Introduction
Schizophrenia is a disabling medical disorder with a global prevalence of approximately 1%. Schizophrenia commonly manifests as positive symptoms (ie, hallucinations, delusions, and disorganized speech and thoughts), negative symptoms (ie, avolition and psychomotor retardation), and cognitive symptoms (ie, impairment of the working memory and attention deficits), and is often accompanied by prominent social and occupational dysfunction.Reference Cannon, Kaprio, Lonnqvist, Huttunen and Koskenvuo1, Reference Insel2 Although the pathophysiology of schizophrenia is unclear, it appears to have a complex etiology. Multiple genetic and environmental factors act together to develop a spectrum of neurobiological vulnerability to schizophrenia.Reference Cannon, Kaprio, Lonnqvist, Huttunen and Koskenvuo1, Reference Insel2
Patients with schizophrenia experience higher mortality and a shorter life expectancy compared with the general population.Reference Saha, Chant and McGrath3 Most of the mortality risk may be due to cardiometabolic dysfunction.Reference Joukamaa, Heliovaara, Knekt, Aromaa, Raitasalo and Lehtinen4 Studies have shown that metabolic disturbances, such as impaired glucose tolerance, insulin resistance, and endothelial dysfunction, may exist prior to the development of schizophrenia.Reference Chen, Du and Yin5–Reference Lizano, Keshavan and Tandon7 However, whether a dysregulated metabolic state and endothelial dysfunction cause or are a consequence of schizophrenia remains unknown.Reference Chen, Du and Yin5, Reference Lizano, Keshavan and Tandon7, Reference Spelman, Walsh, Sharifi, Collins and Thakore8 Increasing evidence found that use of atypical antipsychotics was associated with an elevated likelihood of glucose dysregulationReference Rettenbacher9, Reference Newcomer10 and suggested that changes of molecules linked to insulin resistance may be independent of the effects of atypical antipsychotics among patients with schizophrenia.Reference Spelman, Walsh, Sharifi, Collins and Thakore8, Reference Ryan, Collins and Thakore11–Reference Cohn, Remington, Zipursky, Azad, Connolly and Wolever13 The finding of an elevated risk of type 2 diabetes mellitus (T2DM) in the absence of atypical antipsychotics may imply the shared pathophysiology between schizophrenia and T2DM.Reference Bellivier14–Reference Van Lieshout and Voruganti17
Several family studies have shown that the first-degree relatives of schizophrenia probands had an increased prevalence of T2DM and more impaired glucose tolerance compared with the controls.Reference Fernandez-Egea, Bernardo and Parellada18–Reference Mukherjee, Schnur and Reddy20 Previous studies suggested that a familial link exists between schizophrenia and metabolic disturbances such as T2DM.Reference Fernandez-Egea, Bernardo and Parellada18, Reference Enez Darcin, Yalcin Cavus, Dilbaz, Kaya and Dogan21, Reference Foley, Mackinnon and Morgan22 Spelman et al Reference Spelman, Walsh, Sharifi, Collins and Thakore8 found that effects of schizophrenia and atypical antipsychotics cannot entirely account for the increased risk for T2DM within families. Foley et al Reference Foley, Mackinnon and Morgan22 examined whether there was a familial comorbidity between T2DM and psychosis and reported that a positive family history of T2DM was associated with a positive family history of schizophrenia in those with a psychotic disorder (OR: 1.35). They also found that this association between the family history of T2DM and schizophrenia was stronger in female patients than in male patients.Reference Foley, Mackinnon and Morgan22 Enez Darcin et al Reference Enez Darcin, Yalcin Cavus, Dilbaz, Kaya and Dogan21 tested whether metabolic disturbance was an endophenotype of patients with schizophrenia and their unaffected siblings. They determined that both the drug-free patients with schizophrenia and their siblings exhibited significantly higher mean systolic and diastolic blood pressures and lower levels of high-density lipoprotein and had a greater prevalence of metabolic syndrome compared with the controls.Reference Enez Darcin, Yalcin Cavus, Dilbaz, Kaya and Dogan21 Fernandez-Egea et al Reference Fernandez-Egea, Bernardo and Parellada18 administered a glucose-tolerance test among 6 first-degree siblings of schizophrenia probands and 12 control subjects and reported that the siblings of schizophrenia probands had a significantly higher 2-hour mean glucose concentration compared with the control subjects. However, small sample sizes and cross-sectional study designs were the major limitations in the abovementioned studies.
Patients with schizophrenia exhibited a higher prevalence of metabolic disorders, including T2DM,Reference Hsu, Chien, Lin, Chou and Chou23 hypertension,Reference Carliner, Collins, Cabassa, McNallen, Joestl and Lewis-Fernandez24 and hyperlipidemia,Reference Bai, Su, Chen, Chen and Chang25, Reference Liao, Chang and Wei26 compared with the non-schizophrenic controls. Those metabolic disorders may further increase the risk of subsequent stroke and cardiovascular diseases.Reference Joukamaa, Heliovaara, Knekt, Aromaa, Raitasalo and Lehtinen4, Reference Li, Fan, Tang and Cheng27–Reference Cohn, Prud’homme, Streiner, Kameh and Remington29 As mentioned, a familial association between schizophrenia and metabolic disorders, such as T2DM, was suggested. However, no study has investigated the risk of metabolic disturbance–related physical complications, especially stroke and ischemic heart disease, among the unaffected siblings of patients with schizophrenia. Additional study would be necessary to elucidate the risk of cerebrocardiovascular diseases among the siblings of patients with schizophrenia.
In current study, we investigated the risk of metabolic disorders, namely T2DM, hypertension, and dyslipidemia, and the risk of cerebrocardiovascular diseases, namely stroke and ischemic heart disease, among the unaffected siblings of patients with schizophrenia by using the Taiwan National Health Insurance Research Database (NHIRD) with a large sample size and a longitudinal study design. We hypothesized that the unaffected siblings of schizophrenia probands would have a higher prevalence of metabolic disorders and cerebrocardiovascular diseases compared with the control group.
Method
Data source
The Taiwan National Health Insurance Research Database (NHIRD), which consists of healthcare data from >99% of the entire Taiwan population, is audited and released by the National Health Research Institute for scientific and study purposes. Comprehensive information on insured individuals is included in the database, including demographic data, dates of clinical visits, disease diagnoses, and medical interventions. Individual medical records included in the NHIRD are anonymous to protect patient privacy. In this study, using each resident’s unique personal identification number, all of the information was linked. Subsequently, following the method of Kuo et al,Reference Kuo, Grainge and Valdes30 family kinships in the NHIRD were used for genealogy reconstruction. The diagnostic codes used were based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). The NHIRD has been used extensively in many epidemiologic studies in Taiwan.Reference Chen, Hsu and Huang31–Reference Cheng, Chang and Chen34
Inclusion criteria for the unaffected siblings of schizophrenia probands and the control group
Individuals who were born before 1990 and had no diagnosis of schizophrenia (ICD-9-CM code: 295) at any time but had siblings with schizophrenia were enrolled as the schizophrenia sibling cohort. The age-, sex-, and birth time-matched (1:4) control cohort was randomly identified after eliminating the study cases, those who had been given a diagnosis of major psychiatric disorders at any time, and those with any sibling with major psychiatric disorders. Schizophrenia sibling cohort and control cohort were followed from 1996 to the end of 2011 for the investigation of occurrence of metabolic disorders (T2DM, hypertension, dyslipidemia, obesity) and cerebrocardiovascular diseases (stroke, ischemic heart disease). Metabolic and cerebrocardiovascular disorders were diagnosed by board-certificated physicians. In addition, we identified the presence of metabolic disorders (T2DM, hypertension, dyslipidemia, obesity) of schizophrenic probands as the family history of metabolic disorders. Level of urbanization (level 1 to level 5; level 1: most urbanized region; level 5: least urbanized region) was also assessed for our study.Reference Liu, Hung, Chuang, Chen, Weng and Liu35 This study was approved by the Institutional Review Board of Taipei Veterans General Hospital.
Statistical analysis
For between-group comparisons, the F test was used for continuous variables and Pearson’s X2 test for nominal variables, where appropriate. Logistic regression models with the adjustment of demographic data (age, sex, income, and level of urbanization) were performed to calculate the odds ratios of developing metabolic disorders (T2DM, hypertension, dyslipidemia, and obesity) between schizophrenia sibling cohort and control cohort. Logistic regression models with the additional adjustment of metabolic disorders were used to investigate the risks of developing cerebrocardiovascular diseases (stroke, ischemic heart disease) between study and control cohorts. Furthermore, logistic regression models were used to examine the association between presence/absence of metabolic disorders of schizophrenic probands (family history) and the risks of developing metabolic disorders and cerebrocardiovascular diseases among siblings of schizophrenic patients compared with the control group. Finally, logistic regression analyses stratified by sex were also examined to investigate the role of sex in the risks of subsequent metabolic and cerebrocardiovascular disorders. A 2-tailed P-value of less than 0.05 was considered statistically significant. All data processing and statistical analyses were performed with Statistical Package for Social Science (SPSS) version 17 software (SPSS Inc.) and Statistical Analysis Software (SAS) version 9.1 (SAS Institute, Cary, NC).
Results
The demographic characteristics and prevalence of metabolic disorders and cerebrocardiovascular diseases of the study sample are listed in Table 1. A total of 3135 unaffected siblings of schizophrenia probands and 12,540 age- and sex-matched controls were enrolled in our study, with an average age of 33.18 ± 7.85 years. The unaffected siblings of schizophrenia probands had a higher prevalence of T2DM (3.4% vs. 2.6%, p = 0.010) and a higher level of income (p <0.001) than the controls.
Table 1 Demographic data and prevalence of the metabolic and cerebrocardiovascular disorders among siblings of schizophrenia probands and controls
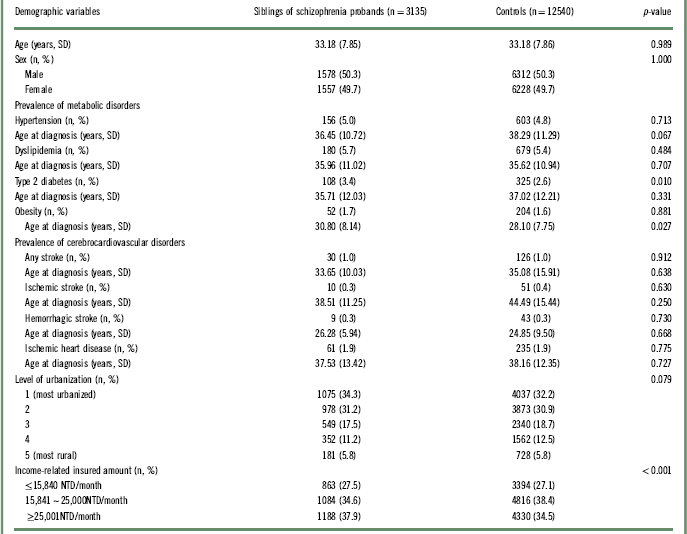
NTD: new Taiwan dollar; SD: standard deviation.
Logistic regression analyses with an adjustment of demographic data (age, sex, level of urbanization, and income) found that the unaffected siblings of patients with schizophrenia were more likely to develop T2DM (OR: 1.39, 95% CI: 1.10–1.75) later in life compared with the control group (Table 2. Subanalyses stratified by sex further reported that only female siblings of schizophrenia probands had an increased risk of developing T2DM compared with the controls. In addition, female siblings of patients with schizophrenia were prone to developing hypertension (OR: 1.47, 95% CI: 1.07–2.01) (Table 2). The prevalence of obesity, dyslipidemia, stroke, and ischemic heart diseases did not differ between the unaffected siblings and the controls (Table 3).
Table 2 Logistic regression analyses of the metabolic disorders among siblings of schizophrenia probands and controls*

OR: Odds ratio; CI: Confidence interval. Bold type indicates statistical significance.
* : adjusted for demographic data.
Table 3 Logistic regression analyses of the cerebrocardiovascular disorders among siblings of schizophrenia probands and controls*

OR: Odds ratio; CI: Confidence interval. Bold type indicates statistical significance.
* : adjusted for demographic data and metabolic disorders.
Finally, we found a significant relationship between family history of metabolic disorders and with the occurrence of subsequent metabolic disorders among siblings of patients with schizophrenia (Table 4). Siblings with family history of metabolic disorders were more likely to develop hypertension (OR: 1.45, 95% CI: 1.05–2.00), dyslipidemia (OR: 1.50, 95% CI: 1.12–2.02), T2DM (OR: 1.77, 95% CI: 1.21–2.57), and obesity (OR: 2.22, 95% CI: 1.41–3.49) compared with siblings without family history and controls (Table 4).
Table 4 Logistic regression analyses of the metabolic* and cerebrocardiovascular# disorders among siblings of schizophrenia probands with/without family history of metabolic disorders and controls

OR: Odds ratio; CI: Confidence interval. Bold type indicates statistical significance.
* : adjusted for demographic data.
# : adjusted for demographic data and metabolic disorders.
Discussion
Our findings confirmed that the unaffected siblings of schizophrenia probands, especially sisters, had a higher prevalence of T2DM and hypertension compared with healthy controls. Especially, those with family history of metabolic disorders were more likely to develop hypertension, dyslipidemia, obesity, and T2DM later in life compared with those without family history and the control group. However, the prevalence of stroke and ischemic heart disease did not differ between the unaffected siblings with or without family history of metabolic disorders and the controls.
The finding of an increased risk of T2DM in the unaffected siblings of patients with schizophrenia may imply a shared familial risk of schizophrenia and T2DM. This could be due to risk factors linking T2DM and schizophrenia, such as common environmental factors, genetic liability, and epigenetic interactions.Reference Gough and O’Donovan15, Reference Andreassen, Djurovic and Thompson36, Reference Hansen, Ingason and Djurovic37 The hypothesis of genetic overlap between schizophrenia and T2DM has been supported by studies describing shared chromosome loci and associated genes between schizophrenia and metabolic disorders, including T2DM.Reference Gough and O’Donovan15, Reference Andreassen, Djurovic and Thompson36, Reference Hansen, Ingason and Djurovic37 Liu et al Reference Liu, Li, Zhang, Deng, Yi and Shi38 performed a pathway analysis of T2DM and schizophrenia risk genes and found that several proteins, such as GRB2 and PLCG1, may interact with networks of proteins in both disorders. The genome-wide association study also reported that schizophrenia shared substantial polygenetic components with T2DM.Reference Lin and Shuldiner16 One study suggested that glucose transporter 1 and 3 may be involved in the association between T2DM and psychosis.Reference McDermott and de Silva39
In addition to the genetic liability between T2DM and schizophrenia, use of atypical antipsychotics was associated with a higher likelihood of subsequent metabolic disorders among patients with schizophrenia.Reference Perez-Iglesias, Mata and Pelayo-Teran40–Reference Haddad42 Previous studies revealed that olanzapine and clozapine were associated with the highest rate of developing T2DM compared with other atypical antipsychotics.Reference Sernyak, Leslie, Alarcon, Losonczy and Rosenheck43 Several pathophysiologies of antipsychotics–related T2DM included drug-induced weight gainReference McIntyre, McCann and Kennedy44 and drug-related insulin resistance.Reference Johnson, Yamazaki and Ward45 However, in the current study, we further suggested that T2DM risk may be independent of atypical antipsychotics because the unaffected siblings also had an elevated risk of T2DM during the follow-up period.
Among patients with schizophrenia, female patients are particularly associated with a higher prevalence of metabolic syndrome compared with male patients. Bener et al Reference Bener, Al-Hamaq and Dafeeah46 reported that female patients also showed a higher prevalence of hypertension than male patients (53.1% vs. 48.6%). As previously mentioned, Enez Darcin et al Reference Enez Darcin, Yalcin Cavus, Dilbaz, Kaya and Dogan21 found that the siblings of patients with schizophrenia had significantly higher mean systolic and diastolic blood pressures compared with the controls. The above-mentioned findings are consistent with our observations that female siblings of patients with schizophrenia had a higher risk of hypertension and T2DM compared with the male siblings. Additional studies are required to investigate the effect gender has on metabolic disorders among patients with schizophrenia and their relatives.
Finally, family history of metabolic disorders played a crucial role in the risk of metabolic disorders among the unaffected siblings of patients with schizophrenia. In our study, we found that the unaffected siblings who had family history of metabolic disorders were prone to developing obesity, hypertension, dyslipidemia, and T2DM during the follow-up compared with those who had no family history and the control group. Our finding may remind clinicians to closely monitor the risk of metabolic disturbance among the siblings of patients who had dual diagnoses of schizophrenia and metabolic disorders.
Our findings provide several research opportunities. First, we suggest that schizophrenia and T2DM may be caused by shared etiological factors, which is consistent with other studies that have shown that schizophrenia and T2DM are caused by multiple genetic variants. Future genetic research should examine whether there is a possible link between schizophrenia and T2DM risk alleles. Second, our data revealed a medical morbidity in the relatives of schizophrenia probands, which should motivate studies on the physical illness burden not only in patients but also in their relatives, such as siblings. Early detection and developing preventive strategies for these comorbidities are also required. Third, the influence of gene–environment interactions on the risk of metabolic disorders on schizophrenia warrants further investigation.
This study has several limitations. First, the prevalence of T2DM may be underestimated because only those who sought medical help and consultation were enrolled. This limitation also may result in an underestimate of the familial risk of comorbidities. However, the diagnosis of T2DM was made by board-certificated physicians on the basis of laboratory examinations; therefore, the diagnostic validity is high. Second, certain data were not available in the Taiwan NHIRD, namely disease severity of schizophrenia, personal lifestyle, education, occupation, dietary pattern, and engagement in physical activity. Ukkola et al Reference Ukkola, Sun and Bouchard47 found that the subjects with insulin-like growth factor (IGF)-2 and IGF-binding protein 1 gene variants had decreased insulin sensitivity after long-term caloric surplus. It was postulated that insulin resistance may result from the interplay of genetic factors and dietary pattern.Reference Ukkola, Sun and Bouchard47 Furthermore, dysglycemia may be associated with negative and depressive symptoms of schizophrenia,Reference Sicras-Mainar, Maurino, Ruiz-Beato and Navarro-Artieda48, Reference Saatcioglu, Kalkan, Fistikci, Erek and Kilic49 while positive symptoms of schizophrenia may affect self-healthcare behaviors among patients with schizophrenia.Reference Ogawa, Miyamoto and Kawakami50 Examining the influence of these factors may shed more light on the role of genetic predisposition and environmental factors in the development of neurobiological vulnerability to schizophrenia.
Conclusion
The unaffected siblings, especially sisters, of patients with schizophrenia had a higher risk of developing T2DM and hypertension later in life compared with the controls. Our study revealed a familial link between schizophrenia and T2DM in a large sample. Additional studies are required to investigate the shared pathophysiology of schizophrenia and T2DM.
Disclosures
Mao-Hsuan Huang, Kai-Lin Huang, Chih-Ming Cheng, Tung-Ping Su, Cheng-Ta Li, Shih-Jen Tsai, Wei-Chen Lin, Albert Yang, and Tzeng-Ji Chen have nothing to disclose. Ju-Wei Hsu declares that the study was supported by a grant from Taipei Veterans General Hospital (V107C-181). Ya-Mei Bai declares that the study was supported by grants from Taipei Veterans General Hospital (V103E10-001, V104E10-002, V105E10-001-MY2-1, V106B-020). Mu-Hong Chen declares that the study was supported by grant from Taipei Veterans General Hospital (V105A-049, V107B-010).






