Introduction
The use of a banker plant system (BPS) is one of the strategies available to conserve natural enemies and improve biological control. This approach consists in providing natural plant hosts in the vicinity from cash crops where they can disperse into the cash crop (Huang et al., Reference Huang, Enkegaard, Osborne, Ramakers, Messelink, Pijnakker and Murphy2011; Parolin et al., Reference Parolin, Bresch, Ruiz, Desneux and Poncet2013). A BPS is composed of three basic elements, namely the banker plant, alternative food, and beneficials (Huang et al., Reference Huang, Enkegaard, Osborne, Ramakers, Messelink, Pijnakker and Murphy2011; Parolin et al., Reference Parolin, Bresch, Ruiz, Desneux and Poncet2013). Several plants have been studied as banker plants for controlling important pests such as spider mites, aphids, and whiteflies, mostly on vegetable crops (Huang et al., Reference Huang, Enkegaard, Osborne, Ramakers, Messelink, Pijnakker and Murphy2011). However, most studies focused on a single (or a few) plant species and specialist natural enemy species as candidates to use in the BPS (Kumar et al., Reference Kumar, Xiao, McKenzie and Osborne2015; Sun et al., Reference Sun, Song and Zhao2020).
A BPS composed of a variety of plants could, in theory, be more suitable to maintain or even increase natural enemies in the agroecosystems. This is because diverse plants can harbor different natural enemies simultaneously instead of only a few plants (Parolin et al., Reference Parolin, Bresch, Ruiz, Desneux and Poncet2013). Weeds (hereafter called non-crop plants) can eventually be used for this purpose, since they provide shelter, microclimatic conditions, oviposition sites, and food to different natural enemies (Parolin et al., Reference Parolin, Bresch, Ruiz, Desneux and Poncet2013; Ottaviano et al., Reference Ottaviano, Cédola, Sánchez and Greco2015; Amaral et al., Reference Amaral, Venzon, dos Santos, Sujii, Schmidt and Harwood2016).
Generalist natural enemies are expected to thrive in BPS because they can prey upon phytophagous species and feed on other sources of food (Venzon et al., Reference Venzon, Amaral, Togni, Chiguachi, Souza, Vázquez and Marucci2019). Predatory mite (PM) species from the Phytoseiidae family are important biological control agents and present different feeding habits. Some species are generalist omnivores feeding on pollen, insects, and honeydew, while other species may feed only on Tetranychidae species (specialists) (McMurtry et al., Reference McMurtry, Moraes and Sourassou2013). Pollen constitutes an important supplementary food source to maintain the predator in the absence of its prey (Marques et al., Reference Marques, Sarmento, Lemos, Pedro-Neto, Sabelis, Venzon, Pallini and Janssen2015) and increases the reproductive performance of N. californicus when feeding on the two-spotted spider mite (TSSM) Tetranychus urticae Koch (Acari: Tetranychidae) (Vacacela et al., Reference Vacacela Ajila, Colares, Lemos, Marques, Franklin, Santos do Vale, Oliveira, Venzon and Pallini2019). Although active prey mixing can increase the foraging time, a diverse diet usually benefits reproduction (Marques et al., Reference Marques, Sarmento, Lemos, Pedro-Neto, Sabelis, Venzon, Pallini and Janssen2015). Some plants may also host alternative prey for maintaining PMs in the field. In general, specialist PMs are more efficient in suppressing a target pest in the short term, but generalist species can persist longer, so their combined action may reduce phytophagous pests in the crops even more (Vacacela et al., Reference Vacacela Ajila, Colares, Lemos, Marques, Franklin, Santos do Vale, Oliveira, Venzon and Pallini2019).
Therefore, the use of BPS to increase PMs for TSSM management could be a suitable conservation biological control strategy in several crops. This could be especially important in more controlled environments where TSSM occurs. TSSM is a polyphagous mite, which infests several cultivated plants worldwide (Rioja et al., Reference Rioja, Zhurov, Bruinsma, Grbic and Grbic2017). Under protected environments, TSSM is even more destructive and can be the principal strawberry pest. Strawberry cultivation under protected environments is an increasing practice due to higher yields and reduction of fungal diseases. Chemical pesticides are still the most used strategy for TSSM control. However, there is a demand to reduce or even eliminate chemical residues on food and negative effects on non-target insects. Releasing commercial products based on PMs such as N. californicus or Phytoseilus macropilis (Banks) (Acari: Phytoseiidae), is current practice for TSSM control (Togni et al., Reference Togni, Lagôa, Venzon and Sujii2019a).
When growing strawberries under protected environments, farmers usually suppress the non-crop vegetation below the wooden structure that supports the bags. However, we hypothesize that these plants growing below the wooden structure may provide shelter and food for PMs. Non-crop vegetation could be used as BPS composed of multiple plant species. This strategy could contribute to more sustainable management of phytophagous mites and other pest species (e.g., thrips) by valuing the different components of biodiversity naturally occurring in agroecosystems. Hence, this study was conducted to investigate if a diverse assemblage of non-crop plants could be used as a BPS in strawberry crops.
Materials and methods
Study areas description
This study was conducted on five farms growing strawberries in Marialva city, Paraná, Brazil, from July 2015 to December 2016 (table 1). The climate of this region is humid sub-tropical. Mean temperatures range from 10 to 30 °C and the mean annual precipitation is 1632 mm (Instituto Agronômico do Paraná, 2020). The region is located in the Atlantic Rainforest biome (Instituto Brasileiro de Geografia e Estatística, 2004). The highly fragmented landscape is characterized by natural vegetation sites interspersed with agricultural and urban areas (Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, 2016). The crops cultivated around the farms were mainly soybean (spring-summer) and maize (autumn-winter).
Table 1. Dimension of high tunnels (m2), geographic coordinates, and altitudes (m) of experimental areas in which strawberry (cv. Albion) was grown.
Marialva, Paraná, Brazil.
The study was performed in strawberry (Albion cv.) cultivated in five greenhouse high tunnels. Plants were grown in a semi-hydroponic suspended cropping system (table 1) in which slabs containing substrate (soil, humus, and rice husk ash) and the plants are placed on a suspended wooden structure. The wooden structures supporting strawberry bags were 1.0 m high and 0.70 m wide. Two bags (0.30 m width) containing substrate spaced 0.1 m from each other were placed on the wooden structures. Plants were spaced 0.25 m in a double row design. Non-crop vegetation was allowed to grow below the wooden structures. PMs developing on the non-crop vegetation could access the strawberry plants by climbing on the wooden structures or by shifting from non-crop plants whose inflorescence or leaves were in contact with strawberry plants or slabs (Supplementary fig. S1).
Crop management
Despite the differences in the farming systems (organic and conventional), all farms conducted similar crop management, except fertilization. On conventional farms, synthetic fertilizers via fertigation were used. Boiled chicken manure and biofertilizers were used for organic fertilization, and the technical recommendations followed the organic law for vegetable production (Brasil, 2003). Such differences in fertilization are not expected to affect the occurrence of non-crop plants and mite species, since the semi-hydroponic system was directed to the plants above the suspended system and the non-crop vegetation grew below this.
Pest and disease management for all the five farms were performed similarly and according to technical recommendations for a strawberry crop with eco-friendly products (neem oil, sulphur- and Bacillus subtilis-based products, PMs, and copper hydroxide) (Ronque, Reference Ronque2010).
Neoseiulus californicus was released on all strawberry farms four times (June and September 2015 and June and September 2016). PMs were manually released by farmers over the strawberry plants by shaking the commercial containers containing (Promip®) each of which had 5000 specimens of N. californicus. One container was used on each application on farms 2 and 3 and one and a half container was used for farms 1, 4, and 5.
Sampling and experimental design
To evaluate how different plants contributed to PMs conservation, we collected the non-crop vegetation below the wooden structures and surveyed the mites occurring on these plants. All plants in a 1.8 m2 quadrat were collected and were defined as one replicate (fig. 1). Three samples of 1.8 m2 each were randomly selected per high tunnel on each farm in July and December 2015 and in March, November, and December 2016 (one sample with three replicates per month), totaling 15 samples for each farm during the experiment.
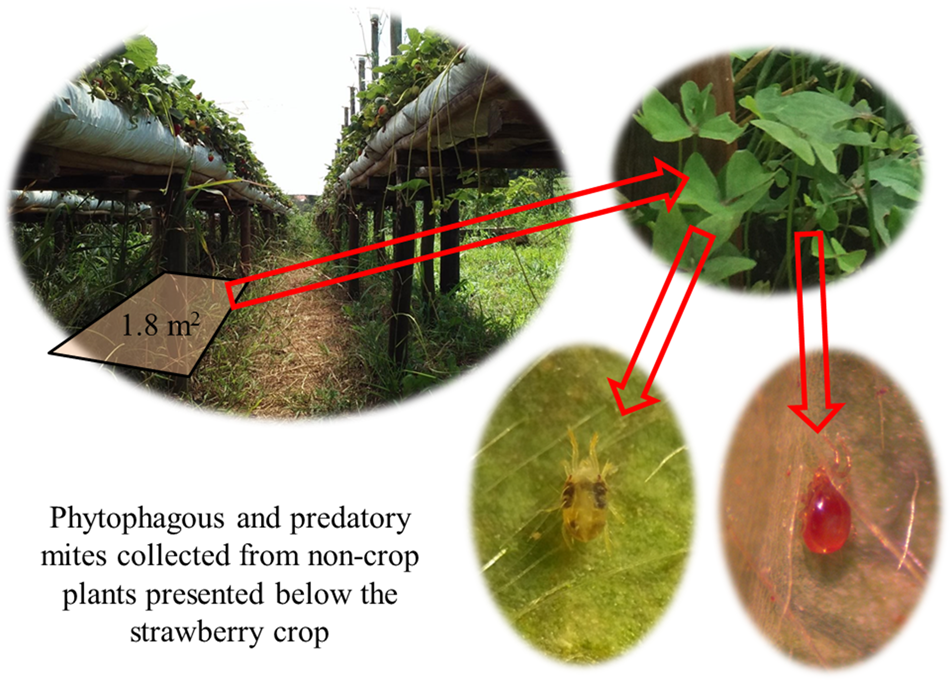
Figure 1. Scheme of phytophagous and predatory mites collection from the non-crop plants presented on an area of 1.8 m2.
For mite identification and quantification, 20% of the biomass (including all plant structures) of each plant was stored separately in paper bags and taken to the laboratory for mite counting and identification. Plant species were identified by using taxonomic characteristics and morphological comparison in a herbarium (Universidade Estadual de Londrina, Department of Animal and Vegetable Biology) (Lorenzi, Reference Lorenzi2014). Vouchers specimens were deposited in the herbarium and zoological collection of the Department of Animal and Vegetable Biology. Floristic analyses were performed by calculating the abundance (percentage of collecting spots that the plant was present) and total area occupied by each non-crop plant species2. The abundance and richness of mite species on each plant were also determined.
After field collection, the non-crop plants were kept in a refrigerator at 10 ± 3 °C to maintain mites at low activity. Next, plants were individually observed to count and identify mites using a stereoscopic microscope (40 × magnification). Mites were mounted on slides and fixed in Hoyer's medium (Moraes and Flechtmann, Reference Moraes and Flechtmann2008) and maintained in an incubator (Logen LS 1.6) at 50 °C. Chaetotaxic characters were examined under a phase-contrast microscope for taxonomic identification (Krantz and Walter, Reference Krantz and Walter2009). After mite identification, we determined their abundance, frequency, and species richness. The percentage of plants that hosted mites was calculated by the percentage of plants that harbored at least one mite species throughout all the collections.
Statistical analyses
To investigate how the abundance of plants and phytophagous mites affected the abundance of PMs on different plants, a generalized linear mixed-effect model (GLMM) was fitted. The abundance of PMs on different plant species was used as a response variable. The abundance of each plant species, phytophagous mites on these plants, and their interactions were the explanatory variables. The farms in which samples were taken from, the management system of each farm, and the time in which the sample occurred were used as random factors in the full model. F-test was used to assess the significance of variables included in the model (Crawley, Reference Crawley2007). A model contrast analysis was also performed to assess the differences between phytophagous mite abundances. Finally, an analysis for modeling the residuals was performed.
Log abundance (to weight the common and rare species) of plants and PMs were arranged in rank-abundance plots. We compared the observed data in the rank-abundance plots with a theoretical predicted abundance distribution model (Chi-square test) (Hammer et al., Reference Hammer, Harper and Ryan2001). Patterns of the spatial distribution of plants harboring PMs within the farms, and the distribution of PMs within these plants on the farms were estimated by calculating the Morisita Index of dispersion (Iδ) for each of these variables separately. F-ratio test was used to evaluate the significance of the results (Hammer et al., Reference Hammer, Harper and Ryan2001).
Linear discriminant analysis (LDA) using the Jaccard Index of similarity (with jackknifed data) was employed to analyze if PMs species were grouped by plant species. The significance of the groups was studied by fitting a one-way analysis of similarity (ANOSIM) and using the Jaccard Index with 9999 permutations (Clarke and Warwick, Reference Clarke and Warwick1994). Through this analysis, the significant associations of different plant species with groups of PMs could also be estimated.
Association between mites on non-crop plants was estimated by the Sorensen index (SI) (Sorensen, Reference Sorensen1948). SI values equal to ‘−1’ indicates no association between species, from ‘0’ to ‘ + 1’ suggest a partial association, and ‘ + 1’ indicates a total association between species (when a mite species was found in one sample, the correspondent phytophagous mite was also found in the same sample).
Spearman correlation (t-test at 5% significance) was used between every pair of mite species (Wei et al., Reference Wei, Simko, Levy, Xie, Jin and Zemla2017). Mite species with more than five specimens collected throughout the experiments were selected to investigate the association between mite fauna on the specific non-crop plant. Analyses were performed using PAST (Hammer et al., Reference Hammer, Harper and Ryan2001) and R statistical software (R Core Team, 2017).
Results
Plants and mites collected
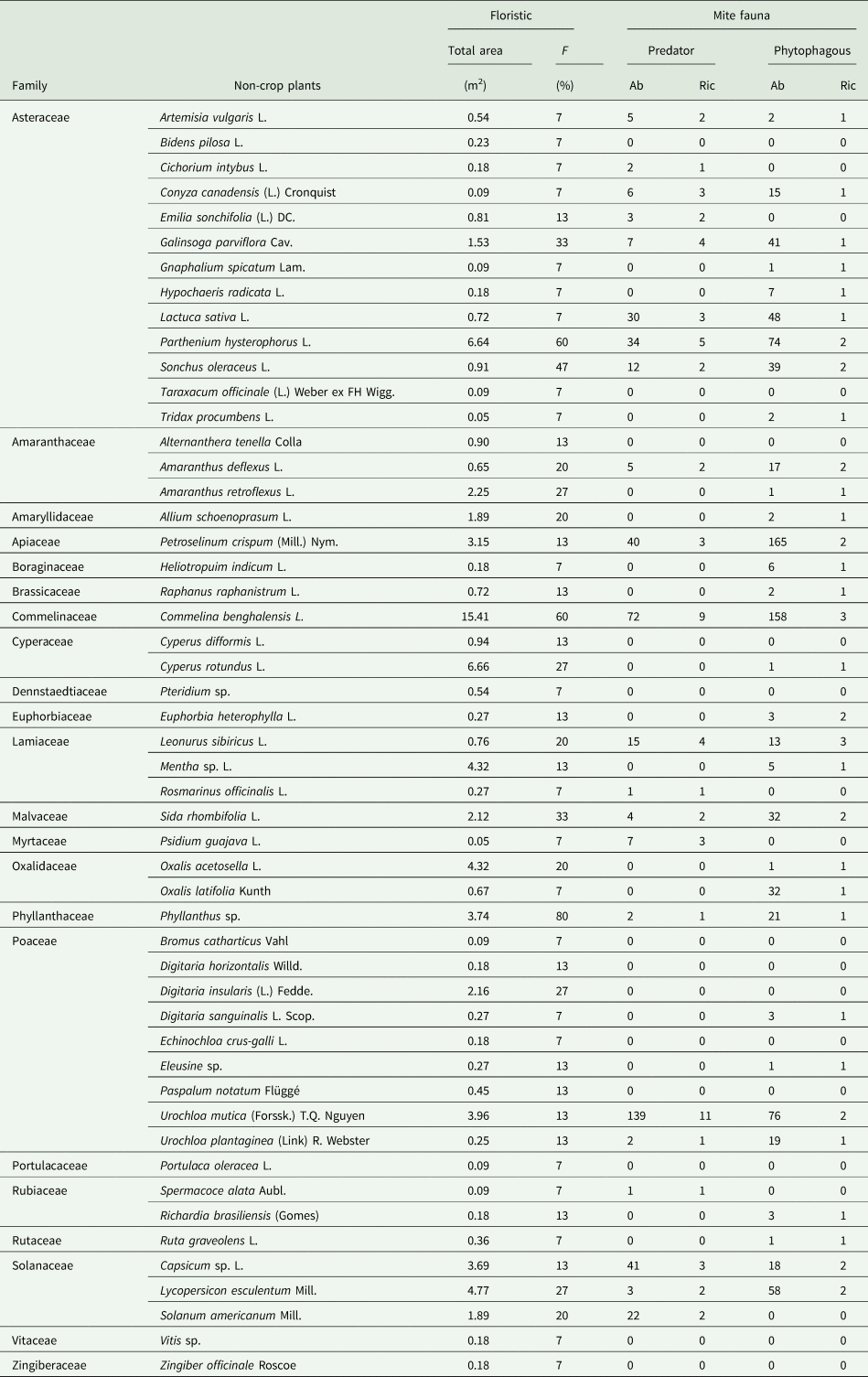
Plant inventory found 51 plant species belonging to 22 families. The two main families found were Asteraceae (13 species) and Poaceae (nine species). The most frequent were Phyllanthus sp., P. hysterophorus, C. rotundus, which were found in 80, 60, and 60% of collection points, respectively (table 2).
Table 2. Species of non-crop plants in different botanical families found below the wooden structure of strawberry (Albion cv.) in a semi-hydroponic system, considering the total area occupied per plant, frequency of plants in collecting points (F), abundance (Ab), and species richness (Ric) of mites found on non-crop plants.
Marialva, Paraná, Brazil – July 2015 to December 2016.
The plant species that hosted the highest abundance of phytophagous mites were Petroselinum crispum Mill. Nym., C. benghalensis, Urochloa mutica (Forssk.) T.Q. Nguyen, and P. hysterophorus (table 2). The plant species hosting most PMs were U. mutica, C. benghalensis, Capsicum sp. L., and P. crispum (table 2).
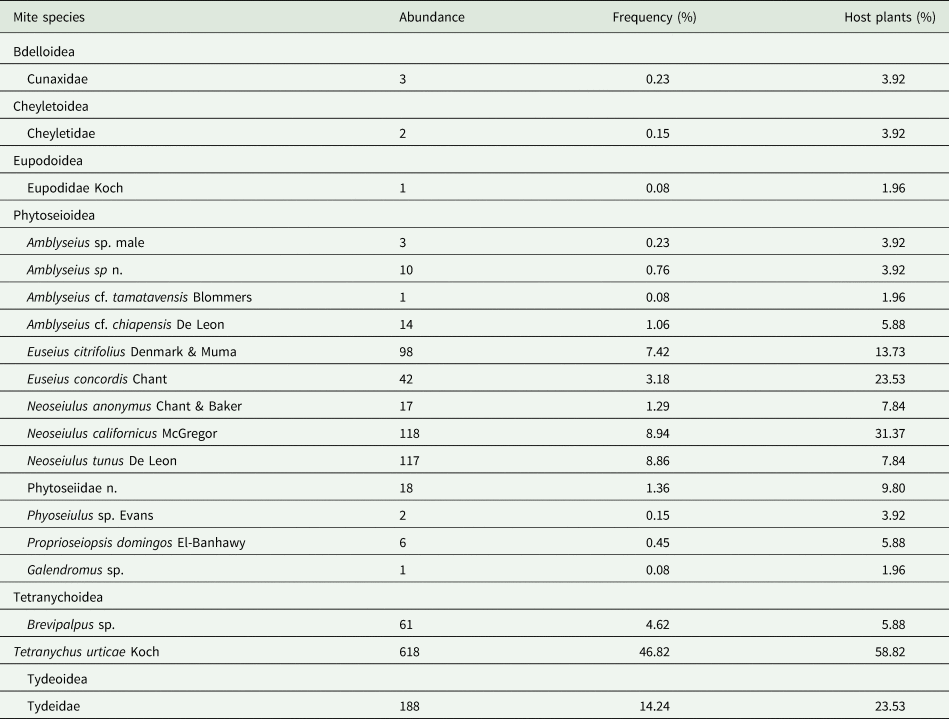
Phytophagous mites comprised 51.44% of the mites collected; Tydeidae, 14.24%; and Phytoseiidae, 33.86%. The phytophagous TSSM and Brevipalpus sp. were found on 58.82 and 5.88% of non-crop plants sampled, respectively. The most abundant Phytoseiidae were N. californicus (8.94%), Neoseiulus anonymus Chant & Baker (8.86%), Euseius citrifolius Denmark & Muma (7.42%), and Euseius concordis Chant (3.18%), found on 31.37, 7.84, 13.73, and 23.53% of hosts, respectively (table 3).
Table 3. Mite fauna found associated with non-crop plants below the wooden structure of strawberry (Albion cv.) crops in the semi-hydroponic system considering the abundance of mites (Ab), frequency in percentage (F), and percentage of host plant species (H) that harbor mites.
Marialva, Paraná, Brazil – July 2015 to December 2016.
The list of all non-crop plants and mites on plants are presented in Supplementary table S1.
Factors affecting the abundance of PMs
The abundance of PMs on different plant species was affected by the abundance of plants (F 1,64 = 37.02, P < 0.001), by the interaction of plant species abundance and the abundance of phytophagous mites on these plants (F 1,64 = 6.22, P = 0.015), but not solely by the abundance of phytophagous mites (F 1,64 = 0.81, P = 0.3728). The most prominent effect on PMs observed was the influence of plant abundance (fig. 2). The distribution of abundance among plant species harboring PMs fitted a logseries distribution (fig. 3a) with a clustered population distribution (Iδ = 1.97, F = 1.87, P < 0.001). In contrast, the distribution of abundance among PMs species fitted a geometric series distribution (fig. 3b) and presented a random distribution (Iδ = 0.49, F = 0.41, P = 0.043).
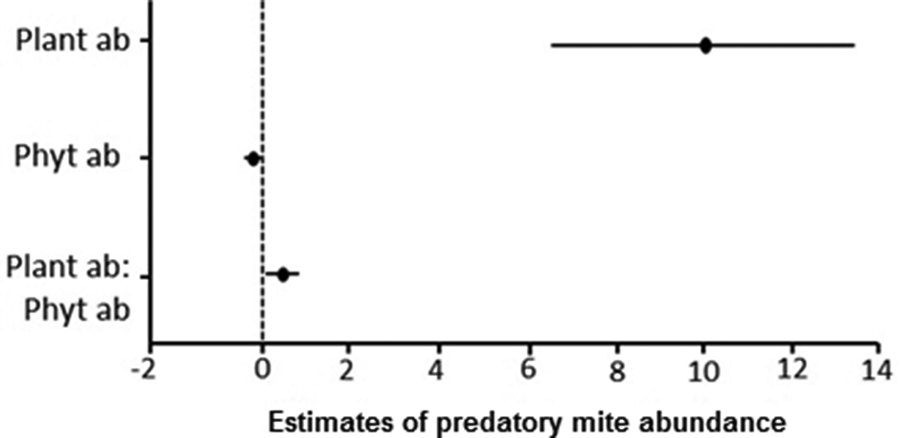
Figure 2. Estimated effects of non-crop plants (Plant ab), phytophagous mite abundance (Phyt ab), and the interaction between these variables (Plant ab: Phyt ab) on the abundance of predatory mites on non-crop plants below the wooden structure of strawberry crops in a semi-hydroponic system in Marialva, Paraná, Brazil. The results are based on a fitted generalized mixed effect model.
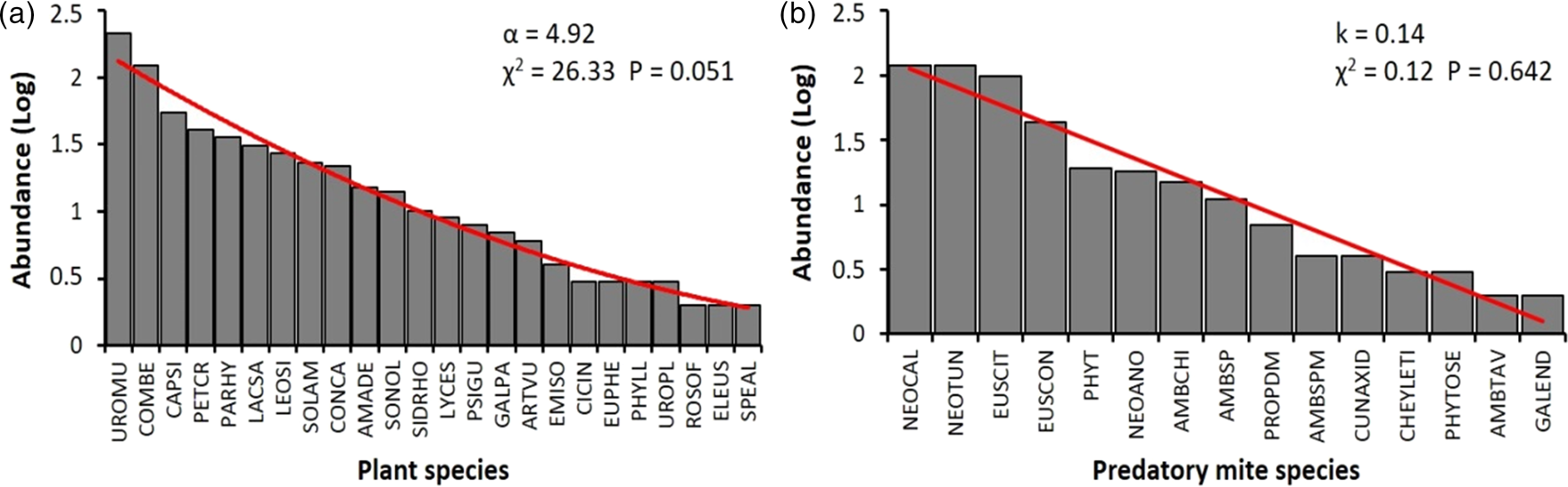
Figure 3. Abundance distribution of non-crop plants harboring predatory mites (a) and abundance distribution of predatory mites hosted in different plant species (b) found below the wooden structure of strawberry in a semi-hydroponic system. The values above the graphs represent the coefficient of the log-series distribution (α) and the geometric distribution (k). The Chi-square tests indicate the adjustment of the observed data to the predicted values from the theoretical models of abundance distribution. UROMU: Urochloa mutica; COMBE: Commelina benghalensis; CAPSI: Capsicum sp.; PETCR: Petroselinum crispum; PARHY: Parthenium hysterophorus; LACSA: Lactuca sativa; LEOSI: Leonurus sibiricus; SOLAM: Solanum americanum; CONCA: Conyza canadensis; AMADE: Amaranthus deflexus; SONOL: Sonchus oleraceus; SIDRHO: Sida rhombifolia; LYPES: Lycopersicon esculentum; PSIGU: Psidium guajava; GALPA: Galinsoga parviflora; ARTVU: Artemisia vulgaris; EMISO: Emilia sonchifolia; CICIN: Cichorium intybus; EUPHE: Euphorbia heterophylla; PHYLL: Phyllanthus sp.; UROPL: Urochloa plantaginea; ROSOF: Rosmarinus officinalis; ELEUS: Eleusine sp.; SPEAL: Spermacoce alata; TYDEY: Tydeidae; NEOCAL: Neoseiulus californicus; NEOTUN: Neoseiulus tunus; EUSCIT: Euseius citrifolius; EUSCON: Euseius concordis; PHYT: Phytoseiidae n.; NEOANO: Neoseiulus anonymus; AMBCHI: Amblyseius cf. chiapensis; AMBSP: Amblyseius sp. male; PROPDM: Proprioseiopsis domingos; CUNAXID: Cunaxidae.; CHEYLETI: Cheyletidae; PHYTOSE: Phytoseiulus sp.; AMBTAV: Amblyseius cf. tamatavensis; GALEND: Galendromus sp.
Association of plants with mites
The LDA analysis showed the formation of different groups of plants with PMs. The species E. citrifolius, N. californicus, N. anonymus, and Neoseiulus tunus De Leon were the species that most contributed to the formation of the different groups of PMs within plant species (fig. 4). Due to the differences found in the LDA analysis, the similarity in PMs species occurring on different plants was very low (ANOSIM R = 0.096, P = 0.0423), so that only a few PM species shared the same plant (fig. 5). The only significant associations found were from C. benghalensis that shared PMs species with other four plant species (Galinsoga parviflora Cav., Lycopersicon esculentum Mill., P. hysterophorus, and Solanum americanum Mill.), and the PMs species that occurred on S. oleraceus was similar to those species that occurred on S. americanum (Supplementary table S2).
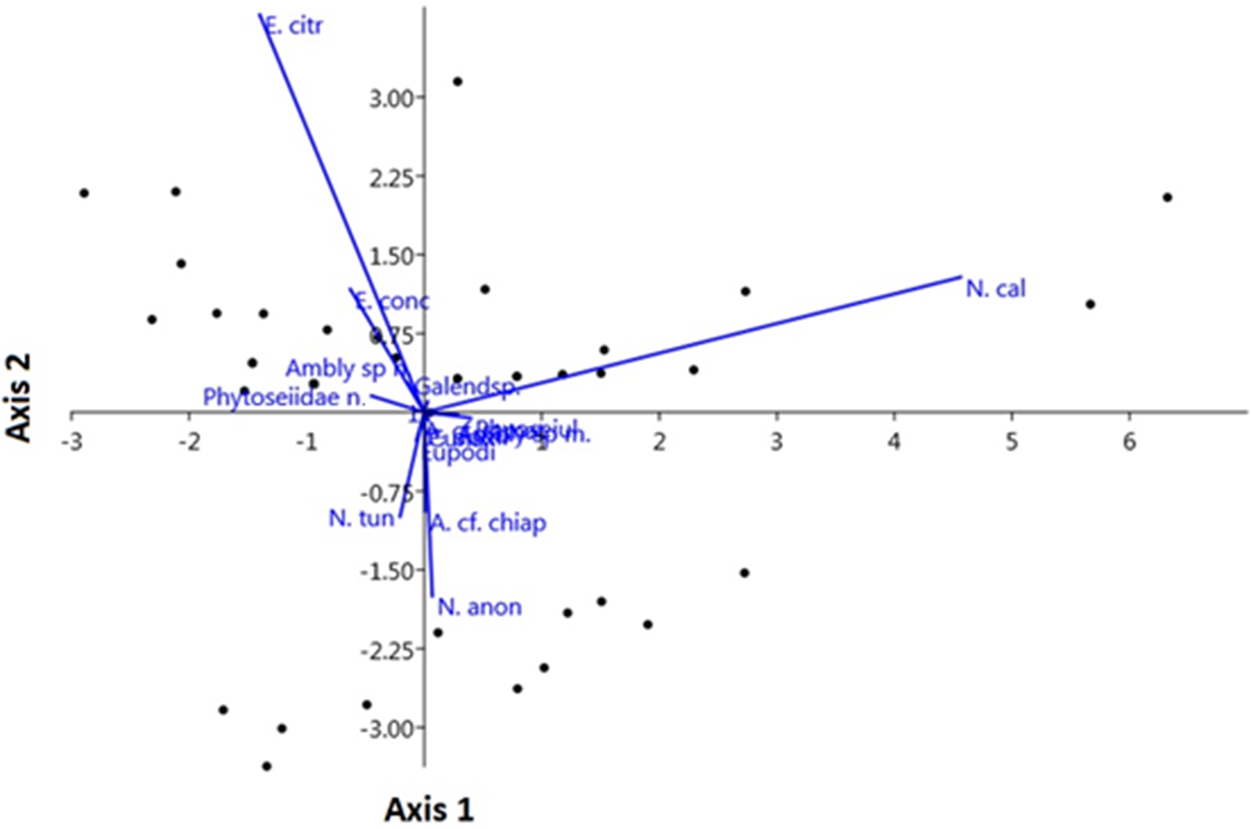
Figure 4. Linear Discriminant Analysis (LDA) of mite species occurring on different non-crop plants found below the wooden structure of strawberry in a semi-hydroponic system, Marialva, Paraná, Brazil. Both axes explained 80.42% of the variance observed in the data. N. cal: Neoseiulus californicus; N. tun: Neoseiulus tunus; E. citri: Euseius citrifolius; E. conc: Euseius concordis; Phytoseiid: Phytoseiidae n.; N. anon: Neoseiulus anonymus; A. cf chiap: Amblyseius cf. chiapensis; P. dom: Proprioseiopsis domingos; Ambly sp. m: Amblyseius sp. male; Cunaxid: Cunaxidae; Cheyletid: Cheyletidae; Phytoseiulus: Phytoseiulus sp.; A. cf. tamat: Amblyseius cf. tamatavensis; Galendsp.: Galendromus sp.; Eupodi: Eupodidae.
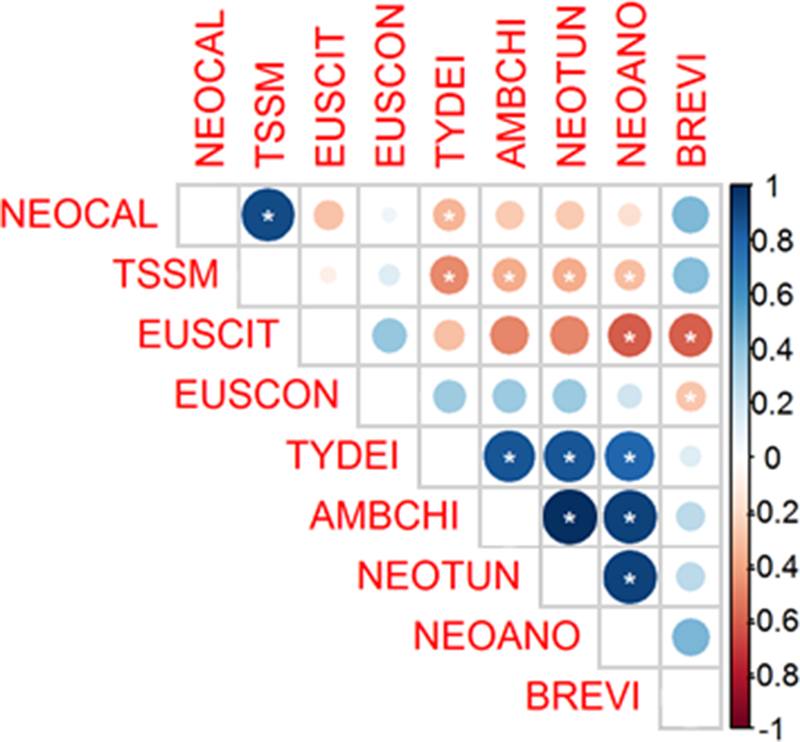
Figure 5. Spearman correlations between mite species on non-crop plants found below the wooden structure of strawberry in a semi-hydroponic system. Asterisks indicate a significant correlation by T-test (P < 0.05). Colors varied from dark blue to red indicating positive (+1) and negative (−1) correlation, respectively. TSSM: Tetranychus urticae, EUSCIT: Euseius citrifolius, TYDEI: Tydeidae, NEOTUN: Neoseiulus tunus, EUSCON: Euseius concordis, AMBCHI: Amblyseius chiapensis, NEOANO: Neoseiulus anonymus, BREVI: Brevipalpus sp., NEOCAL: Neoseiulus californicus.
Complete association (SI: 1.00) was found between phytoseiidmites and TSSM on Lactuca sativa L. and C. benghalensis; Phytoseiidae mites and Brevipalpus sp. on P. crispum; and phytoseiid mites and Tydeidae on Leonurus sibiricus L. (table 4). Partial associations were found between Phytoseiidae and TSSM on P. crispum (0.98) and Sonchus oleraceus L. (0.87); Phytoseiidae and Brevipalpus sp. on C. benghalensis (0.64); and Phytoseiidae and Tydeidae on C. benghalensis (0.87) and U. mutica (0.72). No association was found between Phytoseiidae and Tydeidae on Capsicum sp. (−0.86).
Table 4. Associations between mite species and family on non-crop plants found below the wooden structure of strawberry (Albion cv.) in semi-hydroponic system, measured by Sorensen index (SI).
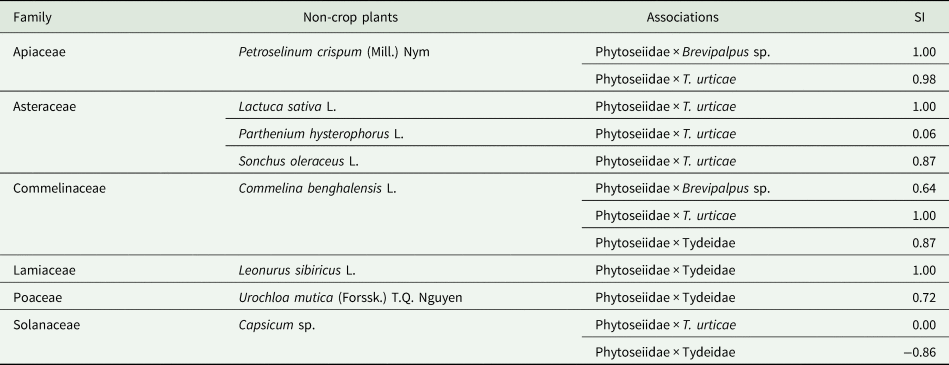
Marialva, Paraná, Brazil – July 2015 to December 2016.
We observed a strong positive correlation between N. californicus and TSSM by Spearman correlation coefficient (0.895) (P < 0.05). Positive correlations were also found for Tydeidae and A. chiapensis, N. tunus and N. anonymus 0.858, 0.858, 0.796, respectively (P < 0.05; fig. 5).
Discussion
The findings reported in this study suggest that a diverse assemblage of non-crop plants could be used as a BPS to conserve PMs in strawberry crops. PMs are organisms with low mobility, and the maintenance of a diverse assemblage of non-crop plants could increase their presence near the crops, possibly preventing pest damage, especially the damage by phytophagous mites. Clearly, preventing pest damage demands knowledge about how PMs are distributed in the field and their association with specific plants as the first step toward the implementation of management strategies using PMs. Additionally, we demonstrated that conservation strategies could be integrated with augmentative biological control because commercially available species, such as N. californicus (Togni et al., Reference Togni, Lagôa, Venzon and Sujii2019a), were found on different plant species.
To combine conservation strategies with augmentative releases, there is a need to know whether the introduced PMs can be found on the non-crop vegetation. The PM N. californicus is commercially available for TSSM control on several crops worldwide (Demite et al., Reference Demite, Moraes, McMurtry, Denmark and Castilho2018; Togni et al., Reference Togni, Lagôa, Venzon and Sujii2019a). This PM could eventually fall from strawberry plants onto non-crop plant leaves where they could find favorable resources for development on non-crop plants, such as Convolvulus arvensis L., Galega officinalis L., or Lamium amplexicaule L. (Ottaviano et al., Reference Ottaviano, Cédola, Sánchez and Greco2015; Vacacela et al., Reference Vacacela Ajila, Colares, Lemos, Marques, Franklin, Santos do Vale, Oliveira, Venzon and Pallini2019). These associations suggest that a BPS is a suitable strategy to combine augmentative and conservation biological control (Parolin et al., Reference Parolin, Bresch, Ruiz, Desneux and Poncet2013), which increases the possibilities in managing multiple target pests simultaneously (Togni et al., Reference Togni, Lagôa, Venzon and Sujii2019a). Although part of N. californicus populations could have been present before the augmentative releases, the greater abundance of this species on all farms studied suggested that the augmentative releases probably increased their abundance and persistence on non-crop plants.
Our results also highlight the role of plants in maintaining the population levels of introduced and naturally occurring PMs. The abundance of PMs was mostly affected by the abundance of non-crop plants. This indicates that the presence of non-crop plants is even more important than the prey (Venzon et al., Reference Venzon, Amaral, Togni, Chiguachi, Souza, Vázquez and Marucci2019). Depending on the lifestyles of phytoseiid species, they feed on prey species and pollen (McMurtry et al., Reference McMurtry, Moraes and Sourassou2013). Providing suitable microclimatic conditions and shelter could also help to increase PMs abundance on non-crop plants (Gontijo, Reference Gontijo2019). For example, the presence of domatia in some plant species can reduce mite cannibalism and improve the abundance of PMs as well as the coexistence with other species (Ferreira et al., Reference Ferreira, Cunha, Pallini, Sabelis and Janssen2008). Also, habitat architecture and complexity can reduce the encounter rates of predators sharing the same resources, reducing intraguild predation and favoring predator abundance (Snyder, Reference Snyder2019; Venzon et al., Reference Venzon, Amaral, Togni, Chiguachi, Souza, Vázquez and Marucci2019).
The role of plants in maintaining higher densities of predators is confirmed by the patterns of abundance distribution of plants and predators. Plant abundance distribution fitted a logseries distribution, which is typical in disturbed environments (Matthews and Whittaker, Reference Matthews and Whittaker2015). Species abundance distribution models fitting a logseries model highlighted that PMs showed specific habitat requirements. These specific habitat requirements reflect the cultivation practices used in the farms, such as weeding and fertilization. Therefore, these practices influence the seed bank within the farm with consequences on plant distribution. For PMs, the abundance distribution fitted a geometric series with a random distribution. Theory predicts that, in assemblages fitting a geometric series, the abundance of each species is proportional to the amount of limiting resources (Matthews and Whittaker, Reference Matthews and Whittaker2015). Therefore, the abundance of PMs species is influenced mostly by the distribution of plant resources, the conditions they provide, and the availability of prey.
Since distinct plant traits (chemical, morphoanatomical and phenological characteristics of plants) favor different phytoseiids(McMurtry et al., Reference McMurtry, Moraes and Sourassou2013), a more diverse assemblage of non-crop plants increases the possibility of coexistence of several species in the same habitat (Amaral et al., Reference Amaral, Venzon, dos Santos, Sujii, Schmidt and Harwood2016; Gontijo, Reference Gontijo2019; Venzon et al., Reference Venzon, Amaral, Togni, Chiguachi, Souza, Vázquez and Marucci2019). This could reduce the temporal and spatial overlap in species occurring in the field. Maintaining the diversity of predatory species in the agroecosystem is expected to increase biological control efficiency (Dainese et al., Reference Dainese, Martin, Aizen, Albrecht, Bartomeus, Bommarco, Carvalheiro, Gagic, Garibaldi, Ghazoul, Grab, Jonsson, Karp, Kennedy, Kleijn, Kremen, Landis, Letourneau, Marini, Poveda, Rader, Smith, Tscharntke, Andersson, Badenhausser, Baensch, Bezerra, Bianchi, Boreux, Bretagnolle, Caballero-Lopez, Cavigliasso, Ćetković, Chacoff, Classen, Cusser, Silva e Silva, Groot, Dudenhöffer, Ekroos, Fijen, Franck, Freitas, Garratt, Gratton, Hipólito, Holzschuh, Hunt, Iverson, Jha, Keasar, Kim, Kishinevsky, Klatt, Klein, Krewenka, Krishnan, Larsen, Lavigne, Liere, Maas, Mallinger, Martinez Pachon, Martínez-Salinas, Meehan, Mitchell, Molina, Nesper, Nilsson, O´Rourke, Peters, Plećaš, Potts, Ramos, Rosenheim, Rundlöf, Rusch, Sáez, Scheper, Schleuning, Schmack, Sciligo, Seymour, Stanley, Stewart, Stout, Sutter, Takada, Taki, Tamburini, Tschumi, Viana, Westphal, Willcox, Wratten, Yoshioka, Zaragoza-Trello, Zhang, Zou and Steffan-Dewenter2019) due to complementary or redundant effects when one species is absent (Snyder, Reference Snyder2019).
Despite the role of plant assemblages, specific plants should be considered in the BPS in strawberry crops. We observed several specific interactions among plants, phytophagous, and predatory species. For safe use of non-crop plants as BPS, the non-crop plant must not be a suitable host for phytophagous mites, especially TSSM, and be a suitable host for PMs. Four plants were found to combine such traits and could be considered during the selective weeding on the farms.
Neoseiulus tunus and E. concordis were the most abundant phytoseiids found on U. mutica. PMs from Pronematus sp. and Asca sp., and the generalist mite Lorria sp. (Tydeidae) were also reported on U. mutica in a previous study (Cruz et al., Reference Cruz, Sarmento, Teodoro, Erasmo, Neto, Ignacio and Junior2012). In the present study, individuals of this plant harbored a great abundance and diversity of PMs species and few TSSM. The predators were probably feeding on U. mutica pollen and tydeid mites on plant leaves, since many of these species have previously been reported on non-crop plants in Brazil (Demite et al., Reference Demite, Feres and Lofego2015).
Two other plant species showed promised results as banker plants. Leonurus sibiricus and S. americanum. Both hosted relatively high populations of PMs and had low TSSM incidence. Phytoseiidae on L. sibiricus presented a 100% association with tydeid mites, indicating a potential predator-prey interaction that can benefit the conservation and maintenance of these species. On the other hand, no associations of Phytoseiidae and phytophagous mites on S. americanum were observed. However, S. americanum was previously suggested as a suitable plant to host Phytoseiulus longipes Evans due to its relationship with T. evansi (Ribeiro et al., Reference Ribeiro, Gondim Júnior, Melo and Delalibera Júnior2012). This indicates that S. americanum is probably beneficial to PMs when prey is present. An overview of Phytoseiidae on solanaceous plants indicated 32 predatory species on S. americanum (Tixier et al., 2020).
Phytoseiidae were mostly E. citrifolius, N. californicus, and N. concordis were also associated with pepper plants. However, these PMs were not associated with mites occurring on pepper plants, which may indicate that predators were feeding on pepper pollen or even using pepper domatia as shelter. Pepper has been studied as a banker plant mostly due to the occurrence of domatia on leaves, which shelters PMs and offer complementary food (Kumar et al., Reference Kumar, Xiao, McKenzie and Osborne2015).
Although P. crispum, C. benghalensis, P. hysterophorus, and L. sativa hosted large populations of PMs, they also hosted significant populations of TSSM. Thus, the use of the above-mentioned plants in BPS should only be considered if an efficient measurement of TSSM control is established otherwise they should be avoided. Similarly, plants with no PMs or with high densities of TSSM should also be avoided. In our study, 16 plant species did not harbor PMs but hosted phytophagous mites. Of these, Oxalis latifolia Kunth, Hypochaeris radicata L., and Heliotropuim indicum L. hosted TSSM specimens, indicating that these plants should not be maintained close to strawberry crop or other crops that TSSM is a key-pest species.
As vegetable crops are ephemeral in time and space due to crop-harvest cycles, they cannot sustain the populations of predatory species throughout seasons (Togni et al., Reference Togni, Venzon, Souza, Sousa, Harterreiten-Souza, Pires and Sujii2019b). Non-crop plants may be a suitable strategy to maintain the populations of PMs naturally occurring or introduced by augmentative releases during the offseason. Weeds may be selectively managed by eliminating non-crop vegetation, which is not suitable for biological control, and should be investigated in future studies. In addition, non-crop vegetation in which PMs normally are found, may be collected and placed on plants where TSSM was found in the crop. This strategy should be considered due to the low labor and investment required, which are important conditions for farmers.
In summary, the findings reported in this study support that non-crop plant diversity plays a fundamental role in the conservation of PMs and can be proposed as a BPS. From 51 plant species identified (22 families), four may be suggested as the most suitable bankers – Capsicum sp. (pepper plant), L. sibiricus, S. americanum, and U. mutica –these plants hosted high PMs density and reduced abundance of the key phytophagous pest, TSSM. In addition to the importance of the plant assemblage in conservation biological control, this approach could also be associated with augmentative biological control due to the maintenance of N. californicus, which is frequently mass released in strawberry crops. Future studies should evaluate the advantages of using these plants to improve their management and use selective weeding to conserve natural enemies in organic and conventional strawberry crops. We also suggest that new studies should be done to develop strategies to release or force PMs movement into the crop to make a clear connection between PMs conservation and biological control of pests. This approach could contribute to the acceptance of these plants as a functional part of the agroecosystem instead of being seen merely as weeds.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485321000973.
Acknowledgements
The authors thank Adilson Gallo, Maria do Carmo Argenton Volpato ‘Dona Carminha’ (in memoriam), Nelson Tamura, Sandra de Assis, and Roberley Martins Faria families, for providing the research areas, the research funding agencies the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES, for the fellowships.












