Introduction
Squamous cell carcinoma (SCC) of the external auditory canal and middle ear is an extremely rare entity, with an annual incidence estimated at between one and six cases per million of the population.Reference Morlon, Stell and Derrick1 While early stage SCC of the external auditory canal has been successfully treated by sleeve resection or lateral temporal bone resection, more advanced cancer requires subtotal temporal resection, resulting in facial palsy, hearing impairment and balance disorder, with severe post-operative complications such as cerebral infarction and meningitis.
The modified Pittsburgh classification proposed by Moody et al.Reference Moody, Hirsch and Myers2 in 2000 has been most commonly used for SCC of the external auditory canal and middle ear. In Moody's classification, tumours limited to the temporal bone are defined as T1 or T2 disease. Tumours extending to the middle ear or apparently eroding the temporal bone are defined as T3 disease. Tumours with invasion into the cochlea, petrous apex, middle-ear medial wall, carotid canal, jugular foramen or dura, or with extensive soft tissue involvement, such as the temporomandibular joint (TMJ) or styloid process, or evidence of facial paresis, are defined as T4 disease. Thus, T4 disease covers a fairly wide range of invasion, from a small extent (to the middle-ear wall) to a large extent (to the brain).
According to this classification, in the late twentieth century, while the reported oncological outcomes of patients with T1, T2 and T3 disease were favourable, the survival rates of patients with T4 disease were extremely poor.Reference Bibas, Ward and Gleeson3–Reference Essig, Kitipornchai, Adams, Zarate, Gandhi and Porceddu7 However, during the last two decades, advances in surgical techniques for skull base surgery and multidrug concomitant chemoradiotherapy with docetaxel, cisplatin and 5-fluorouracil have improved oncological results for patients with advanced SCC of the external auditory canal and middle ear, especially when oncological resection is feasible.Reference Shinomiya, Hasegawa, Yamashita, Ejima, Kenji and Otsuki8–Reference Shiga, Ogawa, Maki, Amano and Kobayashi10 Nevertheless, the prognosis of patients with unresectable T4 disease remains poor.
Considering this background, this study investigated the prognostic factors for patients with advanced SCC of the external auditory canal and middle ear, to update the staging system and ensure ongoing relevance to advances in surgical and non-surgical treatments.
Materials and methods
Patients
Between 1998 and 2017, 102 consecutive patients with SCC of the external auditory canal and middle ear were treated at Kobe University Hospital, Japan. Among the 102 patients, we retrospectively reviewed 95 patients who were pathologically diagnosed with SCC of the external auditory canal and middle ear and treated with curative intent. The remaining seven patients were excluded from this study. Two patients aged 90 years or older refused definitive therapy and were treated with palliative radiotherapy (RT) for pain relief. Three patients had other advanced cancer simultaneously and underwent palliative RT as best supportive care. Another two patients with severe dementia were not suitable for treatment with curative intent. Patients who had unresectable tumours and had undergone non-surgical treatment were considered to have undergone radical treatment and were included in this study.
Diagnosis and treatment
At the initial diagnosis, the extent of disease was assessed with the aid of contrast-enhanced computed tomography, magnetic resonance imaging and 18-fluoro-2-deoxyglucose positron emission tomography. Disease was staged according to the most recent version of the modified Pittsburgh classification (2000).Reference Moody, Hirsch and Myers2 Sites of invasion were determined by pre-operative imaging study.
For patients with T1 and T2 disease, we principally recommended surgical treatment. Radiotherapy was employed for patients who refused surgery. Sleeve resection or lateral temporal bone resection was performed for T1 and T2 disease. For patients with T3 disease, we recommended subtotal temporal bone resection or lateral temporal bone resection, depending on the extent of the disease. When patients refused surgery, concurrent chemoradiotherapy with cisplatin, or the combination of docetaxel, cisplatin and 5-fluorouracil,Reference Shinomiya, Hasegawa, Yamashita, Ejima, Kenji and Otsuki8 was recommended. For patients with resectable T4 disease, we recommended subtotal temporal resection. Invasion of the carotid artery and extensive dural invasion were considered as contraindications, while minor dural and/or brain invasion was considered as resectable. For patients with unresectable T4 disease and patients who refused subtotal temporal resection, chemoradiotherapy with cisplatin, or the combination of docetaxel, cisplatin and 5-fluorouracil,Reference Shinomiya, Hasegawa, Yamashita, Ejima, Kenji and Otsuki8 was performed. Particle beam therapy (carbon or proton) was employed in patients who strongly requested this therapy. Post-operative RT was given to surgically treated patients with positive or close surgical margins.
Surgical procedures
In lateral temporal bone resection, principally, the bony external auditory canal, tympanic membrane, malleus and incus were resected with extended mastoidectomy in an en bloc manner. The superficial lobe of the parotid gland was resected in 3 out of 11 patients with T1 disease and in 12 out of 20 with T2 disease. If parotid gland invasion or parotid lymph node involvement was identified, total parotidectomy was performed. The facial nerve was preserved in all cases. Neck dissection was not performed in any case of lateral temporal bone resection.
In subtotal temporal resection, after total parotidectomy and prophylactic neck dissection (levels II–III), the temporal bone was resected in an en bloc manner with temporo-suboccipital craniotomy. Resection lines were anteriorly along the internal carotid artery and posteriorly at the sigmoid sinus. The medial resection line was along the internal auditory canal. The mandibular condyle was removed to obtain the surgical field, and the facial nerve was sacrificed. Principally, the jugular bulb, sigmoid sinus and dura were preserved, but were resected according to the extent of disease. The defect was reconstructed using a rectus abdominis musculocutaneous free flap.
Tumours with extension to the carotid artery, extensive dura and/or brain were considered as contraindications to subtotal temporal resection. While tumours with limited infiltration to the jugular vein could be successfully resected by sacrificing the jugular vein in selected cases, it was difficult to ensure negative surgical margins in most cases. Thus, we consider tumours with invasion of the jugular vein as relatively inoperable. Limited dural invasion, TMJ invasion and facial nerve invasion were considered resectable. Our treatment strategies are summarised in Table 1.
Table 1. Treatment strategies of our institute
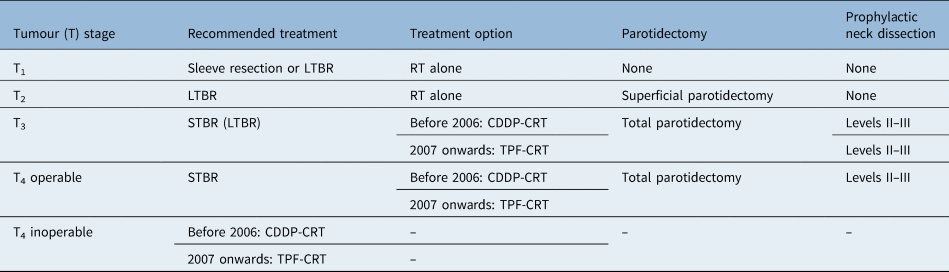
LTBR = lateral temporal bone resection; RT = radiotherapy; STBR = subtotal temporal bone resection; CDDP-CRT = cisplatin-based chemoradiotherapy; TPF-CRT = chemoradiotherapy with docetaxel, 5-fluorouracil and cisplatin
Statistical analysis
Medical records were retrospectively reviewed to obtain information concerning characteristics of the patients, extent of disease, treatment, surgical procedures, surgical margins, post-operative RT, treatment period and oncological results. The treatment period was divided into two time periods: 1998–2005 and 2006–2017. This is because we started to apply chemoradiotherapy with docetaxel, cisplatin and 5-fluorouracil to patients with SCC of the external auditory canal and middle ear from 2006 when applicable.
Kaplan–Meier plots were used to summarise time to event, measured from the end of the first treatment. The log-rank test was used for univariate analysis of survival rates, and the Cox proportional hazards regression analysis was used for multivariate analysis of survival rates. A p-value of 0.05 or less was defined as a significant difference. R software (version 3.0.2. 2013; R foundation for Statistical Computing, Vienna, Austria) was used for the statistical analysis.
This study was approved by Kobe University Hospital Internal Review Board.
Results
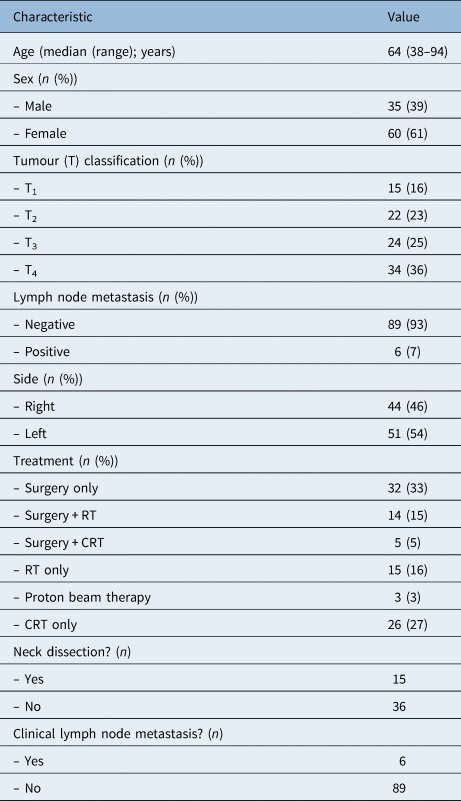
The characteristics of the patients are summarised in Tables 2 and 3. The patients’ ages ranged from 38 to 94 years, with a median age of 64 years. Follow-up periods ranged from 7 to 144 months (median of 50 months, mean of 49.7 months). The numbers of the patients with T1, T2, T3 and T4 disease were 15, 22, 24 and 34, respectively. Only six patients had metastatic lymph nodes.
Table 2. Patients’ characteristics

RT = radiotherapy; CRT = chemoradiotherapy
Table 3. Treatment method according to tumour stage

Data represent numbers of patients. RT = radiotherapy; CRT = chemoradiotherapy
The most common treatment was surgery, which was selected mainly for early stage disease. Among T1 and T2 patients, 11 out of 15 patients with T1 disease and 20 out of 22 patients with T2 disease underwent surgical resection, while 5 patients underwent RT alone, and only 1 patient underwent proton beam therapy. Among T3 patients, 11 had surgical resection, 6 underwent chemoradiotherapy, 6 received RT alone and 1 patient underwent proton beam therapy. Among T4 patients, 9 had surgical resection, 20 underwent chemoradiotherapy (12 with cisplatin, and 8 with docetaxel, cisplatin and 5-fluorouracil), 4 patients received RT alone and 1 patient underwent proton beam therapy.
The data for patients treated with (chemo)radiotherapy are summarised in Table 4. Fifteen patients were treated with RT alone. Eighteen patients were treated with cisplatin-based chemoradiotherapy, and eight patients were treated with chemoradiotherapy with docetaxel, cisplatin and 5-fluorouracil. Three patients had proton beam therapy. Nineteen patients received post-operative RT.
Table 4. Summary of patients treated with radiotherapy

Proton beam therapy was excluded from radiotherapy dose. RT = radiotherapy; TPF = docetaxel, cisplatin and 5-fluorouracil; 3D = three-dimensional; IMRT = intensity-modulated radiotherapy; SD = standard deviation
The details for univariate analysis of survival rates are summarised in Table 5. Significant differences were found in terms of: original tumour site (p = 0.011), T classification (p < 0.001), surgical margin status (p = 0.001), post-operative RT (p = 0.004) and treatment period (p = 0.013). Surgical margin status was obtained from medical records in 46 out of 51 surgically treated patients. The results of multivariate analysis for the 46 surgically treated patients for whom information on surgical margins was available are shown in Table 6, and the results of all 95 patients are shown in Table 7. Regardless of treatment modality, T classification (T4) was found to be a significant independent prognostic factor, as was treatment period.
Table 5. Univariate analysis of survival rates

*Indicates statistical significance (p < 0.05). OS = overall survival; post-op = post-operative; RT = radiotherapy; CRT = chemoradiotherapy
Table 6. Multivariate analysis for 51 operated patients

Surgical margin status was obtained in only 46 patients. *Indicates statistical significance (p < 0.05). CI = confidence interval; post-op = post-operative; RT = radiotherapy
Table 7. Multivariate analysis for all 95 patients

*Indicates statistical significance (p < 0.05). CI = confidence interval
The five-year overall survival rates of the patients with T1, T2, T3 and T4 disease were 93.3, 95.2, 84.7 and 42.9 per cent, respectively. The five-year disease-specific survival rates of the patients with T1, T2, T3 and T4 disease were 100, 100, 84.7 and 48.3 per cent, respectively. Kaplan–Meier plots of overall survival according to T classification are shown in Figure 1. According to the survival curve, the survival rate of patients with T4 disease was markedly worse than the survival rates of patients with T1, T2 and T3 disease. Thus, next, we further analysed the prognostic factors for patients with T4 disease in detail.

Fig. 1. The Kaplan–Meier curves according to the tumour (T) staging of the modified Pittsburgh classification. The five-year survival rate of T4 patients was markedly worse than the survival rates of T1, T2 and T3 patients.
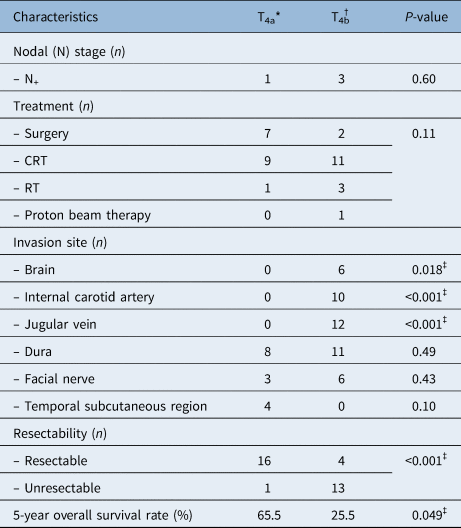
The results of univariate analysis according to the invasion sites of 34 patients with T4 disease are shown in Table 8. Brain invasion (p = 0.024), internal carotid artery invasion (p = 0.049) and internal jugular vein invasion (p = 0.040) were found to be significant predictors of poor prognosis. From these results, we subclassified T4 disease invading the brain, carotid artery or jugular vein as T4b, and T4 disease without these features as T4a. Characteristics of T4a and T4b patients are shown in Table 9.
Table 8. Univariate analysis of tumour stage T4 patients according to invasion site

*Indicates statistical significance (p < 0.05). OS = overall survival
Table 9. Characteristics of tumour stage T4a and T4b patients

*n = 17; †n = 17. ‡Indicates statistical significance (p < 0.05). CRT = chemoradiotherapy; RT = radiotherapy
Chemoradiotherapy or RT tended to be applied in patients with T4b disease, as most T4b diseases were unresectable. The Kaplan–Meier curves of patients with T4a and T4b disease, as well as those with T1, T2 and T3 disease, are shown in Figure 2. The overall survival rate of T4a patients was significantly higher than that of T4b patients (65.5 per cent vs 25.5 per cent, p = 0.049). In addition, the overall survival rate of T4a patients undergoing chemoradiotherapy was significantly higher than that of T4b patients undergoing chemoradiotherapy (five-year overall survival rate = 100 per cent vs 36.4 per cent, p = 0.020).

Fig. 2. The Kaplan–Meier curves of the new classification. The five-year survival rate of tumour stage T4a patients was significantly higher than that of T4b patients (65.5 vs 25.5 per cent, p = 0.049).
Discussion
Because of its rarity and aggressive oncological behaviour, no standard treatment for SCC of the external auditory canal and middle ear has yet been established. For most reported cases, the selected treatment comprised surgical resection and post-operative RT.Reference Chi, Gu, Dai, Chen and Li5–Reference Essig, Kitipornchai, Adams, Zarate, Gandhi and Porceddu7,Reference Leong, Youssef and Lesser11–Reference Gidley, Roberts and Sturgis15 While the cure rates for patients with early stage lesions (T1 and T2) treated by en bloc resection were near to 100 per cent,Reference Leong, Youssef and Lesser11–Reference Gidley, Roberts and Sturgis15 treatment of locally advanced cancers is still challenging.
In previous literature, T classification has been reported to be the most important prognostic factor, as local recurrence is a cause of death in most cases of SCC of the external auditory canal and middle ear. The T classification,Reference Chi, Gu, Dai, Chen and Li5,Reference Gidley, Roberts and Sturgis15–Reference Zanoletti, Lovato, Stritoni, Martini, Mazzoni and Marioni18 N classification,Reference Gidley, Roberts and Sturgis15,Reference Ogawa, Nakamura, Hatano, Uno, Fuwa and Itami17 surgical margins,Reference Chi, Gu, Dai, Chen and Li5,Reference Yin, Ishikawa, Honda, Arakawa, Harabuchi and Nagabashi16,Reference Ogawa, Nakamura, Hatano, Uno, Fuwa and Itami17 dural invasion,Reference Zanoletti, Lovato, Stritoni, Martini, Mazzoni and Marioni18 facial palsyReference Chi, Gu, Dai, Chen and Li5,Reference Zanoletti, Lovato, Stritoni, Martini, Mazzoni and Marioni18 and post-operative RTReference Ogawa, Nakamura, Hatano, Uno, Fuwa and Itami17 were described as prognostic factors in patients with SCC of the external auditory canal and middle ear, as previously reported. In the present study, T classification by the modified Pittsburgh staging system was also confirmed as a prognostic factor by multivariate analysis of all 95 patients. Of note, the oncological outcome of the patients with T4 was extremely poor compared with that for patients with T1, T2 and T3 disease. The five-year overall survival rate of T4 patients was 42.9 per cent, while that of T1, T2 and T3 patients was 93.3, 95.2 and 84.7 per cent, respectively.
Following recent advances in surgical techniques, surgical navigation systems and diagnostic imaging, the oncological outcome of SCC of the external auditory canal and middle ear has gradually improved. In the 1970s, Lewis reported a five-year overall survival rate of 25 per cent in a review of 100 cases.Reference Lewis19 In contrast, in 2006, Yin reported a five-year overall survival rate of 66 per cent.Reference Yin, Ishikawa, Honda, Arakawa, Harabuchi and Nagabashi16 In a meta-analysis, five-year overall survival rates of patients with T3 and T4 disease were 57.5 and 22.9 per cent, respectively, for the period covering 1976–2008.Reference Higgins and Antonio20 These rates increased to 72.5 and 35.8 per cent, respectively, for 2006–2013.Reference Takenaka, Cho, Nakahara, Yamamoto, Yasui and Inohara21 In addition, chemoradiotherapy with docetaxel, cisplatin and 5-fluorouracil has provided a promising oncological outcome for advanced SCC of the external auditory canal and middle ear, including unresectable far-advanced cancers.Reference Shinomiya, Hasegawa, Yamashita, Ejima, Kenji and Otsuki8–Reference Shiga, Ogawa, Maki, Amano and Kobayashi10 These reports and ours demonstrate the necessity for revising the tumour–node–metastasis (TNM) classification.
• The modified Pittsburgh tumour (T) classification can predict the prognosis of squamous cell carcinoma arising from the auditory canal
• Invasion of the brain, internal carotid artery and internal jugular vein predicted (p < 0.05) poor prognosis among T4 patients
• A new method of categorising T4 patients regarding the modified Pittsburgh classification into two groups (T4a and T4b) was proposed
• The overall survival rate of T4a patients was significantly higher than that of T4b patients (65.5 vs 25.5 per cent, p = 0.049)
• The new T4 tumour classification may be useful for predicting both prognosis and therapeutic effects
Mazzoni et al.Reference Mazzoni, Danesi and Zanoletti22 proposed dividing T3 of the modified Pittsburgh classification into T3a (tumour extending less than 5 mm from cartilage to peri-auricular soft tissues, or tumour strictly limited to the anterior bone wall and growing less than 5 mm into the parotid space) and T3b (same as for T3a, but extending more than 5 mm). Also, they divided T4 into T4a (tumour growing into mastoid without facial nerve paresis) and T4b (tumour growing into mastoid with facial paresis, or infratemporal space, or medial wall of tympanum, labyrinth, or petrous bone). Although Mazzoni's classification is useful in the case of surgical resection, there was no consideration of resectable and unresectable tumours treated by intensified chemoradiotherapy such as that with docetaxel, cisplatin and 5-fluorouracil, as shown in the present study and our previous studies.Reference Shinomiya, Hasegawa, Yamashita, Ejima, Kenji and Otsuki8–Reference Shiga, Ogawa, Maki, Amano and Kobayashi10
To address this limitation, we subclassified T4 disease into two subclasses according to the prognostic factors of brain invasion, internal carotid artery invasion and internal jugular vein invasion. As shown in Figure 2, patients with T4 disease were clearly divided into patients without these factors (T4a) and patients with at least one of these factors (T4b). As the majority of T4b diseases were unresectable, patients with T4b disease were mostly treated with RT or chemoradiotherapy. However, the oncological outcomes of patients with T4b disease treated by intensive chemoradiotherapy (with docetaxel, cisplatin and 5-fluorouracil) were still poor. On the other hand, almost all T4a disease was oncologically resectable, and the five-year overall survival rate of patients with T4a disease treated by intensified chemoradiotherapy was 100 per cent. Our new classification of T4a and T4b disease may be useful not only for predicting prognosis but also for predicting therapeutic effects.
In the present series, treatment period was also found to be a significant independent prognostic factor on multivariate analysis. The most likely reason for the improved oncological outcome with time is the amendment of our treatment policy for non-surgical treatment, which changed from cisplatin chemoradiotherapy to docetaxel, cisplatin and 5-fluorouracil chemoradiotherapy. Advances in imaging and surgical technique supported by surgical navigation might also have contributed to the improved survival, as shown in the meta-analysis.Reference Takenaka, Cho, Nakahara, Yamamoto, Yasui and Inohara21
One of the limitations of the present study, which has a level of evidence of 4, is its retrospective nature, which may contain several biases in terms of choice of treatment and patient selection. Although the present study is one of the largest series from a single-institute, based on long-term follow up, the number of patients was still small. Currently, we are conducting a multi-institutional retrospective study to draw more definitive conclusions.
Conclusion
We propose a new classification, classifying T4 of the modified Pittsburgh classification into two groups according to the prognostic factors of invasion of the brain, internal carotid artery and jugular vein.
Competing interests
None declared