999; Birnbaum et al., Reference Birnbaum, Jaffe, Hyde, Kleinman and Weinberger2014; Kunii et al., Reference Kunii, Zhang, Xu, Hyde, McFadden, Shin, Deep-Soboslay, Ye, Li, Kleinman, Wang and Lipska2015). During this gestational period interneurons begin their development by migrating from ganglionic eminence and expressing GAD; by 20 weeks they are synthesizing synaptophysin and the mature chloride pump KCC2 that will generate the chloride gradient (Vanhatalo et al., Reference Vanhatalo, Palvas, Andersson, Rivera, Voipio and Kaila2005; Bayatti et al., Reference Bayatti, Moss, Sun, Ambrose, Ward, Lindsay and Clowry2008; Zecevic et al., Reference Zecevic, Hu and Jakovcevski2011).
P50 inhibition has the advantage as a biomarker that it can be assessed near birth when the parents' effect on the child's development is still minimal and it is directly dependent on the development of the interneurons that co-expression CB1 and CHRNA7 (Miller and Freedman, Reference Miller and Freedman1995). However, a limitation is that P50 inhibition is also multi-determined. In addition to the influence of CB1 and α7-nicotinic receptors, the inhibitory effect on limbic pyramidal neurons is dependent on GABAA and GABAB presynaptic and postsynaptic receptors, feed-forward activation of the interneurons by NMDA-type glutamate receptors, presynaptic release of acetylcholine modulated by 5HT3 receptors, and inactivation of the inhibition by α1-noradrenergic receptors (Freedman, Reference Freedman2014). Other genes involved are DISC1, ERB4, GRID2, GRM3, and GRIK4 (Greenwood et al., Reference Greenwood, Lazzeroni, Murray, Cadenhead, Calkins, Dobie, Green, Gur, Gu, Hardiman, Kelsoe, Leonard, Light, Nuechterlein, Olincy, Radant, Schork, Seidman, Siever, Silverman, Stone, Swerdlow, Tsuang, Tsuang, Turetsky, Freedman and Braff2011).
A second limitation of this observational study is that the effects of marijuana and choline cannot be rigorously isolated from other environmental and genetic influences on fetal development. Factor analysis of socio-economic, maternal health, and neonatal status parameters was used to construct summary covariates of these many possible influences. None of the three factors had significant effects on marijuana–choline interaction. Marijuana usage, despite its adverse effects on the offspring's later cognitive and behavioral development, had few effects on general fetal growth in this and other samples (El Marroun et al., Reference El Marroun, Tiemeier, Steegers, Jaddoe, Hofman, Verhulst, van den Brink and Huizink2009). Prenatal vitamins with folate were strongly advised; lower usage has been associated with marijuana use, but was not generally found here (Knight et al., Reference Knight, James, Edwards, Spurlock, Oyemade, Johnson, West, Cole, Westney and Westney1994). Depression was common in all women who used marijuana, but only women who continued marijuana for 10 gestational weeks or more had affected offspring. Obesity and alcohol use were often comorbid with marijuana use, but they had no significant effect when assessed with marijuana in joint analyses of outcome. The younger age of mothers who use marijuana is consistent with population-wide studies (Brown et al., Reference Brown, Sarvet, Shmulewitz, Martins, Wall and Hasin2017). Cigarette smoking has been comorbid with marijuana use in other samples, but not in the present one. Parental ratings of the child's behavior might be influenced by the parent's postnatal marijuana use. However, few mothers used marijuana immediately postpartum, and effects on ratings were not significant.
P50 inhibition was selected as a biomarker because, in addition to its mechanistic significance, the loss of inhibition predicts early childhood problems in attention and social function Ross et al., Reference Ross, Hunter, Hoffman, McCarthy, Chambers, Law, Leonard, Zerbe and Freedman2016. These early childhood behaviors, including self-regulation and problems with attention and social withdrawal, are recognized as a pre-disposing factor for psychosis, substance abuse, and depression (Rutter, Kim-Cohen, and Maughan, Reference Rutter, Kim-Cohen and Maughan2006; Pine and Fox, Reference Pine and Fox2015; Kertz et al., Reference Kertz, Belden, Tillman and Luby2016; Olson et al., Reference Olson, Choe and Sameroff2017). Self-regulation has been shown to be a mediating factor between prenatal maternal substance abuse and the child's later psychopathology (Lin et al., Reference Lin, Ostlund, Conradt, Lagasse and Lester2018). Lower IBQ-R Regulation is specifically associated with decreased reading readiness at age 4 years and decreased conscientiousness, organization, and increased distractibility at age 9 years (Gartstein et al., Reference Gartstein, Putnam and Kliewer2016; Slobodskaya and Kozlova, Reference Slobodskaya and Kozlova2016). As the child matures, Regulation positively modulates both Surgency and Negativity to help the child meet social expectations (Ahadi et al., Reference Ahadi, Rothbart and Ye1993). Positive effects of higher prenatal maternal choline persist for at least 7 years (Boeke et al., Reference Boeke, Gillman, Hughes, Rifas-Shiman, Villamor and Oken2013). Thus, the children in this study are likely to be influenced by the effect of their mothers' marijuana and choline for much of their development. Longer-term behavioral and cognitive consequences of marijuana and choline in pregnancy unfold over decades as the infant matures (Fried et al., Reference Fried, Watkinson and Gray2003; Goldschmidt et al., Reference Goldschmidt, Richardson, Willford, Severtson and Day2012).
Improved maternal prenatal nutrition to promote fetal development to prevent adverse effects on the child's mental function and mental health is now recognized as a global public health priority (Stephenson et al., Reference Stephenson, Heslehurst, Hall, Schoemaker, Hutchinson, Cade, Barrett, Crozier, Barker, Kumaran, Yajnik, Baird and Mishra2018). Specifically for prenatal choline, the American Medical Association now recommends ‘evidence-based amounts of choline in all prenatal vitamins’ (American Medical Association, 2017). Prenatal vitamins currently contain as little as 10–50 mg choline. Phosphatidylcholine supplements up to 6300 mg (equivalent to 900 mg choline) raise maternal choline to the highest levels in this study without serious adverse effects (Cheatham et al., Reference Cheatham, Goldman, Fischer, da Costa, Reznick and Zeisel2012; Food and Drug Administration, 2016; Ross et al., Reference Ross, Hunter, Hoffman, McCarthy, Chambers, Law, Leonard, Zerbe and Freedman2016). This intervention assures maternal choline intake above the FDA minimum recommended intake (550 mg) and is less than half the maximum amount considered safe (3000–3500 mg) (Hoffman et al., Reference Hoffman, Olincy, D'Alessandro, Reisz, Hansen, Hunter, Freedman and Ross2019). Ideally, expectant parents will heed warnings about the adverse effects of prenatal marijuana use on their child, but, regardless of the parents' decision, clinicians have a dual obligation to respect their autonomy and to provide the best possible health care for the mother and fetus (American College of Obstetricians and Gynecologists, 2016). Care would appear from this study to include enhancing the mother's choline level to protect the fetus's brain development.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003329171900179X.
Data
Data from the study are available by request.
Acknowledgements
Randal G. Ross (MD), who died during the course of this study, was responsible for its initial conceptualization and design.
Financial support
The study was supported by the Institute for Children's Mental Disorders; The Anschutz Foundation; National Institutes of Health NIH/NCATS grant number UL1 TR001082 (all authors); NICHD grant number K12HD001271-11 (Dr Hoffman).
Conflict of interest
The authors report no conflict of interest.







