Published online by Cambridge University Press: 19 January 2004
This investigation quantifies some aspects of the parasite–host relationship between the digenean Microphallus piriformes and its intermediate host Littorina saxatilis, the rough periwinkle. M. piriformes has an abridged life-cycle with no free-living stages, metacercariae remain within host viscera. Noticeable differences in shell shape of parasitized and uninfected periwinkles were investigated. These differences in shell shape were defined by growth parameters of height, diameter and β angle. The relationship between these parameters was examined together with their impact on parasite reproduction. All 3 shape parameters were altered in periwinkles infected by M. piriformes. The alteration in β angle and height increased the available volume for parasites in the shell spire by about 12%. As metacercarial production per sporocyst has been shown to depend on host size, the increased volume enables considerable additional life-time reproduction by the parasite, of approximately 550–850 additional metacercariae in hosts of the usual size range. The form of gigantism found in this study is discussed in relation to previous concepts. It is suggested that gigantism in permanently castrated hosts is adaptive parasite manipulation of host physiology, favoured in parasites with abbreviated life-cycles, when host viability increases parasite transmission, and when an initially small host individual is infected.
During a 3-year study of Microphallus piriformes and its marine gastropod intermediate host, the rough periwinkle Littorina saxatilis, it was noticed that the shell shape (but not overall shell height) of parasitized periwinkles differed perceptibly from those not infected with this parasite. This paper describes the pattern of altered host shell growth and estimates its likely impact on parasite reproduction within the modified host, interpreting the changes as an aspect of gigantism. There have been many debates over gigantism in molluscs infected by trematodes, but no universal agreement. Many examples of trematode-induced gigantism have been recorded (Rothschild & Rothschild, 1939; McClelland & Bourns, 1969; Mouritsen & Jensen, 1994) but other workers have observed stunting of growth (Sturrock & Sturrock, 1970; Sousa, 1983; Crews & Yoshing, 1989). Fernandez & Esch (1991) found no indication of change of growth in Helisoma anceps following parasitic castration by trematode larvae. Gorbushin (1997) found that, in the mud snail Hydrobia ulvae, parasites in the families Microphallidae and Heterophidae caused gigantic growth whereas Notocotylid and Bunocotylid trematodes had no effect on size achieved.
Gigantism is frequently interpreted in terms of the redistribution of the resources made available by parasitic castration – itself a host manipulation of adaptive significance to the parasite (Baudoin, 1975). Resources that the infected host would have allocated to reproduction are free for reallocation to host maintenance and growth, or diversion into parasite growth and reproduction. The magnitude of the pool of reallocatable resources may depend on the life-history of the host (Sousa, 1983) and food availability (Mouritsen & Jensen, 1994). Whether or not increased host growth occurs would be expected to vary according to both the reallocatable resource pool and the outcome of host–parasite competition for its utilization. Thus, within the same host species, different species of parasites may have different effects on host growth (Gorbushin, 1997), presumably by diverting varying proportions of the resource pool into themselves, and the effects of a particular parasite on host growth may differ between populations of that host (Mouritsen, Gorbushin & Jensen, 1999). In the special case where parasite castration is reversible, gigantism may be an adaptive strategy of the host, prolonging its life and potential for post-infection reproduction (Minchella, 1985). Here, a selective advantage of gigantism for the host is present, whereas in permanently castrated hosts there can be no further selection on host mechanisms of response or resistance to the castrating parasite. Changes occurring in the host are therefore either under selection by the parasite, by-products of other selected features of the parasite's mode of host utilization, or stem from general host responses selected for by other agents of damage or disease.
In addition to conflicts over the utilization of resources, hosts and their parasites have conflicts over the utilization of space within the host's body. This conflict is particularly acute in hosts anatomically confined by an exoskeleton or shell. Increased growth of a parasitized host could therefore facilitate the spatial integration of host and parasite by providing additional volume for accommodation of the parasite, without (or with less) reduction of host organs (Theron, 1985; Probst & Kube, 1999). Envisaged in this context, gigantism may be beneficial to host (and hence parasite) viability and enable increased parasite reproduction. An increase in the volume of mainly the shell-constrained part of the host occupied by the parasite would be predicted from this concept, rather than a general increase in total host size. Increase in the volume of only the parasite-utilized parts of the host would minimize the allocation of resources to host growth necessary to achieve optimal spatial integration of this parasite.
In this study we investigated the parasite–host relationship between the microphallid digenean trematode fluke Microphallus piriformes and its intermediate host Littorina saxatilis, the rough periwinkle, an intertidal gastropod. M. piriformes is part of the ‘pygmaeus’ group of parasites that is predominantly found in arctic regions. M. piriformes does not have any free-living larval stages (either cercarial and/or miracidial) and development to transmissible unencysted metacercariae occurs within L. saxatilis.
M. piriformes sporocysts occupy the periwinkle's gonads, and in mature infections, metacercariae-containing sporocysts are found throughout the digestive gland too. Parasitic castration appears to be complete, through early destruction of the gonad. No infected L. saxatilis, even with immature infections, were found with broods during 3 years of sampling (N=774 infected L. saxatilis). L. saxatilis is ovoviviparous, i.e. it broods its eggs through their development into young periwinkles in a brood pouch; in the study population reproduction occurs throughout the year.
Collection of monthly samples from Muck Island, Co. Antrim, N. Ireland gave rise to the observation that some L. saxatilis specimens had narrow and elongated shells. The surface of the gastropod shell is generated by a revolution around a fixed axis of a closed curve that is always geometrically similar to itself and increases its dimensions continually (D'Arcy Thompson, 1961). In most gastropods such as L. saxatilis, growth follows a skew pattern in relation to the axis of revolution and the curve in space generated by any given point makes a constant angle to the axis of the enveloping cone. The curve is similar to a helix structure as well as a logarithmic spiral and is therefore known as a helicon-spiral (D'Arcy Thompson, 1961). The β angle can be measured and is the enveloping angle of the cone formed from tangents to the whorls on either side of the shell axis. Those gastropods with a smaller β angle tend to have much narrower spirals and a thinner shell (D'Arcy Thompson, 1961).
The purpose of this study was to determine the relationship between the growth parameters of height, diameter and β angle for infected and uninfected L. saxatilis and to ascertain the extent to which altered shell growth might influence parasite reproduction. The implications for our understanding of the phenomenon of gigantism are discussed.
The L. saxatilis used for this study were a subsample of the monthly samples collected randomly from Muck Island shore during the summer months of 1999 and 2000 when M. piriformes prevalence was highest (McCarthy, Fitzpatrick & Irwin, 2000).
L. saxatilis were measured in the laboratory using vernier callipers (precision±0·1 mm). The measurement of the overall shell height was taken from the base of the aperture to the top of the spire. The diameter was at the widest part of the shell (Fig. 1A). The β angle was also measured (Fig. 1B) using a custom-built instrument consisting of 2 flat pieces of metal (each 8·1×0·7 cm) hinged together to allow movement to fit on either side of the periwinkle's uppermost whorl. The angle between the 2 metal sides was measured with a protractor, estimated accuracy±1° (3 measurements, averaged).

Fig. 1. (A) Measurements of height and diameter of Littorina saxatilis. (B) β angle measurement of L. saxatilis. (C) The typical shell shape of uninfected L. saxatilis. (D) The typical shell shape of L. saxatilis infected with Microphallus piriformes.
The shell of L. saxatilis was cracked using forceps and the periwinkle soft tissue was removed. Using mounted needles the reproductive tissue and digestive gland were teased apart to ascertain the presence/absence of periwinkle brood or trematode presence. M. piriformes were identified using the descriptions of Saville et al. (1997).
Preliminary analysis of uninfected periwinkles indicated that the relationship between overall shell height and diameter was allometric. Log transformation linearized their relationship, so log shell height and log diameter were used in all analyses. β angle did not require transformation. Log shell height, log diameter and β angle will be referred to as shape parameters.
The effects of infection on periwinkle growth and shape were investigated by constructing a series of generalized linear models (using JMP SAS Institute, 1995), with each shape parameter in turn as the dependent (y) variable and with x variables being infection status (infected or uninfected, categorized variable), the remaining 2 shape parameters (continuous variables), and interactions between these. The initial model was fully factorial with all interactions included. Non-significant interactions (P>0·05) were removed by a backwards-stepwise procedure until only significant factors remained in the model.
The whorls of L. saxatilis were approximated by the shape of a cone the base of which equalled the maximum diameter of the periwinkle shell, and of vertical height (h) (i.e. perpendicular to this base) calculated as this radius divided by the tangent of half the β angle. Note that h is not the measured overall shell height, as direct measurement is impractical.
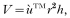
where V is the volume, r the radius and h the vertical height. Volumes were calculated for typical values of β angle and diameter for infected and uninfected periwinkles, using regression equations resulting from the analysis of periwinkle shape (shown in Fig. 4).
The previously described relationships between M. piriformes reproduction within its host and the size of the host (McCarthy, Fitzpatrick & Irwin, 2002) were used to calculate the increased metacercariae production per sporocyst expected from the observed alteration in shell shape. The number of metacercariae per sporocyst increased linearly with host shell height (see Fig. 4 of McCarthy et al. 2002) with a steep slope such that a 1 mm increment in shell height produces an average additional 11·18 metacercariae per sporocyst.
The generalized linear models of the effects of infection on the 3 shell shape parameters are shown in Table 1. Log diameter was significantly related to shell height, β angle and infection. Log shell height was significantly related to log diameter, β angle, infection and the infection*log diameter interaction. β angle was significantly related to log diameter, log shell height and infection (Table 1). Infection therefore had an influence on all 3 shape parameters after the effects of the other shape parameters were statistically controlled. The directions of these effects are illustrated in Figs 2–4.

Fig. 2. The relationship between log diameter and log height for Microphallus piriformes infected (n=60) and uninfected (n=144) Littorina saxatilis sampled from Muck Island. The lines of best fit are computer generated.

Fig. 3. The relationship between β angle and log height for Microphallus piriformes infected (n=60) and uninfected (n=144) Littorina saxatilis sampled from Muck Island. The lines of best fit are computer generated.

Fig. 4. The relationship between log diameter and β angle for Microphallus piriformes infected (n=60) and uninfected (n=144) Littorina saxatilis sampled from Muck Island. The lines of best fit are computer generated.

Fig. 2 is a plot of log shell height against log diameter for infected and uninfected periwinkles. The steeper linear slope of infected L. saxatilis shows that they were taller for their diameter than uninfected periwinkles. Fig. 3, log height plotted against β angle, shows that infected L. saxatilis had a steeper slope in this relationship than uninfected. On closer examination the individual points show that infected L. saxatilis were distributed at the lower end of the β angle scale, uninfected periwinkles therefore tended to have larger β angles. Uninfected periwinkles with a smaller β angle tended to be smaller in shell height than infected periwinkles with the same β angle. Fig. 4, β angle plotted against log diameter, shows that the regression slopes for infected and uninfected periwinkles were nearly parallel, but their intercepts were different indicating that infected L. saxatilis had a smaller β angle at all diameters than uninfected.
It is clear that infection with M. piriformes affected all the periwinkle growth shape parameters but not by simply increasing or decreasing size uniformly. The shape was altered, and infected L. saxatilis were affected differently in all 3 parameters. We also made the incidental observation, while cracking L. saxatilis for dissection, that the shells of altered shape were thinner and cracked more easily than those with the normal morphology. We did not, however, quantify this effect.
The alteration of β angle shown in typical parasitized periwinkles produced increases in both vertical height (h) and volume of about 12% compared to those of uninfected periwinkles of the same shell diameter (Table 2).

The altered shape of the shell of typical periwinkles infected with M. piriformes produced an expected increase in metacercarial production per sporocyst compared to the unaltered shape (Table 2). The shape-related increment in parasite reproduction itself increased with shell diameter (Table 2).
The change in shell shape associated with infection is informative about the relationship between L. saxatilis and M. piriformes. There are 2 possible causal directions underlying this correlation: either L. saxatilis with a small β angle growth form are more susceptible to infection by M. piriformes, or else infection with this parasite influenced the subsequent growth of its host. We reject the first possibility because this small β angle growth form was not present in other nearby populations of L. saxatilis; at sites with very low overall parasite prevalence and at sites with a high prevalence of other parasites, all periwinkles had the shell shape associated with uninfected L. saxatilis at the Muck Island site. The elongated spire shape was thus only found at the site associated with high M. piriformes prevalence (McCarthy et al. 2000).
We therefore conclude that infected L. saxatilis grew differently in shell shape for the measured parameters of shell height, diameter and β angle, (Fig. 1A–D). Infected L. saxatilis had a smaller β angle, and growth around the spiral axis must have been altered at a young age because the top whorls, those first grown by the periwinkle, were elongated. Hence the smaller β angle of infected periwinkles produced a taller shell with a greater volume than that of uninfected periwinkles of the same diameter. However, there was a scatter of points and a low r2 when the β angle and log height were plotted, indicating that not all infected L. saxatilis had an extremely altered shell shape. The dynamics of gastropod growth suggest that the degree of alteration in shape will be greatest in L. saxatilis infected when juvenile and will diminish in periwinkles infected when adult. This altered growth indicates that infection of L. saxatilis by M. piriformes can occur at any age, and thus may differ from the post-reproductive susceptibility to infection of Littorina littorea by its common trematode parasites (Hughes & Answer, 1982). In the Muck Island samples L. saxatilis were infected with M. piriformes from a size class of 4·4–4·6 mm up to 15·2–15·4 mm and showed a normal distribution with no statistical difference between infected and uninfected L. saxatilis size distributions (Kolmogorov–Smirnov test, z=0·846, P=0·40) (McCarthy, 2000). Further work is needed to clarify the infection dynamics of this parasite.
The change in shell shape observed in L. saxatilis, especially those presumably infected as juveniles, may represent a form of gigantism. However, the age-dependence of this shape alteration differs from the age-related changes associated with gigantism proposed by Sousa (1983). He argued that those snails infected during their pre-reproductive stages are stunted or grow at the same rate until they reach reproductive age. In contrast, those snails infected at a reproductive age display enhanced growth compared to those uninfected, because resources have been allocated to somatic growth rather than reproduction (Sousa, 1983). Our results clearly do not support such mechanisms of gigantism based solely on reallocation of resources that would otherwise have been allocated to host reproduction.
It is likely that the changes observed in periwinkles infected with M. piriformes are related specifically to this parasite–host combination. Changes in shell shape were not observed in periwinkles infected with the other species of trematode on Muck Island or elsewhere (McCarthy et al. 2000).
M. piriformes establishes itself as sporocysts within the tissues of the digestive gland and gonad of L. saxatilis and develops to metacercarial stages in the periwinkle. The presence of M. piriformes metacercariae retained in the periwinkle host may do more damage to the host than daughter sporocysts alone. However, Probst & Kube (1999) demonstrated that retained metacercariae in Hydrobia ventrosa tissues caused the same histopathological abnormalities as daughter sporocysts; the only difference they observed was that the digestive gland was compressed due to the spatial requirements of the metacercariae. M. piriformes sacrifices its daughter sporocyst wall in order to meet the nutritional requirements of the higher metabolic rate of the metacercariae (Popiel & James, 1978). Production of metacercariae in M. piriformes has been shown to be less than cercarial production in 2 species of cercariae-releasing microphallids also using L. saxatilis as an intermediate host (McCarthy et al. 2002). Parasite demands on the host thus seem to be lessened in M. piriformes, suggesting low pathogenicity – presumably selected because of the importance of host viability for transmission of this parasite.
The growth effects observed in the relationship between M. piriformes and L. saxatilis may not be an expression of gigantism, but one of spatial integration in which the volume of parasites is accommodated within the periwinkle. As highlighted by Probst & Kube (1999) spatial integration has 2 patterns: integration by substitution in which the volume of parasites occupies a space normally used for host reproduction, or integration by addition in which the volume of the parasite is added to that of the host organs. There may be a greater spatial requirement in the two-host life-cycle of M. piriformes because there is no cercarial release that would reduce the parasitic volume inside the host. Other microphallid trematodes with abbreviated life-cycles, M. pirum and M. pseudopygmaeus which infect and produce metacercariae in Hydrobia ulvae, and Onoba aculeus respectively, produce gigantism in their hosts (Gorbushin, 1997; Gorbushin & Levakin, 1999) with H. ulvae growth rates greater than those infected with cercariae-releasing microphallids (Gorbushin, 1997).
The increase in volume inside the whorls of the shape-changed host shell therefore has a particular advantage for those microphallids with abbreviated life-cycles, by enabling greater parasite reproduction to be accommodated without further constriction of host tissue. Our calculations, based on parasite production data already published (McCarthy et al. 2002), provide a quantitative estimate of the adaptive value of a parasite-related change in the growth of its host. Total number of sporocysts was related to the size of host in M. piriformes (McCarthy et al. 2002) and averaged 108. The shape change is thus, on average, associated with additional production (in round figures) of an estimated 550–850 additional metacercariae in hosts of 5–8 mm shell diameter. The growth changes are therefore advantageous to the parasite and, in this host with permanent parasitic castration, must be interpreted as manipulation of host physiology and hence growth by M. piriformes, with the manipulation probably ensuing from an early phase of parasitic infection and having greatest effect on juvenile hosts.
M. piriformes also alters host behaviour (McCarthy et al. 2000): hosts (with mature infections only) climb higher on intertidal rocks and remain there, thereby becoming more vulnerable to predation by gull definitive hosts. The further manipulations presented here may have additional implications for this transmission: larger-spired periwinkles might be perceived as more profitable prey by the gulls, and their thinner shells may enhance prey processing by the definitive host, either externally by cracking in the beak, or internally during digestion of prey or parts swallowed within the shell.
In conclusion, we suggest that gigantism in permanently castrated hosts should be construed as the outcome of parasite manipulation of host physiology for its own reproduction, within the constraints posed by resource availability, within-host spatial integration, and the time-scale of host viability necessary for parasitic transmission. The parasite may be viewed as optimizing its spatial environment by at least initially allowing its host to allocate its freed resources to particular forms of growth, producing space for subsequent occupation by parasite biomass. Gigantism – any form of increased host growth – may be favoured (1) in parasites with abbreviated life-cycles and no cercarial release, (2) when a small host individual is infected, (3) when host longevity increases parasite reproduction and/or transmission. On the other hand, parasite appropriation of most host resources for its own (rather than host) growth may be favoured when (1) the host is already large and offers sufficient space, (2) rapid parasite reproduction is advantageous, (3) host longevity is not particularly advantageous to the parasite. The changes in host shape demonstrated here indicate a subtlety in the growth effects of parasites on their hosts. Greater sophistication in the measurement of hosts and analysis of their growth is clearly needed in future studies.

Fig. 1. (A) Measurements of height and diameter of Littorina saxatilis. (B) β angle measurement of L. saxatilis. (C) The typical shell shape of uninfected L. saxatilis. (D) The typical shell shape of L. saxatilis infected with Microphallus piriformes.
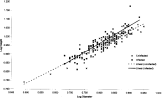
Fig. 2. The relationship between log diameter and log height for Microphallus piriformes infected (n=60) and uninfected (n=144) Littorina saxatilis sampled from Muck Island. The lines of best fit are computer generated.
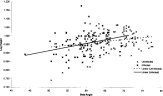
Fig. 3. The relationship between β angle and log height for Microphallus piriformes infected (n=60) and uninfected (n=144) Littorina saxatilis sampled from Muck Island. The lines of best fit are computer generated.

Fig. 4. The relationship between log diameter and β angle for Microphallus piriformes infected (n=60) and uninfected (n=144) Littorina saxatilis sampled from Muck Island. The lines of best fit are computer generated.

Table 1. Results of linear regression models to examine the relationship between log diameter, log shell height, β angle and the infection status of Littorina saxatilis with 1 degree of freedom

Table 2. Relationship between β angle, vertical height (h) and volume of periwinkles and the resultant increase in Microphallus piriformes in Littorina saxatilis