Published online by Cambridge University Press: 16 June 2005
Triclabendazole (TCBZ) is a halogenated benzimidazole compound that possesses high activity against immature and adult stages of the liver fluke, Fasciola hepatica. The intensive use of TCBZ in endemic areas of fascioliasis has resulted in the development of liver flukes resistant to this compound. TCBZ sulphoxide (TCBZSO) and TCBZ sulphone (TCBZSO2) are the main molecules recovered in the bloodstream of TCBZ-treated animals. In order to gain some insight into the possible mechanisms of resistance to TCBZ, the goals of the work described here were: to compare the ex vivo transtegumental diffusion of TCBZ parent drug and its sulpho-metabolites (TCBZSO and TCBZSO2) into TCBZ-susceptible and -resistant liver flukes; and to assess the comparative pattern of TCBZ biotransformation by TCBZ-susceptible and -resistant F. hepatica. For the tegumental diffusion studies, TCBZ-susceptible (Cullompton) and -resistant (Sligo) adult flukes collected from untreated infected sheep were incubated (15–180 min) in KRT buffer containing either TCBZ, TCBZSO or TCBZSO2 (5 nmol.ml−1). For the metabolism studies, microsomal fractions obtained from TCBZ-susceptible and -resistant flukes were incubated for 60 min with TCBZ (40 μM), and the amount of the formed metabolic product (TCBZSO) was measured. Drug/metabolite concentrations were quantified by HPLC. All the assayed TCBZ-related molecules penetrated through the tegument of both TCBZ-susceptible and -resistant flukes. However, significantly lower (approximately 50%) concentrations of TCBZ and TCBZSO were recovered within the TCBZ-resistant flukes compared to the TCBZ-susceptible ones over the 180 min incubation period. The rate of TCBZ sulphoxidative metabolism into TCBZSO was significantly higher (39%) in TCBZ-resistant flukes. The flavin-monooxigenase (FMO) enzyme system appears to be the main metabolic pathway involved in the formation of TCBZSO in both TCBZ-susceptible and -resistant flukes. The altered drug influx/efflux and enhanced metabolic capacity identified in TCBZ-resistant liver flukes may account for the development of resistance to TCBZ.
The trematode Fasciola hepatica is a cosmopolitan parasite which causes considerable loss in sheep and cattle production systems all over the world (Boray, 1994). In severe infections, adult liver flukes cause anaemia, hypoalbuminaemia and often depressed total protein contents (Soulsby, 1987). Additionally, fascioliasis is an important zoonotic disease, particularly in underdeveloped countries (Mas-Coma, 2004). Chemotherapy, based on the use of flukicidal compounds, is the main tool to control liver flukes. Triclabendazole (5-chloro-6(2-3 dichlorophenoxy)-2-methylthio-1H-benzimidazole) (TCBZ), an halogenated benzimidazole (BZD) thiol derivative, shows excellent efficacy against both juvenile and adult F. hepatica, which is unlike other available flukicidal drugs (Boray et al. 1983). TCBZ parent drug is not detectable in plasma after its oral administration to sheep (Hennessy et al. 1987) and cattle (Sanyal, 1995), TCZB-sulphoxide (TCBZSO) and TCBZ-sulphone (TCBZSO2) being the metabolites recovered from the bloodstream of treated animals (Fig. 1). Furthermore, extremely low concentrations of TCBZ were recovered in bile, TCBZSO, TCBZSO2 and the hydroxy-TCBZ derivatives being the major biliary metabolites found in sheep (Hennessy et al. 1987). As is true of other nematodicidal BZD sulphoxides, the sulphoxide metabolite of TCBZ is believed to be an active moiety. In fact, the tegument of F. hepatica is highly susceptible to TCBZSO, since the compound causes severe surface damage after relatively short time exposure (Stitt & Fairweather, 1993, 1994).

Fig. 1. Chemical structures of triclabendazole and its sulphoxide and sulphone metabolites.
Since TCBZ is the most widely used flukicide due to its excellent activity against both the mature and immature stages of F. hepatica, the selection of TCBZ-resistant populations is now an emerging problem in several areas of the world (data reviewed by Fairweather, 2005). The nematodicidal action of BZDs is based on their binding to β-tubulin, which produces subsequent disruption of the tubulin-microtubule dynamic equilibrium (Lacey, 1988). BZD resistance in Haemonchus contortus has been associated with the loss of high-affinity binding (Lubega and Prichard, 1991a) and an alteration of the β-tubulin isoform pattern (Lubega and Prichard, 1991b), correlated with a conserved mutation at amino acid 200 (phenylalanine to tyrosine) in β-tubulin isotype 1 (Kwa, Veenstra and Roos, 1994). Although the flukicidal activity of TCBZ remains to be fully understood, there are data to support a microtubule-based action of this compound (Stitt and Fairweather, 1992, 1993, 1994, 1996). However, it has been shown that the TCBZ-resistant phenotype in F. hepatica is not associated with residue changes in the primary amino acid sequence in β-tubulin (Robinson et al. 2002). This suggests that there may be an alternative mechanism of TCBZ resistance. Additionally, Coles and Stafford (2001) reported that albendazole (ABZ), a methylcarbamate BZD compound, was highly effective against adult TCBZ-resistant F. hepatica. If it is assumed that both TCBZ and ABZ act on the β-tubulin of F. hepatica, then some alternative resistance mechanisms need to be advanced to explain the differential fasciolicidal activity of ABZ and TCBZ observed in field efficacy trials.
Flukicidal drugs can reach target sites within F. hepatica either by oral ingestion of blood or by transtegumental diffusion. The large absorptive surface area of the fluke's tegument may have a major role in drug diffusion from the surrounding medium. The entry of a drug into a parasite may mainly depend on the diffusion surface area, the concentration gradient across the membrane, the pH/pK relationship and the lipophilicity of the molecule. Studies on ex vivo drug diffusion into TCBZ-susceptible F. hepatica, demonstrated that TCBZ, TCBZSO and TCBZSO2 had the capability to penetrate the fluke's tegument (Alvarez, Mottier and Lanusse, 2004; Mottier et al. 2004b). Additionally, it has been demonstrated that adult TCBZ-susceptible F. hepatica is able to sulphoxidate TCBZ into TCBZSO (Mottier et al. 2004b).
In order to gain further insight into the mechanism implicated in TCBZ resistance, the comparative ex vivo tegumental diffusion of TCBZ, TCBZSO and TCBZSO2 into TCBZ-susceptible and -resistant F. hepatica was investigated in the current work. Additionally, the pattern of TCBZ biotransformation by the microsomal fraction of both TCBZ-susceptible and -resistant liver fluke isolates was compared.
Reference standards (97–99% pure) of TCBZ and its metabolites were provided by Novartis Animal Health (Basel, Switzerland). The reduced form of nicotinamide adenine dinucleotide phosphate (NADPH), tris[hydroxymethyl]aminomethane hydrochloride (Tris), methimazole (MTZ) and piperonyl butoxide (PB) were purchased from Sigma-Aldrich Chemical Company (St Louis, USA). The solvents used for the chemical extraction and chromatographic analysis were HPLC grade (Baker Inc., Phillipsburg, USA). Buffer salts (NaHCO3, Na2HPO4 and CH3COONH4) were purchased from Baker Inc. (Phillipsburg, USA).
Ten (10) parasite-free Corriedale weaned lambs were orally infected with 200 metacercariae of F. hepatica contained in a gelatine capsule. Five animals were infected with a TCBZ-susceptible isolate (named Cullompton), and the other 5 with a TCBZ-resistant isolate (named Sligo). For details of the history of the two isolates, see Robinson et al. (2004). Sixteen weeks after infection the animals were stunned and exanguinated immediately. Animal procedures and management protocols were approved by the Ethics Committee according to Animal Welfare Policy (act 087/02) of the Faculty of Veterinary Medicine, Universidad Nacional del Centro de la Provincia de Buenos Aires (UNCPBA), Tandil, Argentina (http://www.vet.unicen.edu.ar), and to internationally accepted animal welfare guidelines (AVMA, 2001).
Adult F. hepatica specimens (TCBZ-susceptible or -resistant) were recovered from the common bile ducts and the gall-bladder of each lamb. The parasites were rinsed extensively with saline solution (NaCl 0·9%, 37 °C) to remove bile and/or adhering materials.
The collected flukes (susceptible or resistant) were maintained for 2 h before starting the incubation process in a Kreb's Ringer Tris (KRT) buffer (pH 7·4) at 37 °C (McCracken and Lipkowitz, 1990). Fluke specimens (approximately 0·1 g) of each isolate were incubated at 37 °C in KRT buffer (1 ml), spiked with 10 μl of either TCBZ, TCBZSO or TCBZSO2 (previously dissolved in methanol, at a concentration of 500 μM) at a final concentration of 5 nmol.ml−1. Incubation times assayed were 15, 45, 90, 120 and 180 min. For each fluke isolated, there were 4 replicates for each drug at each incubation time. Blank samples containing parasite material and incubation medium without drug (spiked with 10 μl of methanol), and drug-spiked medium without parasite material were incubated during the same time-intervals. Once the incubation time had elapsed, the flukes were rinsed thoroughly with saline solution, blotted on coarse filter paper and stored at −20 °C until their preparation for high-performance liquid chromatography (HPLC) analysis to measure drug concentrations. The parasite material was processed within 15 days after the incubation assays.
Parasite specimens (1 g) of the TCBZ-susceptible or -resistant isolates of F. hepatica were rinsed with cold KCl (1·15%) and then transported to the laboratory in flasks filled with phosphate buffer (0·1 M, pH 7·3) at 4 °C. All subsequent operations were performed between 0 and 4 °C. Parasite material was homogenized in the phosphate buffer using an Ultra-Turrax homogenizer (IKA Works Inc., Wilmington, USA), centrifuged at 10000 g for 20 min and the resulting supernatant was further centrifuged at 100000 g for 60 min. The pellet (microsomal preparation) was suspended in phosphate buffer and stored at −70 °C until used for incubation assays. An aliquot of the microsomal preparation was used to determine protein content using bovine serum albumin as a control standard (Smith et al. 1985). (ii) F. hepatica enzyme assays. The metabolic activity was assessed by the amount of TCBZSO formed in the presence of NADPH. A typical reaction mixture contained: 250 μl of NADPH solution (1 nmol.ml−1) prepared in phosphate buffer (pH 7·3), 100 μl of tissue preparation (3 mg of microsomal protein) and 15 nmol of TCBZ dissolved in 10 μl of methanol. The incubation mixture was adjusted to a final volume of 500 μl with phosphate buffer (0·1 M, pH 7·3). The initial concentration of the incubated substrate (TCBZ) was 40 μM. Incubations (60 min) were carried out at 37 °C in glass vials in an oscillating water bath under aerobic conditions. The drug substrates were also incubated, under the same conditions, either with inactivated (boiled) microsomes or without NADPH. These incubations were used as controls for possible non-enzymatic drug conversion. All reactions were stopped by the addition of 200 μl of ice-cold acetonitrile and stored at −20 °C until analysis.
The in vitro oxidation of TCBZ by F. hepatica was studied after pre-incubation of the microsomal preparation (5 min at 37 °C) with 100 μM of the well known flavin-monooxigenase (FMO) substrate methimazole (MTZ) in the absence of NADPH (Dixit and Roche, 1984). Then, the incubated substrate (TCBZ) was added and the reaction started with the addition of NADPH. MTZ was dissolved in 25 μl of distilled water.
The oxidation of TCBZ was also studied in the presence of 100 μM piperonyl butoxide (PB), a cytochrome P450 inhibitor. Incubation mixtures containing PB were pre-incubated for 5 min at 37 °C without NADPH, followed by the addition of TCBZ or TCBZSO. The reaction started with the addition of NADPH. PB was dissolved in 10 μl of methanol and parallel control tubes contained the same volume of the solvent. Both MTZ and PB were also incubated in the absence of the substrates under the same conditions to ensure that their presence in the incubation mixture did not interfere with the chromatographic determination of TCBZ and its metabolites. All the incubated microsomal mixtures had the same volume (20 μl) of methanol.
HPLC analysis for TCBZ and its metabolites was carried out as previously described (Mottier et al. 2004a).
Drug concentrations are expressed as nmol.g F. hepatica wet weight−1. Drug-free F. hepatica material (0·1 g) was spiked with each target molecule (TCBZ, TCBZSO or TCBZSO2) to reach the following final concentrations: 0·5, 1, 2·5, 5, 10, 20 and 30 nmol.g F. hepatica wet weight−1, and with the internal standard (IS) mebendazole (MBZ) (10 μl, stock solution of 500 μM). Validation of the analytical procedures for extraction and quantification of TCBZ and its metabolites from fluke material was carried out as previously described (Mottier et al. 2004a).
MBZ (IS) (5 nmol dissolved in 20 μl of methanol) was added to inactivated incubation mixtures. Spiked samples (500 μl), fortified with TCBZ and its metabolites, were mixed with 200 μl of cold acetonitrile followed by the addition of the IS. Experimental and fortified samples were mixed with 1·5 ml of ethyl acetate and shaken on a mechanical shaker for 5 min. This extraction/clean-up step was repeated once and the combined ethyl acetate extracts were evaporated using an Automatic Environmental Speed Vac System (Savant, Holbrook, USA). The dry residue was re-dissolved in 300 μl of mobile phase and 50 μl of the solution were injected into the HPLC system (Shimadzu Corporation, Kyoto, Japan). Validation of the analytical procedures for extraction and quantification of TCBZ and metabolites was performed before starting the analysis of the experimental samples from the incubation assays. Known amounts of each analyte (1–60 nmol.ml−1) were added to aliquots of boiled (inactivated) parasite microsomal preparations, extracted and analysed by HPLC (triplicate determinations) to obtain calibration curves and percentages of recovery. Calibration curves were prepared using the least squares linear regression analysis (Instat 3.00, Graph Pad Software, Inc., San Diego, USA) of HPLC peak area ratios of analytes/IS and nominal concentrations of spiked samples. Correlation coefficients (r) for the different analytes ranged between 0·995 and 0·999. A lack-of-fit test was also carried out to confirm the linearity of the regression line of each analyte. The concentrations in the experimental samples were determined following interpolation using the standard curves. Absolute recoveries were established by comparison of the detector responses (peak areas) obtained for spiked microsomal samples and those of direct standards prepared in mobile phase. Drug/metabolite absolute recoveries were 89–92% (TCBZ), 84–98% (TCBZSO) and 91–99% (TCZSO2). Inter-assay precision coefficients of variation (CVs) were <15% and the limits of quantification (nmol.ml−1) were 0·38 (TCBZ), 0·37 (TCBZSO) and 0·39 (TCZSO2).
The determination of parasite protein concentrations was adapted from that described by Smith et al. (1985). The working reagent was prepared with 50 volumes of bicinchoninic acid (Sigma-Aldrich Chemical Company, St Louis, USA) and 1 volume of cupric sulphate (CuSO4) (Baker Inc., Phillipsburg, USA). The bicinchoninic acid complexes with the Cu2+ ion and with the sample proteins, giving a purple colour that can be measured by spectrophotometry. The colour obtained is directly proportional to the sample protein concentration.
Once the concentration values (expressed as nmol. g F. hepatica wet weight−1) for each compound within the target parasites (TCBZ-susceptible or -resistant isolates) were determined for each individual incubation assay, the area under the concentration versus time curve (AUC) was calculated by the trapezoidal rule method (Gibaldi and Perrier, 1982), using the PkSolution 2·0 program (Summit Research Services, Ashland, Ohio, USA). The AUC value (expressed as nmol.min.g F. hepatica wet weight−1) was considered as an indicator of the total drug availability within the liver fluke. The individual concentrations and AUC values are presented as mean±S.D. (4 replicates). The individual concentrations and AUC values obtained for each molecule assayed in each parasite isolate were also compared by Student's t-test (Instat 3.0 Software, Graph Pad Software, San Diego, California, USA) with P<0·05 as the limit of significance. The data of the metabolic assays are expressed as mean±S.D. Metabolic rates of the products formed are expressed in nmol.min−1.mg−1 of microsomal protein. Statistical comparisons were carried out using Student's t-test (Instat 3.0 Software, Graph Pad Software, San Diego, California, USA). A value of P<0·05 was considered statistically significant.
TCBZ, TCBZSO and TCBZSO2 were recovered from both TCBZ-susceptible and -resistant F. hepatica as early as 15 min post-incubation after their ex vivo incubation, demonstrating a fast diffusion of TCBZ/metabolites from the medium to the parasite tissues. The concentrations (mean±S.D.) of TCBZ and TCBZSO, measured in TCBZ-susceptible and -resistant F. hepatica at different times post-incubation are shown in Fig. 2. The diffusion of TCBZSO into the TCBZ-susceptible flukes was significantly greater than that observed for the TCBZ-resistant ones at all the incubation times assayed. Additionally, higher concentrations of TCBZ were measured within the TCBZ-susceptible liver flukes at 45, 90 and 120 min of incubation. Thus, the amounts of both TCBZ and TCBZSO recovered over time were significantly higher within the TCBZ-susceptible compared to the TCBZ-resistant flukes. Total TCBZ and TCBZSO availabilities, expressed as AUC0–180min obtained from the TCBZ-susceptible and -resistant F. hepatica, are compared in Fig. 3. TCBZ and TCBZSO AUC values (1983±121 nmol.min.g−1 and 3046±119 nmol.min.g−1, respectively) obtained for the TCBZ-susceptible flukes were significantly higher than those observed for the TCBZ-resistant ones (1079±110 and 1557±137 nmol.min.g−1 for TCBZ and TCBZSO, respectively). In contrast, TCBZSO2 concentrations measured within the susceptible liver flukes were higher only at 15 and 45 min of incubation. There were no statistically significant differences between the total amount of TCBZSO2 recovered from the TCBZ-susceptible (3791±178 nmol.min.g−1) and the TCBZ-resistant (3486±236 nmol.min.g−1) isolates. Similar total protein contents were measured in both isolates of F. hepatica (between 90 and 94 mg of protein.g−1 parasite).

Fig. 2. Comparative ex vivo diffusion of triclabendazole (TCBZ) (A) and triclabendazole sulphoxide (TCBZSO) (B) in TCBZ-susceptible and -resistant Fasciola hepatica. Results represent mean concentrations (±S.D.) of each analyte measured in the parasite after different incubation times with 4 replicates. *Values are significantly different from those measured in TCBZ-susceptible Fasciola hepatica at P<0·05.

Fig. 3. Comparison of the amount of triclabendazole (TCBZ) and triclabendazole sulphoxide (TCBZSO) measured over 180 min of incubation in TCBZ-susceptible (S) and -resistant (R) Fasciola hepatica incubated with TCBZ or TCBZSO (n=4). Results are expressed as area under the concentration versus time curves (AUC). *Values are significantly different from those measured in TCBZ-susceptible Fasciola hepatica at P<0·05.
The microsomal fraction of F. hepatica was capable of oxidizing TCBZ into its TCBZSO metabolite. TCBZ sulphoxidative activity observed in both TCBZ-susceptible and -resistant F. hepatica is shown in Fig. 4. There was no measurable oxidation activity in control incubations using inactivated (boiled) microsomes or in the absence of NADPH. The sulphoxidative metabolic rate was significantly lower in the TCBZ-susceptible (0·012±0·0003 nmol.min−1.mg−1) compared to the TCBZ-resistant isolate (0·017±0·0006 nmol.min−1.mg−1). In both cases, only trace amounts of TCBZSO2 were recovered in the parasite microsomal incubations.

Fig. 4. Comparative in vitro sulphoxidation of triclabendazole (TCBZ) by the microsomal fraction of TCBZ-susceptible and -resistant isolates of Fasciola hepatica. The results shown concentrations (nmol.mg−1 of microsomal protein) of TCBZ sulphoxide (TCBZSO) formed after 60 min of incubation. Data express the mean±S.D. *Values are significantly different from those measured in TCBZ-susceptible flukes at P<0·05.
FMO inhibition by MTZ reduced TCBZSO production between 34% (TCBZ-susceptible) and 43% (TCBZ-resistant) following TCBZ incubation with liver fluke microsomes (Fig. 5). On the other hand, inhibition of the cytochrome P450 system by PB slightly decreased the sulphoxidation of TCBZ to TCBZSO in microsomes obtained from the TCBZ-susceptible (12%) and -resistant isolate (12%) (Fig. 5). Formation of the sulphone metabolite was not observed after the inhibition of both enzymatic systems.

Fig. 5. Metabolism of triclabendazole (TCBZ) in TCBZ-susceptible (S) and -resistant (R) Fasciola hepatica microsomes. Effects of methimazole (MTZ) (a flavin-monooxigenase (FMO) inhibitor) and piperonyl butoxide (PB) (a cytochrome P450 inhibitor) on the formation of the TCBZ sulphoxide (TCBZSO) metabolite in TCBZ-S and -R flukes. Data express the concentration of TCBZSO formed (nmol.mg−1 of microsomal protein) after 60 min of TCBZ incubation with and without the inhibitor compounds. Values in parentheses express the percentage or reduction of the suphoxidative activity in the presence of the inhibitor substances. Values are significantly different compared to TCBZ alone at (*) P<0·05 and (***) P<0·001. aThe percentage is significantly different from that observed after TCBZ plus MTZ incubation in S Fasciola hepatica at P<0·05.
The present investigation was set out to determine whether differences in the uptake and/or metabolism of TCBZ and its principal sulpho-metabolites occurred between TCBZ-susceptible and -resistant F. hepatica and, if so, whether they might contribute to the development of resistance to TCBZ by the fluke. Following incubation in TCBZ and TCBZSO, significantly higher concentrations of the two compounds were recovered in TCBZ-susceptible flukes. The sulphoxidative metabolism of TCBZ to TCBZSO was significantly higher in TCBZ-resistant flukes. In addition, the FMO system appears to be the main pathway for the metabolism of TCBZ. The results will be discussed in terms of the mechanisms of resistance known to operate in other parasites.
Mechanisms of acquired anthelmintic resistance by parasites can be grouped into two categories, pharmacokinetic-mediated and pharmacodynamic-mediated mechanisms. Pharmacokinetic-mediated mechanisms include processes such as decreased drug uptake, accelerated drug efflux and increased drug inactivation. The consequence of these effects would be to decrease drug accumulation within cells and make it more difficult for the drug to bind to its receptor and trigger an effect. The pharmacodynamic-mediated mechanism(s) involve changes in the receptor molecule (its structure, levels of synthesis, ability to activate downstream elements in pathways leading to a functional response). In this situation, using higher dosages of the drug may have little or no effect on resistant parasites. In contrast, greater possibilities to reverse drug resistance exist if a pharmacokinetic-mediated mechanism is associated with drug resistance. Since drug uptake, efflux or metabolism can be saturated by an excess of substrate (drug), overdosing could result in a recovered deleterious effect on target parasites. However, limitations related to drug toxicity, drug solubility, cost of treatment and environmental impact, for example, would not recommend such a practice under field conditions. Also, higher doses may actually increase the selection pressure for resistant parasites and the risk of magnifying the problem.
The relationship between a trematode parasite such as F. hepatica and its environment involves both the external surface (tegument) and the gastrovascular cavity (gastrodermis), the latter having a single opening that serves as both mouth and anus (Thompson and Geary, 1995). The greater absorption surface area of the tegument probably determines its major relevance in drug diffusion from the surrounding medium. The surface of the fluke is folded into a series of broad plateaux separated by deep valleys. The surface area is further increased by small, flask-shaped intuckings of the apical plasma membrane, known as apical invaginations (Fairweather, Threadgold and Hanna, 1999). The accumulated data show that the main route of acquisition of broad-spectrum anthelmintics by target parasites appears to be through their tegument (cestodes/trematodes) (Alvarez, Sánchez and Lanusse, 1999; Alvarez et al. 2000, 2001) or cuticle (nematodes) (Ho et al. 1990; Sims et al. 1996; Cross, Renz and Trees, 1998; Alvarez et al. 2001). Since the concentration gradient (unpublished observations) and lipid solubility (Mottier et al. 2003) appear to be critical for the penetration of BZD molecules through the external surface of helminths, passive diffusion is probably the main mechanism implicated in the entry of anthelmintic drugs across the trematode tegument. For example, it has been shown that there are differences in the entry of ABZ and albendazole sulphoxide (ABZSO) into F. hepatica: i.e. a higher concentration of the thioether ABZ was measured compared to that for ABZSO (Alvarez et al. 2001).
Total amounts of TCBZ and TCBZSO (estimated as AUC) recovered in the TCBZ-resistant flukes were significantly lower than those observed in the TCBZ-susceptible ones. In fact, significantly (P<0·05) higher concentrations of TCBZSO were obtained at all the incubation times assayed in TCBZ-susceptible F. hepatica. Similarly, higher concentrations of TCBZ parent drug were detected in the TCBZ-susceptible isolate (at 45, 60 and 90 min of incubation) compared to the TCBZ-resistant isolate. These findings will be discussed in relation to the following 3 mechanisms: increased drug efflux in TCBZ-resistant F. hepatica; enhanced oxidative metabolism of TCBZ in TCBZ-resistant flukes and differences in protein content between the two isolates. In relation to the first mechanism, membrane drug transporters participate in efflux of xenobiotics in many vertebrate and invertebrate organisms, as part of a general mechanism of cell protection. In vertebrates, P-glycoprotein (Pgp) is one of the major transmembrane transporters for xenobiotic compounds. Pgp is a member of the ATP-binding cassette (ABC) transporter that functions as an ATP-dependent efflux mechanism that enables substrates to be expelled from cells (Gerlach et al. 1986). Over-expression of Pgp has been implicated in the resistance to macrocyclic lactones (ivermectin, moxidectin) (Pouliot et al. 1997; Xu et al. 1998), closantel and BZDs in nematodes, although the exact nature of the role has yet to be established (Kerboeuf et al. 2003; Wolstenholme et al. 2004). Additionally, an ABC transporter has been identified in F. hepatica (Reed et al. 1998). The relationship between BZD anthelmintics and ABC transporters is controversial. While ABZ was reported to be neither a substrate nor an inhibitor of Pgp (Merino et al. 2002), specific binding between BZD molecules and Pgp has been demonstrated in human cells (Nare et al. 1994). Additionally, verapamil (a Pgp inhibitor) was able to partially reverse BZD resistance in free-living stages of H. contortus (Beugnet, Gauthey and Kerboeuf, 1997). An over-expression of these transporters in the TCBZ-resistant flukes could explain the lower TCBZ and TCBZSO concentrations measured within TCBZ-resistant F. hepatica in the current assays. Although over-expression of ABC transporters may be a possible mechanism of resistance to TCBZ in F. hepatica, further work in the field is required.
The second mechanism to be discussed is the enhancement of the oxidative metabolism of TCBZ in TCBZ-resistant F. hepatica. It has been previously shown that adult liver flukes have the ability to oxidize ABZ into ABZSO and albendazole sulphone (ABZSO2) (Solana, Rodriguez and Lanusse, 2001), supporting the concept that F. hepatica contains the enzymatic capacity to biotransform xenobiotics. Furthermore, the sulphone metabolite of TCBZ has been identified within F. hepatica following incubation in TCBZSO (Robinson et al. 2004). Moreover, the microsomal fraction from adult flukes generated TCBZSO (main product) and TCBZSO2 following incubation with TCBZ parent drug (Mottier et al. 2004b). In the current work, marked differences in the capacity of F. hepatica to oxidize TCBZ into the sulphoxide metabolite have been observed between the TCBZ-susceptible and -resistant strains. That is, the sulphoxidative activity of the TCBZ-resistant parasites was significantly higher compared to the TCBZ-susceptible ones. These results could explain the lower concentrations of TCBZ measured in the TCBZ-resistant flukes. On the other hand, the observed differences in TCBZSO concentrations between the TCBZ-susceptible and -resistant flukes could be related to a greater sulphonating capacity of the resistant isolate, however, this metabolic pathway needs to be further explored. Available data demonstrates that TCBZ parent drug and TCBZSO may be responsible for the flukicidal activity against F. hepatica (Fairweather, 2005). The further oxidized sulphone metabolite (Büscher et al. 1999), as well as the different TCBZ-hydroxy metabolites, may be much less pharmacologically active or inactive. For instance, the sulphone metabolites of the nematodicidal BZD anthelmintics (ABZ and fenbendazole) have been shown to have an extremely low capacity to bind to nematode tubulin (Lubega and Prichard, 1991c). It is likely that further oxidation in the TCBZ metabolic pathway may correlate with a reduced pharmacological potency. Thus, the enhanced oxidative capacity to biotransform TCBZ into its sulphoxide metabolite, observed in the TCBZ-resistant flukes in the current assays, may form the basis for a resistance mechanism to elude the flukicidal effect of TCBZ. In fact, the differences in TCBZ sulphoxidation observed between the TCBZ-susceptible and -resistant flukes were statistically significant, with the metabolic capacity of the TCBZ-resistant isolate being approximately 39% higher compared with that of the TCBZ-susceptible one. However, this difference by itself probably is not enough to fully explain the high level of resistance displayed by the Sligo isolate under field conditions (Coles and Stafford, 2001). Therefore, it is likely that additional mechanism(s) of drug resistance, besides the high metabolic activity observed in the TCBZ-resistant flukes, may be involved in the high degree of resistance to TCBZ shown by F. hepatica.
Xenobiotic metabolism in helminth parasites seems to be predominantly reductive and hydrolytic (Douch and Buchanan, 1979). However, the microsomal and cytosolic fractions of F. hepatica showed higher sulphoxidative capacity than that observed for other helminth parasites (Solana et al. 2001). Both TCBZ-susceptible and -resistant F. hepatica demonstrated the ability to oxidize TCBZ into TCBZSO, but the metabolic rate was lower than that observed for ABZ sulphoxidation by the fluke (Solana et al. 2001). Moreover, since the addition of NADPH to the incubation mixture did not increase the sulphoxidation of ABZ (Solana et al. 2001), but clearly increased the formation of TCBZSO from TCBZ (Mottier et al. 2004b), different metabolic pathways for the two BZD compounds would be indicated. It has been demonstrated that FMO is primarily involved in ABZ hepatic sulphoxidation in sheep (Galtier, Alvinerie and Delatour, 1986; Lanusse and Prichard, 1993) and cattle (Lanusse and Prichard, 1993). The involvement of both FMO and cytochrome P450 enzyme systems in TCBZ sulphoxidation and TCBZSO sulphonation in sheep liver microsomes has been described (Mottier et al. 2004b). Both MTZ and PB pre-incubations are a useful tool to assess the relative contribution of FMO and cytochrome P450, respectively, to the oxidation of a given substrate. In the current work, F. hepatica FMO system was inhibited by MTZ pre-incubation (Dixit and Roche, 1984) whilst PB inhibited the sulphoxidation of TCBZ mediated by cytochrome P450. However, it is clear that the contribution of FMO and cytochrome P450 do not fully explain the total amount of TCBZSO recovered from the incubation mixtures containing TCBZ.
The final mechanism to be discussed concerns differences in protein content between the two isolates of F. hepatica. In contrast to most of the BZD anthelmintics, TCBZ binds strongly to plasma proteins. This non-specific protein binding could account for the differences in colchicine binding (a molecule that binds specifically to microtubular protein) to F. hepatica extracts. Fetterer (1986) examined the interaction between TCBZ and cell-free extracts of adult F. hepatica by assessing the ability of the compound to inhibit the binding of colchicine, and showed that TCBZ failed to inhibit colchicine binding to tubulin in homogenized flukes. However, Bennett and Köhler (1987) described an inhibition of colchicine binding in purified fluke tubulin that resulted in disruption of different microtubule-based secretory processes. A non-specific binding of TCBZ to fluke proteins other than tubulin has been proposed as a cause of these contradictory results. The non-specific protein binding of TCBZ would strongly reduce the amount of drug available to bind to microtubular protein, explaining the negative results earlier reported by Fetterer (1986). A similar mechanism could be used to explain the present results, less TCBZ and TCBZSO being available to bind the target receptor, thereby reducing the therapeutic effect. However, although this non-specific protein binding may also account for the differences in TCBZ and TCBZSO recovered within the TCBZ-susceptible and -resistant flukes, similar total protein contents were measured in both isolates of F. hepatica (between 90 and 94 mg of protein/g parasite).
From the results presented here, it is evident that TCBZ-resistant F. hepatica are exposed to lower concentrations of TCBZ and TCBZSO compared to the TCBZ-susceptible ones. These differences may be by different mechanisms but, most likely, are related to an increased capacity to oxidise TCBZ and TCBZSO to an inert form in the TCBZ-resistant isolate. The lower concentrations of TCBZ and TCBZSO measured in the ex vivo experiments and the higher rate of metabolism observed in the TCBZ-resistant specimens are important findings towards understanding of the mechanisms of TCBZ resistance in F. hepatica. Although the outcome of the work given here is insufficient to fully comprehend resistance to TCBZ in liver flukes, it is an encouraging pharmacological contribution which should be followed by deeper molecular research.
The authors would like to acknowledge Dr Gottfried Büscher, from Novartis Animal Health Inc., Basel, Switzerland, who kindly provided TCBZ, TCBZSO and TCBZSO2 pure reference standards. Lourdes Mottier is a recipient of a fellowship from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina. This research was partially supported by the Agencia Nacional de Promoción Científica y Tecnológica (PICT 08-07277), Fundación Antorchas, Universidad Nacional del Centro de la Provincia de Buenos Aires (UNCPBA) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (all from Argentina).

Fig. 1. Chemical structures of triclabendazole and its sulphoxide and sulphone metabolites.

Fig. 2. Comparative ex vivo diffusion of triclabendazole (TCBZ) (A) and triclabendazole sulphoxide (TCBZSO) (B) in TCBZ-susceptible and -resistant Fasciola hepatica. Results represent mean concentrations (±S.D.) of each analyte measured in the parasite after different incubation times with 4 replicates. *Values are significantly different from those measured in TCBZ-susceptible Fasciola hepatica at P<0·05.

Fig. 3. Comparison of the amount of triclabendazole (TCBZ) and triclabendazole sulphoxide (TCBZSO) measured over 180 min of incubation in TCBZ-susceptible (S) and -resistant (R) Fasciola hepatica incubated with TCBZ or TCBZSO (n=4). Results are expressed as area under the concentration versus time curves (AUC). *Values are significantly different from those measured in TCBZ-susceptible Fasciola hepatica at P<0·05.

Fig. 4. Comparative in vitro sulphoxidation of triclabendazole (TCBZ) by the microsomal fraction of TCBZ-susceptible and -resistant isolates of Fasciola hepatica. The results shown concentrations (nmol.mg−1 of microsomal protein) of TCBZ sulphoxide (TCBZSO) formed after 60 min of incubation. Data express the mean±S.D. *Values are significantly different from those measured in TCBZ-susceptible flukes at P<0·05.
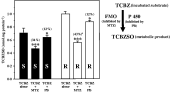
Fig. 5. Metabolism of triclabendazole (TCBZ) in TCBZ-susceptible (S) and -resistant (R) Fasciola hepatica microsomes. Effects of methimazole (MTZ) (a flavin-monooxigenase (FMO) inhibitor) and piperonyl butoxide (PB) (a cytochrome P450 inhibitor) on the formation of the TCBZ sulphoxide (TCBZSO) metabolite in TCBZ-S and -R flukes. Data express the concentration of TCBZSO formed (nmol.mg−1 of microsomal protein) after 60 min of TCBZ incubation with and without the inhibitor compounds. Values in parentheses express the percentage or reduction of the suphoxidative activity in the presence of the inhibitor substances. Values are significantly different compared to TCBZ alone at (*) P<0·05 and (***) P<0·001. aThe percentage is significantly different from that observed after TCBZ plus MTZ incubation in S Fasciola hepatica at P<0·05.