The Late Devonian (Frasnian) Gogo Formation fauna currently comprises some 50 species of fishes in addition to concavicarid crustaceans, making up approximately a quarter of the fauna, along with rare eurypterids, tentaculatids and other invertebrates (Briggs et al. Reference Briggs, Rolfe, Butler, Liston and Ingham2011). The excellent 3-D preservation of the fish fossils makes them a standard reference for the study of anatomical features in early vertebrates. In this paper we discuss a number of new vertebrate discoveries from the Gogo Formation that have come to light or been featured in various research projects since our previous major review paper (Long & Trinajstic Reference Long, Yu, Maisey and Miao2010). Most of the work on Gogo fishes published prior to 2010 can be found in that publication.
1. Geological setting
The Gogo Formation includes the oldest inter-reef deposits in the southern region of the Lennard Shelf within the Canning Basin. Details of its history of mapping and discovery can be found in Playford & Lowry (Reference Playford and Lowry1966), Long & Trinasjtic (Reference Long, Yu, Maisey and Miao2010) and Playford et al. (Reference Playford, Hocking and Cockbain2009). The outcrops of the Gogo Formation occur over a large area (c.200 km2) between prominent outcrops of the Devonian reef complex to the east of Fitzroy Crossing, on Gogo and Mt Pierre Stations. The Gogo Formation is about 700 m thick, measured from drill core, and is the basinal lateral facies equivalent of the Sadler Limestone Formation (marginal-slope facies), Pillara Limestone (platform facies) and Windjana Formation (reef facies, Playford Reference Playford1980). The Gogo Formation has been dated as early Frasnian (falsiovalis to punctata conodont zones), based on revision of the conodont fauna and radiolarians from the upper part of the formation (Glenister & Klapper Reference Glenister and Klapper1966; Druce Reference Druce1976; Nazarov et al. Reference Nazarov, Cockbain and Playford1982; Nazarov & Ormiston Reference Nazarov and Ormiston1983). Three local hypoxic events suggesting sea level rises are recognised in the early Frasnian, indicative of oxygen depletion at the sea floor (Playford & Wallace Reference Playford and Wallace2001).
2. Preservation of Gogo fishes
The Gogo fish fossils are generally preserved as three-dimensional, undistorted or compressed bone and mineralised cartilage within limestone concretions that rapidly formed after death. Finer resolution preservation occurs of biomineralised soft tissues, which to date have included muscle fibres and bundles, nerve cells and umbilical structures (Trinajstic et al. Reference Trinajstic, Marshall, Long and Bifield2007; Long et al. Reference Long, Trinajstic, Young and Senden2008, 2009), as well as mineralised muscle blocks in ptyctodonts (Trinajstic et al. Reference Trinajstic, Marshall, Long and Bifield2007) and arthrodires (Trinajstic et al. Reference Trinajstic, Sanchez, Dupret, Tafforeau, Long, Young, Senden, Boisvert, Power and Ahlberg2013). The extraordinary preservation of the fossils is the result of microbially-induced encapsulation of the fauna preventing full decomposition (Mendelez et al. 2013a, b).
Biomarkers from the fossil and carbonate nodules indicate the presence of a stratified water column with persistent euxinic conditions in the photic zone supporting the sulphur reducing bacteria which were important for the rapid formation of carbonate nodules that encapsulated of the fauna (Mendelez et al. 2013a, b). These features facilitated the mineralisation of soft tissues, rarely preserved in fossils of this age (Trinajstic et al. Reference Trinajstic, Marshall, Long and Bifield2007, Reference Trinajstic, Sanchez, Dupret, Tafforeau, Long, Young, Senden, Boisvert, Power and Ahlberg2013). In addition, an overall lack of major tectonic forces in the region after burial of the reef complex sedimentary sequence enabled the fossil specimens to retain their 3-D shape without any sign of post-burial compaction (Playford et al. Reference Playford, Hocking and Cockbain2009).
Specimens figured herein are held in the collections of the following Australian museums and university collections: ANU, The Australian National University, Research School of Earth Sciences, Canberra; MV, Museum Victoria; SAM, South Australian Museum; WAM, Western Australian Museum.
3. The search for Gogo fish fossils
The history of Gogo fish expeditions and their outcomes from the initial discovery through to the 2005 Museum Victoria expedition has been summarised in Long (Reference Long2006). The discovery of many new species resulting from the author's (JL) expeditions between 1986 and 2005 was due in part to careful re-examination of known sites mapped by the earlier joint expeditions (1963, 1967) and outlined in Miles (Reference Miles1971), but also due to searching those Gogo outcrops depicted on the geological maps, but not known to have yielded fishes previously. These include sites around Embayment Hill, south of Stromatoporoid Camp, first prospected successfully for fishes on the 2001 expedition, yielding the new actinopterygian Gogosardina coatsi (Choo et al. Reference Choo, Long and Trinajstic2009), plus areas in between Stromatoporoid Camp and Long's Well, which produced the first Gogo acanthodian (Burrow et al. Reference Burrow, Trinajstic and Long2012), chondrichthyans (Long et al. Reference Long, Mark-Kurik, Johanson, Lee, Young, Zhu, Ahlberg, Newman, Jones, Den Blaauwen, Choo and Trinajstic2015) and first coelacanth from the Gogo formation, all found on the 2008 Museum Victoria expedition. In 2011 a Curtin University expedition to Gogo recovered many significant new finds, including arthrodires with soft tissue preservation and a complete specimen of the tetrapodomorph fish Gogonasus.
4. The Gogo fish fauna
The Gogo fish fauna currently comprises some 50 species of placoderms, actinopterygians, dipnoans, acanthodians and single taxon representatives of chondrichthyan, onychodontiform, actinistian and osteolepiform fishes. Of these, some were newly discovered on the 2005 and 2008 Gogo expeditions, and have not yet been formally described (for example, a second chondrichthyan and an actinistian). The most abundant vertebrate group is the placoderms, with the highest diversity exhibited by the small eubrachythoracid arthrodires. Notably absent from the fauna are the agnathans (jawless fish) and one osteichthyan group, the porolepiforms. However, slightly younger (mid-late Frasnian) sediments of the Virgin Hills Formation, representing distal forereef slope deposits that conformably overlay the Gogo Formation, have recently yielded numerous sharks teeth, acanthodian scales and thelodont scales (Trinajstic & George Reference Trinajstic and George2009; Trinajstic et al. Reference Trinajstic, Roelofs, Burrow, Long and Turner2014; Roelofs et al. Reference Roelofs, Playton, Barham and Trinajstic2015; Hairapetian et al. 2016).
Lately, major advances have been achieved in studying these fossils using micro-CT tomography from both synchrotron and micro-CT machines. At first, only prepared material was scanned, with great resolution at the 10–30-micron level using the ANU Vizlab facility (e.g., Gogonasus, Long et al. Reference Long2006). Recent work using the Grenoble synchrotron has been applied to specimens still embedded within limestone to resolve soft-tissue preservation (Trinajstic et al. Reference Trinajstic, Sanchez, Dupret, Tafforeau, Long, Young, Senden, Boisvert, Power and Ahlberg2013; Sanchez et al. Reference Sanchez, Dupret, Tafforeau, Trinajstic, Ryll, Gouttenoirea, Wretman, Zylberberge, Peyrina and Ahlberg2013). Most recently, the neutron beam at ANSTO in Lucas heights, Sydney region, has been applied to image larger scale specimens within thick nodules (c.10 cm) with good resolution.
5. New information on Gogo placoderms
5.1. Antiarchs
Only one antiarch is present at Gogo, the bothriolepidoid Bothriolepis sp., and although undescribed, research on the mouthparts (Young Reference Young1984) and pelvic region (Long et al. Reference Long, Burrow, Ginter, Maisey, Trinajstic, Coates, Young and Senden2015b) has been undertaken. Most recently, the lower jaw bones (inferognathals) and spines of the pectoral fin dermal bone were analysed using synchrotron imaging by Rückelin et al. (2012) to determine if teeth were present. This study concluded that there was no difference in the microanatomy of the ‘tooth-like' structures on the jaws and spines on the pectoral fins and bones. It was suggested that antiarchs might have not secondarily lost dentine (as was implied in the acceptance that they had teeth on their jaws), as dentine cannot be found anywhere in their dermal skeletons (Rückelin et al. 2012). Two specimens of Gogo Bothriolepis were figured in a paper on antiarch reproduction (Long et al. Reference Long, Mark-Kurik, Johanson, Lee, Young, Zhu, Ahlberg, Newman, Jones, Den Blaauwen, Choo and Trinajstic2015a, supplementary information) and also in a book (Long Reference Long2011). One specimen from the MV collections displayed unusual morphological features on the dorsal surface of the subanal lamina of the posterior ventrolateral plate, and another specimen from the ANU collections showed the presence of small paired plates in this region. These were initially interpreted as female ‘genital plates', following identification of similar structures in Pterichthyodes and Microbrachius, but we are now revising this interpretation in the light of new research with our collaborators at the University of Rimouski, Quebec. Further work on the well–preserved Escuminac Formation Bothriolepis canadensis specimens is critical to resolving the issue. Continuation of the description of Gogo Bothriolepis material is now dependent on a number of new finds awaiting to be fully prepared, with others to be synchrotron-scanned prior to acid preparation to search for soft tissues.
5.2. Ptyctodontids
Recently published work on Gogo ptyctodontids has focused on the description of the dermal skeleton, jaws and embryos of Materpiscis and Austroptyctodus (Trinajstic et al. Reference Trinajstic, Long, Johanson, Young and Senden2012; Johanson & Trinajstic Reference Johanson, Trinajstic, Carr and Ritchie2014) and the redescription of the pelvic girdle in Campbellodus (Trinajstic et al. Reference Trinajstic, Boisvert, Long, Maksimenko and Johanson2015). The recognition of dermal and perichondral ossifications within the pelvic skeleton of Campbellodus showed for the first time that, like the pectoral girdle, the pelvic girdle was a composite structure. This suggests that in early jawed vertebrates the formation of paired girdles was an interactive process along the body flank (Trinajstic et al. Reference Trinajstic, Boisvert, Long, Maksimenko and Johanson2015). A new specimen of Campbellodus (WAM 11.9.1) shows the articulated upper and lower jaws in near occlusion, indicating the inferognathals protruded well forward anteriorly of the supragnathals (Fig. 1A), a feature also present in Materpiscis (Trinajstic et al. Reference Trinajstic, Long, Johanson, Young and Senden2012). The description of the synarcual in Campbellodus, with the inclusion of developmental data from chondrichthyans, provided evidence that the synarcual first forms as individual vertebrae and that bone is deposited between vertebrae in development (Johanson et al. Reference Johanson, Trinajstic, Carr and Ritchie2013; Johanson & Trinajstic Reference Johnson and Trinajstic2014). These studies show the utility of combining fossil and extant taxa to inform on the evolution of the vertebrate skeleton.
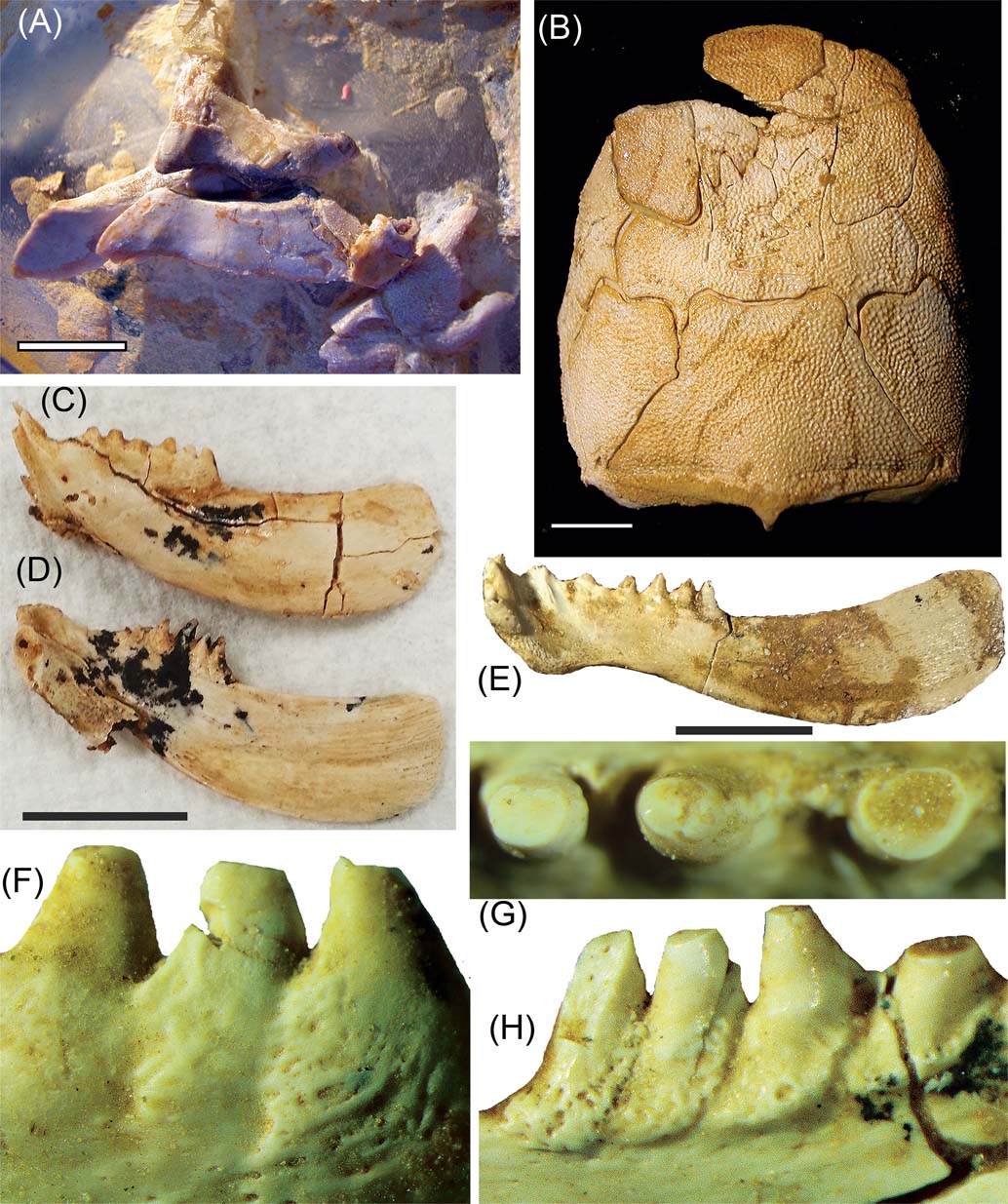
Figure 1 Gogo placoderms. (A) ptyctodontid Campbellodus decipiens, WAM 11.9.1, showing articulated upper and lower jaws. (B), (E) ?Torosteus sp.: (B) WAM 91.4.31, skull roof in dorsal view; (E) right inferognathal in mesial view. (C), (D) (F–H) Torosteid, sp. indeterminate, SAM P50606: inferognathals in (C) lateral and (D) mesial views; (F–H) close up views showing teeth with resorption pits at bases. Scale bars = 1 cm.
5.3. Arthrodires
Recent work by Rücklin et al. (Reference Rücklin, Donoghue, Johanson, Trinajstic, Marone and Stampanoni2012) and Donoghue & Rücklin (Reference Donoghue and Rücklin2014) have confirmed the earlier hypothesis put forward by Smith & Johanson (Reference Smith and Johanson2003) that placoderms do possess true teeth, characterised by having a true dentine structure that developed in association with a looped vasculature and pulp cavity. The teeth developed successionally ahead of their biting function, with the pulp canals of older teeth becoming infilled with bone; however, both upper and lower teeth lack a hypermineralised capping layer such as the acrodin or enameloid common to the teeth of higher vertebrates.
Detailed synchrotron imaging of Gogo arthrodire bones has revealed Sharpey's fibres, which indicate the position of tendinous attachments of muscles to bone (Sanchez et al. Reference Sanchez, Dupret, Tafforeau, Trinajstic, Ryll, Gouttenoirea, Wretman, Zylberberge, Peyrina and Ahlberg2013). A key finding of this study was that Sharpey's fibres were identified in some bones that lacked muscle scars, the traditional method for identifying where muscles were present, suggesting that previous muscle reconstructions did not capture the full complement of arthrodire muscles. This was shown in Eastmanosteus, where preservation of the neck muscles showed the presence of levator capitas major and minor, where previously only the levator capitas major had been predicted, as well as where transversus abdominis muscles had not been previously predicted (Trinajstic et al. Reference Trinajstic, Sanchez, Dupret, Tafforeau, Long, Young, Senden, Boisvert, Power and Ahlberg2013). Such studies will pave the way for more accurate restoration of major muscle systems in these early vertebrates.
Further work on the pelvic region has advanced knowledge on reproduction in arthrodires. Intromittent organs were first recognised in a single adult male specimen of Incisoscutum (Ahlberg et al. Reference Ahlberg, Trinajstic, Johnason and Long2009) and later were also found to be present within embryos (Johanson & Trinajstic Reference Johnson and Trinajstic2014). The discovery of additional specimens with intromittent organs demonstrated that these paired elements were separate from the pelvic fins, which differs from chondrichthyans where they form part of the pelvic fin (Trinajstic et al. Reference Trinajstic, Boisvert, Long, Maksimenko and Johanson2015). Females have also now been found with embryos of differing sizes, suggestive of different developmental stages occurring (Johanson & Trinajstic Reference Johnson and Trinajstic2014).
Current work on Gogo arthrodires is focusing on the form and function of their sensory line pits and redescription of poorly known taxa, based on more complete new specimens. A possible new species of torosteid arthrodire, represented by two new specimens, is shown in Figure 1B–H, characterised by its short, deep inferognathals (Fig. 1C, D). The dentition is particularly interesting in showing inferognathal teeth cleanly worn down on the cusps (Fig. 1G–H) and with resorption pits at the coalescing tooth bases (Fig. 1F, G).
6. New information on Gogo acanthodians
The first Gogo acanthodian was recently described, based on a single specimen found on the 2008 expedition. It is a small mesacanthid, named as Halmacanthodes ahlbergi by Burrow et al. (Reference Burrow, Trinajstic and Long2012). Although 3-D-preserved, the delicate nature of the perichondral shell enveloping the bones prevented the braincase remaining intact; but the jaws and shoulder girdles were well preserved, as well as the body and head scales and small areas of mineralised tissue, which permitted the body shape to be determined (Burrow et al. Reference Burrow, Trinajstic and Long2012, fig. 1c, h).
7. New information on Gogo Chondrichthyans
Two Gogo sharks were found on the 2005 expedition (Fig. 2), and the first of these has now been formally described as Gogoselachus lynnbeazleyae (Long et al. Reference Long, Mark-Kurik, Johanson, Lee, Young, Zhu, Ahlberg, Newman, Jones, Den Blaauwen, Choo and Trinajstic2015b), based on isolated lower jaws, scapulocoracoids, parts of the visceral skeleton (gill-arches), scales and teeth (Fig. 2E, F). The mineralised cartilage was acid prepared from the limestone intact, and detailed investigation of its microstructure revealed that it retained bony cell spaces within the matrix binding the tesserae. This equates to an almost intermediate condition between modern sharks and stem gnathostomes like placoderms. Modern chondrichthyans have skeletons are made entirely of tessera with a non-cellular matrix (Maisey Reference Maisey2013).
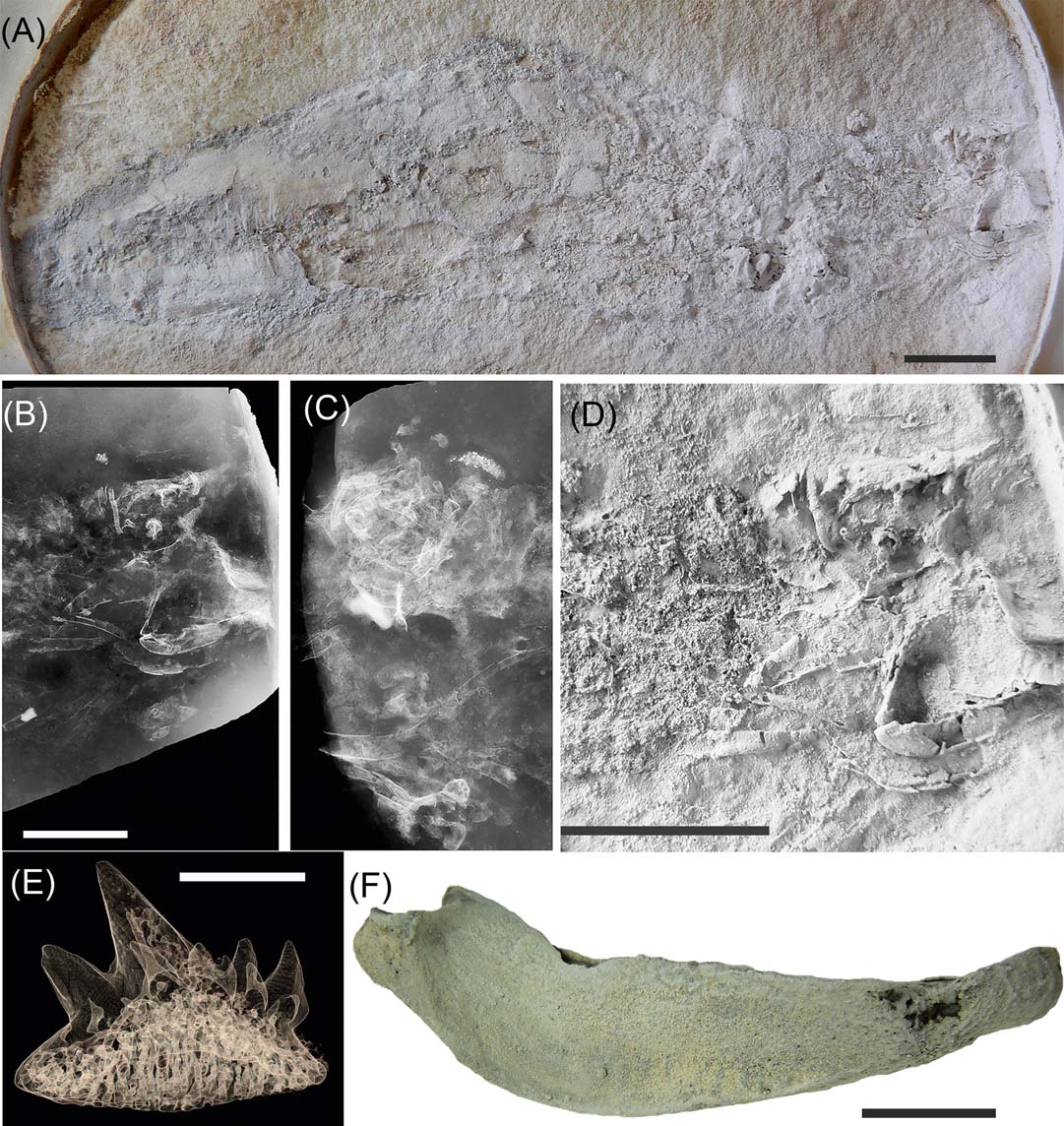
Figure 2 Gogo chondrichthyans. (A–D) Chondrichthyan indet, WAM 08.7.1: (A) in right lateral view; (B), (C) radiographs of the head region of both sides; (D) close-up of head shown in (A), whitened with ammonium chloride. (E), (F) chondrichthyan, Gogoselachus lynnbeazleyae, WAM 09.6.145: (E) tooth shown as micro-CT tomogram; (F) right Meckel's cartilage in lateral view (from Long et al. Reference Long, Burrow, Ginter, Maisey, Trinajstic, Coates, Young and Senden2015b). Scale bars = 1 cm.
A second, more complete shark-like vertebrate has been identified from the Gogo sites, collected on the 2005 expedition and is here shown in detail for the first time (Fig. 2A–D). It represents most of the body of the animal including its head (Fig. 2A, D), and is characterised by the body covered by numerous muscle fibres without scales present. The jaws and braincase are shark-like, but no teeth have been recovered. Radiographs show the gill-arches and some features of the neurocranium clearly (Fig. 2B, C). Long (Reference Long and Warren2007) originally presented the specimen as a possible acanthodiform acanthodian. Trinajstic et al. (Reference Trinajstic, Burrow and Long2011) referred to it as a chondrichthyan based on the jaw articulation, naked skin and pelvic girdle, which had previously not been visible due to extensive soft tissue overlying the skeleton. Although the jaws and visceral skeleton are displaced ventrally from the braincase, the articulated position of the hyomandibular against the palatoquadrate indicates chondrichthyan affinities, because it terminates well before the anterior margin of the postorbital blade of the palatoquadrate, as in Acanthodes and chondrichthyans (Brazeau & De Winter Reference Brazeau and De Winter2015).
8. New information on Gogo osteichthyans
8.1. Actinopterygians
There has been a series of recent papers focusing on the taxonomy of Gogo actinopterygians, first described in detail by Gardiner (Reference Gardiner1984), as well as elucidating their internal anatomy. Choo (Reference Choo2011) formally renamed ‘Mimia' (preoccupied by a moth) as Mimipiscis and described the new species M. bartrami from complete Gogo specimens, one of which swallowed a conodont animal as its last meal (Choo Reference Choo2011, fig. 10a). The ubiquitous genus Moythomasia was also revised by Choo (Reference Choo2015), who presented new reconstructions of the Gogo species M. durgaringa. Giles & Friedman (Reference Giles and Friedman2014) recently described the endocast of Mimipiscis toombsi, noting its morphology as closer to basal osteichthyans than to the well-known Early Carboniferous form Kentuckia. Other recent papers describing new Devonian actinopterygians, or discussing Devonian vertebrate diversity data, routinely cross reference the Gogo specimens (Swartz Reference Swartz2012; Giles et al. Reference Giles, Darras, Clement, Blieck and Friedman2015a; Sallan Reference Sallan2014).
8.2. Onychodontids
Since the description of the Gogo Onychodus by Andrews et al. (Reference Andrews, Long, Ahlberg, Barwick and Campbell2006), there have been numerous phylogenetic analyses of lower vertebrates that utilise the character information derived from this taxon (e.g., Davis et al. Reference Davis, Finarelli and Coates2012; Zhu et al. Reference Zhu, Yu, Ahlberg, Choo, Lu, Qiao, Qu, Zhao, Jia, Blom and Zhu2013; Dupret et al. Reference Dupret, Sanchez, Goujet, Tafforeau and Ahlberg2014), but no new primary work on the Gogo material has been performed. In their description of new Chinese specimens and review of onychodontid functional morphology, Lu & Zhu (Reference Lu and Zhu2010) have reconstructed the possible braincase kinematics of the Gogo Onychodus.
8.3. Dipnomorphans
The lungfish fauna from Gogo is now the most diverse known for any Devonian vertebrate site, with ten genera and 12 species described. Recent additions to knowledge of Gogo dipnoans includes a detailed study of the dermal skeleton of the dipterid Rhinodipterus kimberleyensis by Clement (Reference Clement2012) and an account of the anatomy of the cranial endocast in that species (Clement & Ahlberg Reference Clement and Ahlberg2014), and how the buccal pump mechanism functioned in Rhinodipterus (Clement et al. Reference Clement, Long, Tafforeau and Ahlberg2016). Challands (Reference Challands2015) revised the phylogeny of early lungfishes and his work supports earlier suggestion that the Gogo Chirodipterus is not congeneric with the type material from Germany, or with the Chinese species (Friedman Reference Freidman2007a). This work supports the suggestion that Griphognathus whitei from Gogo could be the plesiomorphic sister taxon to all other rhynchodipterids as first suggested by Friedman (Reference Friedman2007b) and Long (Reference Long, Yu, Maisey and Miao2010). A description of the ontogenetic stages of the posterior neurocranium formation in the rhynchodipterid Griphognathus whitei has also been published (Campbell et al. Reference Campbell, Barwick and Senden2012).
A new kind of osteichthyan, Cainocara (Campbell & Barwick Reference Campbell and Barwick2011), is the most recent new family to be described from the Gogo formation; however, we suggest an alternative interpretation, that the specimen described represents a poorly preserved incomplete dipnoan braincase (cf. Griphognathus sp.).
8.4. Tetrapodomorphans
The MV specimen of the tetrapodomorph fish Gogonasus andrewsae (Long et al. Reference Long2006, Holland & Long Reference Holland and Long2009) has been further studied, shedding additional light on its endocranium (Holland Reference Holland2014), pectoral girdle and fin (Holland Reference Holland2013). An unusual median cavity was identified within the endocranial walls of Gogonasus that was suggested as a possible homologue of the rostral cavity in actinistians (Holland Reference Holland2014, p. 13). Ongoing work is describing the gill-arches, pelvic girdle and caudal fin structure based on the MV specimen (Fig. 3A-D), along with a new specimen found on the 2011 Curtin University expedition.
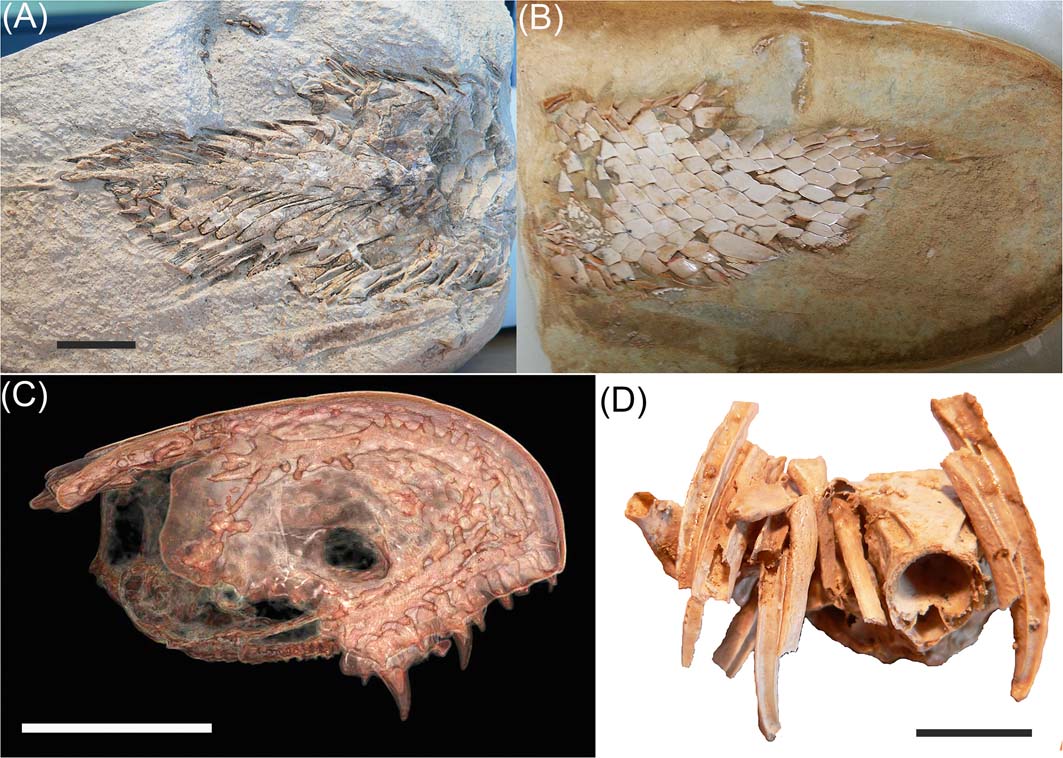
Figure 3 Gogo tetrapodomorphan, Gogonasus andrewsae, MV P221807. (A, B) caudal fin region: (A) as found; (B) acid-prepared. (C), (D) Micro-CT tomograms: (C) showing ethmosphenoid division of the head, highlighting the laterosensory system of the dermal bones; (D) showing some of the gill-arches in association with the oticco-occipital region of the braincase. Scale bars = 1 cm.
The large spiracular openings on the dorsal surface of the head of Gogonasus were recently confirmed as likely used for accessory breathing, based on observations of the spiracles for air-breathing in the most primitive living osteichthyan, Polypterus, by Graham et al. (Reference Graham, Wegner, Miller, Jew, Lai, Berquist, Frank and Long2014).
9. Future directions
There are still large numbers of unprepared Gogo fishes held in collections of the Western Australian Museum, Museum Victoria, Curtin University and Flinders University, based on collecting expeditions spanning the past 30 years. As new imaging techniques are developed and employed (e.g., synchrotrons, micro-CT units), and trialled with unprepared Gogo specimens (e.g., ANSTO neutron beam Dingo facility), so will significant new anatomical features be described. It is increasingly important not to prepare all specimens in acid baths, unless certain significant new specimens clearly show discrete anatomical features necessary for advancing scientific knowledge (i.e., visible in split specimens). There is great potential to await future technological advances for analysing specimens, so storing unprepared specimens is a strategic conservation plan for these collections.
Finally, a study of the significance of the Gogo fossil site has been recently published that proposes a new method, based on various academic and other performance metrics, to assess the scientific significance of a fossil site (Long Reference Long2016).
10. Acknowledgements
The collection of Gogo fishes was supported by the following grants: ARC DP 0558499; DP0772138; DP 1092870; and DP 110101127. JAL would like to thank Nick Fraser and the Palaeontological Society for inviting him to be a keynote speaker at the Stan Wood Symposium in September 2014.





