Introduction
Powdery mildew (PM) of wheat caused by an obligate biotrophic fungus, Erysiphe graminis DC. f. sp. tritici Marchal, is an important and devastating disease problem worldwide, resulting in both yield losses and quality deterioration (Griffey et al., Reference Griffey, Das and Stromberg1993). Resistance breeding requires constant efforts to enrich the reservoir of resistance genes in wheat. Wild species have been widely used as genetic resources for introgression of useful genes into cultivated species by wide hybridization (Mujeeb-Kazi, Reference Mujeeb-Kazi2003; Yao et al., Reference Yao, Zhang, Yang, Xu, Jiang, Xiong, Zhang, Zhang, Ma and Sorrells2007). So far, 58 genes/alleles for resistance to PM in wheat at 39 loci have been identified and located on 16 different chromosomes, of which 21 resistance genes/alleles have been tagged with molecular markers (Huang and Roder, Reference Huang and Roder2004; McIntosh et al., Reference McIntosh, Yamazaki, Dubcovsky, Rogers, Morris, Somers, Appels and Devos2010).
There are numerous examples of successful transfer of genes carrying resistance to various pathogens, environmental stresses and nutritionally useful characteristics from wild diploid relatives into the genome of polyploid wheats (Jiang et al., Reference Jiang, Friebe and Gill1994). At the diploid level, there are two species of einkorn wheat: Triticum monococcum L. and Triticum urartu. Biosystematic treatments and sterility of their hybrids (Johnson and Dhaliwal, Reference Johnson and Dhaliwal1976) indicated that they are valid biological species. T. monococcum comprises both wild and cultivated forms and has important wild subspecies. T. urartu was identified in 1937 and, later, Johnson (Reference Johnson1975) investigated this species as a possible donor of the B genome to polyploid wheats. However, the results revealed that T. urartu chromosomes pair with A-genome chromosomes of the hexaploid wheat. Strong evidence has been reported in favour of Aegilops speltoides (SS) as the female parent of all wild tetraploid wheats (Kilian et al., Reference Kilian, Mammen, Millet, Sharma, Garner, Salamini, Hammer and Ozkan2011); however, the B-genome origin of wheat remains to be confirmed. Of all the genomes analysed, the Ae. speltoides genome is most closely related to the B genome of wheat (Eilam et al., Reference Eilam, Anikster, Millet, Manisterski, Sagi-Assif and Feldman2007).
The maintenance of resistance to obligate pathogens of common wheat over many years has been dependent on the ongoing availability, identification and utilization of resistance genes. As resistance genes became ineffective due to increased virulence in pathogen populations, breeders introduced new genes. These initially came from common wheat, but, subsequently, there was increasing use of resistance genes introduced from wheat with lower ploidy or from related species. Common wheat is an allohexaploid species and genes introgressed into it from species of lower ploidy often confer lower levels of resistance than in the original source genotypes (McIntosh et al., Reference McIntosh, Zhang, Cowger, Parks, Lagudah and Hoxha2011). In some cases, such introgressions do not have adequate resistance to protect hexaploid cultivar derivatives from significant losses. In other instances, the original hybrids with wheat are fully susceptible, indicating evidence of the genetic suppression of resistance. The possibility of the genetic suppression of phenotypic effects in wide crosses of wheat has been a recurrent topic for many years; however, there is no plausible explanation of how suppression actually occurs, assuming that the presumed genes are present.
The present paper reports seedling screening of amphiploids derived from Au/Am genome and S-genome diploid (2n = 2x = 14) progenitor species against PM to identify resistant germplasm.
Materials and methods
Germplasm
The germplasms for this study encompass amphiploid wheats that are genomically AABBAmAm, AABBAuAu and AABBSS. The production protocol of these amphiploids has been reported earlier (Mujeeb-Kazi, Reference Mujeeb-Kazi2006). Amphiploids derived from the crosses of T. durum with either A m or A u genome progenitor species (194 accessions) along with their 24 durum parents (Triticum turgidum, AABB) and 20 accessions of amphiploids derived from the crosses of T. durum with Ae. speltoides (AABBSS) were screened against PM. The pedigree, accessions numbers of T. urartu, T. monococcum and Ae. speltoides along with disease scores are given in Supplementary Table S1 (available online only at http://journals.cambridge.org).
Seedling screening for PM resistance
For the evaluation of seedling resistance, in vitro screening was performed on all genotypes at the seedling stage under glass house conditions at the Crop Diseases Research Station, Murree, Pakistan. The planting had three replicates to form a completely randomized design and replication means were taken. Artificial inoculation was done using bulk inoculum collected during the 2007–8 cropping season. From the initial infections, the inoculum collected was applied on the test materials, and this served as the source of all further testing. Test procedures relative to inoculum collection and increase were essentially similar to those reported by Duggal et al. (Reference Duggal, Jellis, Hollins and Stratford2000). After inoculations, seedlings were maintained at 16–19°C with light for 21 h/d. Infection types were recorded after the appearance of mildew symptoms on a 0–9 scale (McNeal et al., Reference McNeal, Konzak, Smith, Tate and Russell1971). Plants having infection type 0 were considered as completely resistant (immune), those having infection types 1–3 were considered resistant, those with a 4–6 score as intermediate and 7–9 as highly susceptible.
Results
Seedling resistance evaluation
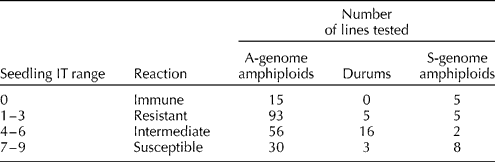
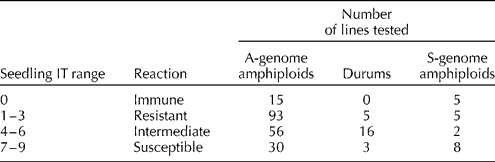
PM development was found to be satisfactory in greenhouse evaluations and readily identifiable variations in disease reactions between resistant and susceptible seedlings were observed. The frequency of genotypes among the A-genome amphiploids, S-genome amphiploids and durum parents is depicted in Table 1. The PM data for individual amphiploids are given in Supplementary Table S1 (available online only at http://journals.cambridge.org).
Table 1 Seedling screening for powdery mildew resistance in amphiploids and their durum parents
IT, infection types.
A-genome amphiploids
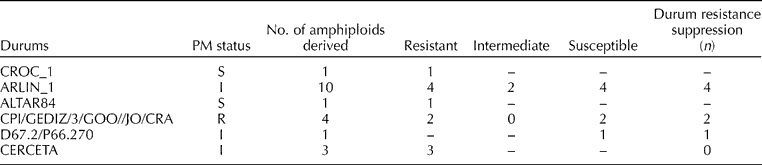
The results revealed that among the 194 A-genome amphiploids, 15 accessions (7.7%) were immune, 93 (47.9%) were resistant, 56 (28.8%) showed an intermediate response and the remaining 30 (15.4%) were found to be completely susceptible to PM. These 194 amphiploids were developed by exploiting 24 durum wheat genotypes and PM evaluation of these durum wheats enabled us to propose putative resistance sources in these synthesized amphiploids. Among the 24 durum wheats, five (20.8%) were completely resistant to PM, 16 (66.6%) showed an intermediate response and the remaining 3 (12.5%) were completely susceptible to PM (Table 2). The comparative analysis of amphiploids and their durum parents identified that in 41 amphiploids, resistance was encoded by an A-genome diploid parent and in 102 cases, both parents were found to be tolerant, and it was difficult to dissect the donor resistance source. The suppression of resistance was identified in 20 amphiploids, in which resistance of the durum parent was not manifested in the amphiploids (Table 2). In 23 amphiploids, both parents (diploid and durum) were susceptible to PM and the amphiploids also did not show any resistance.
Table 2 Comparison of powdery mildew (PM) resistance in durum wheats and their derived A-genome amphiploids

S, susceptible; I, intermediate; R, resistant.
Among the 194 A-genome amphiploids, 120 were developed from the hybridization of 93 T. monococcum ssp. boeoticum accessions and 20 durum cultivars. Similarly, 38 were developed from the hybridization of T. monococcum ssp. monococcum with 12 durum cultivars and 36 from T. urartu. So, the resistance sources from different subspecies of A-genome-related species possess allelic diversity that could enrich the existing Triticum gene pool with the potential to improve both durum and bread wheat.
S-genome amphiploids
The results revealed that among the 20 S-genome amphiploids, five were found to be immune, five were resistant, two showed an intermediate response and eight were found to be completely susceptible to PM. The comparative analysis of the durum parents and respective amphiploids indicated that the resistance source in eight amphiploids was from Ae. speltoides (Table 3). In seven amphiploids, resistance suppression was observed, i.e. the resistant durum parents failed to express resistance in respective amphiploids. In two amphiploids, an additive response was observed, in which the amphiploids showed a higher level of resistance than the durum parent, suggesting that both parents were resistant. In six susceptible amphiploids, both parents were proposed to have susceptibility because the susceptible durum parents and their respective amphiploids did not show any resistance (Table 3).
Table 3 Comparison of powdery mildew (PM) resistance in durum wheats and their derived S-genome amphiploids
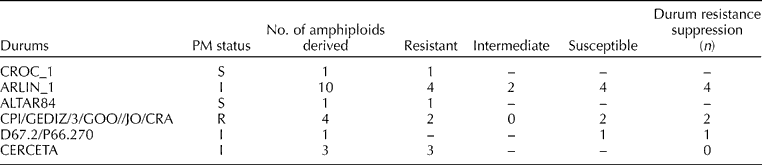
S, susceptible; I, intermediate; R, resistant.
Discussion
Previous studies have identified PM resistance in wild relatives and several genes have been transferred to cultivated wheat such as Pm12 (6B) and Pm32 (1B) from Ae. speltoides (Jia, Reference Jia1996; Hsam et al., Reference Hsam, Lapochkina and Zeller2003), Pm29 (7D) from Aegilops geniculata (Zeller et al., Reference Zeller, Kong, Hartl, Mohler and Hsam2002), Pm34 and Pm35 (5DL) from Aegilops tauschii (Miranda et al., Reference Miranda, Murphy, Marshall and Leath2006, Reference Miranda, Murphy, Marshall, Cowger and Leath2007; Qiu et al., Reference Qiu, Sun, Zhou, Kong, Zhang and Jia2006), Pm39 from Aegilops umbellulata (Zhu et al., Reference Zhu, Zhou, Kong, Dong and Jia2006), and some undesignated genes from Aegilops longissima, Aegilops searsii, Ae. umbellulata (Buloichik et al., Reference Buloichik, Borzyak and Voluevich2008), Aegilops comosa (Bennett, Reference Bennett1984) and Aegilops sharonensis (Olivera et al., Reference Olivera, Kolmer, Anikster and Steffenson2007). From T. monococcum, Pm25 and three temporarily designated genes, Pm2026, Mlm3033 and Mlm80, have been introduced in wheat (McIntosh et al., Reference McIntosh, Yamazaki, Dubcovsky, Rogers, Morris, Somers, Appels and Devos2010). The present investigation identified some new valuable sources of PM resistance which can be introgressed into cultivated wheats through wide hybridization. This initial screening identified novel genes/alleles which need to be further validated at the molecular level. A competitive advantage of studying amphiploids is the availability and identification of user-friendly, genetically compatible germplasm having the potential to improve both durum and bread wheats. A moderate frequency of seedling resistant accessions in both A-genome amphiploids (56%) and S-genome amphiploids (50%) was observed, which could provide diverse sources of resistance to this disease.
Amphiploids that probably had received resistance genes from either of the parents and expressed successfully are important to further use for protecting wheat from PM. The comparative advantage of using a higher number of diploid progenitors with each durum cultivar was to get the optimum number of amphiploids expressing resistance. This is important due to the phenomenon of resistance suppression that has been widely observed in wheat and wide crosses for various biotic stresses. This phenomenon of the dilution of resistance was earlier reported by Kerber and Dyck (Reference Kerber and Dyck1969) who found a reduced expression of resistance to leaf rusts in amphiploids with T. durum compared with the diploid resistant parent Ae. tauschii. Similar results were obtained by Trottet et al. (Reference Trottet, Jahier and Tanguy1982) with PM, leaf and stripe rust, and glume blotch. Bai and Knott (Reference Bai and Knott1992) described the presence of at least four suppressor genes on chromosomes of the D genome inhibiting leaf and stem rust resistance from tetraploid wheats in hexaploid synthetics, and proposed the search for non-suppressing alleles to facilitate the transfer of potentially good sources of genes from relatives into common wheat. There are also certain evidences where resistance in durum is suppressed by the diploid progenitor in amphiploids, e.g. Lr23 (Nelson et al., Reference Nelson, Singh, Autrique and Sorrells1997). However, the complete expression of diploid resistance in common wheat has been reported for green bug resistance (Harvey et al., Reference Harvey, Martin and Livers1980), Hessian fly (Gill et al., Reference Gill, Hatchett and Raupp1987), cereal cyst nematode (Eastwood et al., Reference Eastwood, Lagudah and Appels1991) and resistance to wheat curl mite (Thomas and Connor, Reference Thomas and Connor1986). In several cases, PM resistance of the same durum parent was expressed or suppressed when hybridized with different diploid accessions (Supplementary Table S1, available online only at http://journals.cambridge.org). Therefore, it is not clear whether resistance gene suppressors operated in durum or diploid accessions, which is due to the lack of knowledge about the function, structure, variability and operation of suppressor genes in Triticeae. Thus, prior knowledge of the dilution effect and the search for non-suppressor alleles would enhance the success of transfer of beneficial genes from wild diploid progenitors to common wheat and their use in practical wheat breeding programmes.
In conclusion, this initial PM screening at the seedling stage identified several amphiploids that carry resistance from Am, Au, AB and/or S genomes and justifies the expansion of genetic diversity for PM resistance.