Introduction
Various psychological elements, including emotional, cognitive, attentional, and behavioral aspects have been reported to be important risk factors for chronicity of pain (Simons et al. Reference Simons, Elman and Borsook2014), and neuroimaging studies have examined the mechanisms underlying chronic pain states from various brain-based functional, electrophysiological, and cognitive–emotional abnormality viewpoints (Yoshino et al. Reference Yoshino, Okamoto, Yoshimura, Shishida, Toki and Doi2013; Yoshino et al. Reference Yoshino, Okamoto, Kunisato, Yoshimura, Jinnin and Hayashi2014; Kregel et al. Reference Kregel, Meeus, Malfliet, Dolphens, Danneels and Nijs2015). In terms of psychological interventions for chronic pain, cognitive–behavioral therapy (CBT) is reported to be useful, and is designed to modify maladaptive perceptual and behavioral patterns and to teach self-control techniques for pain management (Williams et al. Reference Williams, Eccleston and Morley2012; Yoshino et al. Reference Yoshino, Okamoto, Horikoshi, Oshita, Nakamura and Otsuru2015). However, to our knowledge, there are only a few neuroimaging studies that have investigated brain mechanisms modulated by CBT for patients with chronic pain. For example, one study has documented that CBT for chronic pain increased activations in the ventrolateral prefrontal cortex (VLPFC) and lateral orbitofrontal cortex (OFC) during pressure-evoked pain (Jensen et al. Reference Jensen, Kosek, Wicksell, Kemani, Olsson and Merle2012). Seminowicz et al. have investigated gray matter (GM) changes after CBT in patients with chronic pain, and after CBT, chronic pain patients show increased GM in various pain-matrix regions including the OFC, and also show decreased GM in the supplementary motor area (Seminowicz et al. Reference Seminowicz, Shpaner, Keaser, Krauthamer, Mantegna and Dumas2013).
Recent evidence indicates that resting-state functional magnetic resonance imaging (R-fMRI) and intrinsic connectivity network (ICN) are useful for investigating human cognitions, behaviors, emotions, and somatic sensations (Shpaner et al. Reference Shpaner, Kelly, Lieberman, Perelman, Davis and Keefe2014; Simons et al. Reference Simons, Elman and Borsook2014; Yoshino et al. Reference Yoshino, Okamoto, Kunisato, Yoshimura, Jinnin and Hayashi2014; Kregel et al. Reference Kregel, Meeus, Malfliet, Dolphens, Danneels and Nijs2015; Wu et al. Reference Wu, Tu, Chao, Li, Low and Chuang2016). This approach should therefore be useful in chronic pain research. The ICNs robustly correspond with the representation of underlying structural connectivity (Fox & Raichle, Reference Fox and Raichle2007; Honey et al. Reference Honey, Sporns, Cammoun, Gigandet, Thiran and Meuli2009). Therefore, we used the independent component analysis (ICA) methods to examine ICN differences between pre- and post-treatment. Most R-fMRI studies in chronic pain have shown changes within the default mode network (DMN) and sensorimotor network (SMN) (Shpaner et al. Reference Shpaner, Kelly, Lieberman, Perelman, Davis and Keefe2014; Yoshino et al. Reference Yoshino, Okamoto, Kunisato, Yoshimura, Jinnin and Hayashi2014; Kregel et al. Reference Kregel, Meeus, Malfliet, Dolphens, Danneels and Nijs2015). For example, Shpaner et al. have compared pre–post differences in seed-based connectivity during resting conditions between CBT and educational control groups in chronic pain patients (Shpaner et al. Reference Shpaner, Kelly, Lieberman, Perelman, Davis and Keefe2014). They found CBT-specific connectivity between the DMN and brain regions such as the amygdala or secondary somatosensory cortex, and compared with control patients, CBT decreased functional connectivity between the DMN and the amygdala or periaqueductal gray, while increasing functional connectivity between the basal ganglia network and the right secondary somatosensory cortex. Dhond et al. also evaluated changes of the DMN and SMN to investigate the effects of acupuncture for pain perception (Dhond et al. Reference Dhond, Yeh, Park, Kettner and Napadow2008). Furthermore, many CBT studies have underscored the importance of prefrontal cortex functioning in treatment effects, including the dorsolateral prefrontal cortex (DLPFC), VLPFC, and OFC (Jensen et al. Reference Jensen, Kosek, Wicksell, Kemani, Olsson and Merle2012; Seminowicz et al. Reference Seminowicz, Shpaner, Keaser, Krauthamer, Mantegna and Dumas2013; Shpaner et al. Reference Shpaner, Kelly, Lieberman, Perelman, Davis and Keefe2014; Crowther et al. Reference Crowther, Smoski, Minkel, Moore, Gibbs and Petty2015). These regions have been identified as parts of the central executive network (CEN). The CEN adjusts self-referential awareness with attention to sensory information, mediates cognitive control processes, and modulates pain perception (Fox et al. Reference Fox, Snyder, Vincent, Corbetta, Van Essen and Raichle2005; Seminowicz & Davis, Reference Seminowicz and Davis2007; Seidel et al. Reference Seidel, Pfabigan, Hahn, Sladky, Grahl and Paul2015), therefore playing an important role in CBT (Jensen et al. Reference Jensen, Kosek, Wicksell, Kemani, Olsson and Merle2012; Seminowicz et al. Reference Seminowicz, Shpaner, Keaser, Krauthamer, Mantegna and Dumas2013; Shpaner et al. Reference Shpaner, Kelly, Lieberman, Perelman, Davis and Keefe2014). Similarly, the dorsal attention network (DAN) is related to goal-directed processing and maintenance or replacement of attention, and is also regarded as an important network in CBT efficacy (Crowther et al. Reference Crowther, Smoski, Minkel, Moore, Gibbs and Petty2015; Mason et al. Reference Mason, Peters and Kumari2016). Although ambiguities remain, these studies demonstrate that the prefrontal cortex is the particularly crucial region, perhaps given its key role in cognitive control processes and attention. Thus, the DMN, SMN, CEN, and DAN are independently proposed as networks that mediate effective CBT treatment. We examined neural changes within these networks, pre- and post-treatment, for patients diagnosed with somatoform pain disorder (APA, 1994). Shpaner et al. primarily investigated the seed-based connectivity between brain networks and brain regions such as the amygdala and no healthy controls were recruited, although a control group of patients was used (Shpaner et al. Reference Shpaner, Kelly, Lieberman, Perelman, Davis and Keefe2014). Therefore, we examined intra-ICN comparisons and set healthy controls. Based on the above findings, we hypothesized that the prefrontal cortex, including the DLPFC, VLPFC, and OFC, would show increased activity in patients with chronic pain after CBT.
Furthermore, CBT post-treatment prediction could enhance access to limited resources for those most likely to gain benefit, helping to allocate patients to the most suitable treatments, in keeping with patient condition (Kumari et al. Reference Kumari, Peters, Fannon, Antonova, Premkumar and Anilkumar2009; Crowther et al. Reference Crowther, Smoski, Minkel, Moore, Gibbs and Petty2015). However, to our knowledge, no neuroimaging studies in chronic pain have addressed this issue. We therefore also examined whether ICNs at baseline are predictive of CBT outcomes. It has been reported that prefrontal cortex functioning is related to predictors of CBT response in psychiatric disorders (Kumari et al. Reference Kumari, Peters, Fannon, Antonova, Premkumar and Anilkumar2009; Crowther et al. Reference Crowther, Smoski, Minkel, Moore, Gibbs and Petty2015), and we hypothesized relationships between ICN connectivity strength in the prefrontal cortex and response to treatment. In this study, we first extracted the brain regions that differed between chronic pain and healthy controls, and then specifically searched these regions for pre–post CBT-related changes. Next, we examined whether these selected regions particular to chronic pain are associated with the likelihood of treatment effects.
Method
Participants
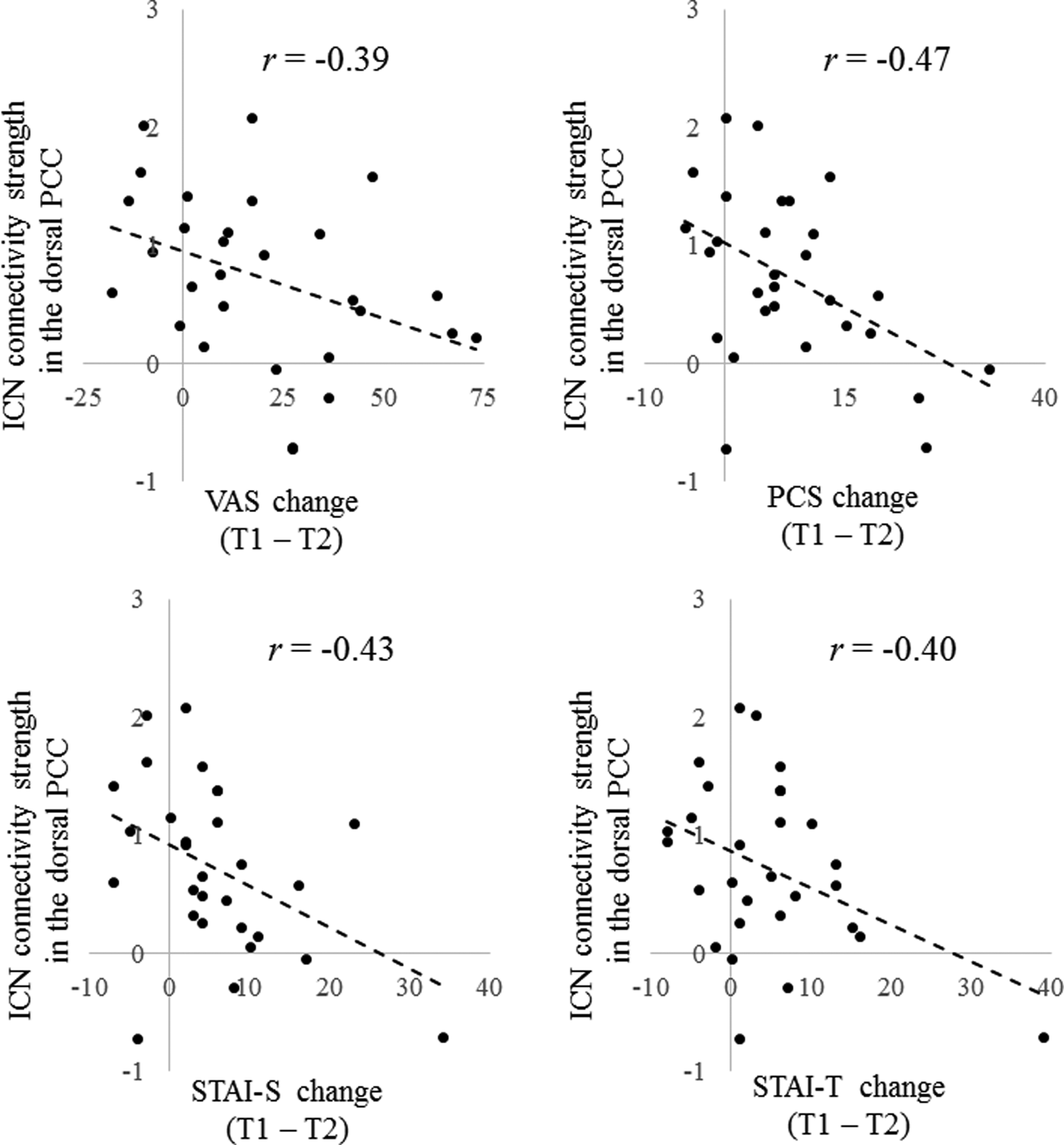
Twenty-nine patients were recruited from the Department of Psychiatry and Neurosciences, Anesthesiology and Critical Care, Orthopedic Surgery or Dental Anesthesiology at the Hiroshima University Hospital. All participants were Japanese and provided their informed written consent to receive treatment and participate in the present study, according to a protocol approved by the ethics committee of the Hiroshima University. We assert that all procedures contributing to this work comply with the ethical standards of the ethics committee of the Hiroshima University and with the Helsinki Declaration of 1975, as revised in 2008. The criterion for inclusion in the study was a diagnosis of somatoform pain disorder as established by a psychiatrist with more than 10 years of experience using the Structured Clinical Interview for DSM-IV (SCID) (First et al. Reference First, Spitzer, Gibbon and Williams2012). Clinical characteristics were assessed and R-fMRI conducted at time 1 (T1; pretreatment) and time 2 (T2; 3 months post-treatment). During MRI scanning, participants were instructed to relax with their eyes closed, without falling asleep. Exclusion criteria included the following: (1) HIV-related pain and cancer pain, because these are associated with malignant diseases, which entail a different symptom trajectory than chronic pain states; (2) difficulty understanding the purpose of study (e.g. presence of dementia, delirium, or psychosis); (3) organic brain disorder (e.g. cerebral hemorrhage, infarction); (4) schizophrenia, bipolar affective disorder, or seizure disorder not adequately controlled by medication or current substance abuse. As indicated in Table 1, all patients were taking medications, but such medications were not changed during the CBT trial. Normal control participants were recruited from a non-clinical population. The control participants endorsed no chronic pain problems and had no history of psychiatric disorders. The controls were assessed at one point in time.
Table 1. Demographic and psychometric variables of patients and controls
BDI-II, Beck Depression Inventory – Second Edition; STAI-S, State-Trait-Anxiety Inventory – State; STAI-T, State-Trait-Anxiety Inventory – Trait; PCS, Pain Catastrophizing Scale; VAS, Visual Analogue Scale; SF-MPQ, Short-Form McGill Pain Questionnaire; T1, Pretreatment; T2, Post-treatment
All variables except gender are presented as the mean ± standard deviation
Clinical assessments
Pain characteristics
The Short-Form McGill Pain Questionnaire (SF-MPQ) was used to assess pain characteristics (Melzack, Reference Melzack1987). The SF-MPQ also includes the Present Pain Intensity index and a visual analog scale (VAS). In addition, the Pain Catastrophizing Scale (PCS) was used (Sullivan et al. Reference Sullivan, Bishop and Pivik1995). The PCS is a 13-item self-report inventory designed to assess the extent to which a person engages in catastrophic thinking in response to pain stimuli.
Psychometric evaluation
The Beck Depression Inventory-Second Edition (BDI-II) was used to measure depressive symptoms (Beck et al. Reference Beck, Brown and Steer1996). The State-Trait-Anxiety Inventory (STAI) was also administered (Spielberger, Reference Spielberger1983).
Treatment procedures
Group CBT was conducted for 12 weekly 90-min sessions. The treatment was manual-based and conducted by one or two psychiatrists with more than 10 years of experience in general clinical practice and more than 8 years of experience in CBT practice. The program was developed over about 2 years through preliminary trials and under the supervision of an experienced clinical psychologist with more than 10 years’ practice experience in the USA. The theoretical model, rationale, and details of the CBT treatment have been previously described (Yoshino et al. Reference Yoshino, Okamoto, Horikoshi, Oshita, Nakamura and Otsuru2015). The major aims of treatment were to develop self-monitoring techniques that facilitate the identification of pain, thinking, behavior, and mood, to learn self-control techniques such as relaxation and behavioral activation, and to modify specific pain-related dysfunctional beliefs.
fMRI acquisition
The fMRI procedure was performed using a Magnex Eclipse 3 T Power Drive 250 (Siemens, Munich, Germany). A time course series of 120 scans was acquired using T2*-weighted, gradient echo, echo planar imaging sequences. Each volume consisted of 30 slices, with a slice thickness of 4 mm with no gaps, which covered the entire cerebral and cerebellar cortices. The time interval between two successive acquisitions of the same image (TR) was 3000 ms, the echo time (TE) was 46 ms, and the flip angle was 90°. The field of view (FOV) was 256 mm, and the matrix size was 64 × 64, giving voxel dimensions of 4 mm × 4 mm × 4 mm. Scan acquisition was synchronized to the onset of each trial. The total experimental duration was 6 min. After functional scanning, structural scans were acquired using a T1-weighted gradient echo pulse sequence (TR = 2160 ms; TE = 3.93 ms; flip angle = 15°; FOV = 256 mm; voxel dimensions of 1 mm × 1 mm × 1 mm) to facilitate localization. After scanning, participants were asked whether they had kept their eyes closed and whether they had remained awake during the scan. All participants confirmed that they had kept their eyes closed and remained awake.
fMRI analysis
Preprocessing
Functional MRI data were preprocessed using the SPM software package (SPM8, Wellcome Department of Cognitive Neurology, London, UK). The first eight volumes were discarded to allow for scanner calibration and the adaptation of participants to the scanning environment. Data were motion corrected, spatially normalized into the stereotactic space of the Montreal Neurological Institute (MNI), and spatially smoothed with a 9 × 9 × 9 mm3 Gaussian kernel.
ICA
Group spatial ICA was performed on all participants (patients and controls) using the fMRI toolbox (GIFT version 3.0a; http://icatb.sourceforge.net) (Calhoun et al. Reference Calhoun, Adali, Pearlson and Pekar2001). First, this analysis was performed in the following steps: Data reduction, application of the ICA algorithm, and back reconstruction for each participant (Calhoun et al. Reference Calhoun, Adali, Pearlson and Pekar2001). Principal component analysis was used to reduce the computational complexity in the data from each participant. We decomposed the preprocessed data into 20 ICNs based on the default setting (Calhoun et al. Reference Calhoun, Adali, Pearlson and Pekar2001). Second, the Infomax algorithm was used to run the ICA. Time courses and spatial maps were computed for each participant, and the obtained mean spatial maps were transformed to z scores (Calhoun et al. Reference Calhoun, Adali, Pearlson and Pekar2001).
To extract the best adapted networks of interest, we ran multiple spatial regressions on the spatial maps of 20 ICNs for all participants, including chronic patients at pretreatment, chronic pain patients at post-treatment, and healthy controls using the established T-maps of the ICNs [e.g. the DMN including primarily the medial prefrontal cortex (MPFC) and posterior cingulate cortex (PCC), the SMN including somatosensory and motor cortices, the CEN including the DLPFC and posterior parietal cortex, and the DAN including the intraparietal sulcus/superior parietal lobule] (Fox & Raichle, Reference Fox and Raichle2007; Menon, Reference Menon2011; Raichle, Reference Raichle2011), which were made using the spatial coordinates of the Anatomical Automatic Labeling (AAL) atlas via the MarsBar SPM Toolbox (http://www.sourceforge.net/projects/marsbar), and according to the largest correlation coefficients, identified the DMN, SMN, CEN, and DAN from 20 ICNs (online Supplementary Fig. S1 and Supplementary Table S1). SPM8 was used to create statistical parametric maps for each network. In this analysis, we used a voxel threshold of p (uncorrected) <0.005 and the cluster correction procedure implemented in SPM8 that computes the number of expected voxels per cluster (10 voxels) according to random field theory (Hayasaka & Nichols, Reference Hayasaka and Nichols2004; Kaichi et al. Reference Kaichi, Okada, Takamura, Toki, Akiyama and Higaki2016). These networks are in accordance with the previously reported segmentations of the DMN, SMN, CEN, and DAN (Dhond et al. Reference Dhond, Yeh, Park, Kettner and Napadow2008; Shpaner et al. Reference Shpaner, Kelly, Lieberman, Perelman, Davis and Keefe2014; Crowther et al. Reference Crowther, Smoski, Minkel, Moore, Gibbs and Petty2015; Chen et al. Reference Chen, Chen, Liu and Shi2016).
Group analyses
Group analysis was performed using SPM8. First, for voxel-based comparisons of each network between the chronic pain patients at T1 and healthy controls, we applied the two-sample t test. In this analysis, we used a voxel threshold of p (uncorrected) <0.005 and the cluster correction procedure implemented in SPM8 that computes the number of expected voxels per cluster (10 voxels) according to random field theory (Hayasaka & Nichols, Reference Hayasaka and Nichols2004; Kaichi et al. Reference Kaichi, Okada, Takamura, Toki, Akiyama and Higaki2016). In this initial analysis, we identified the areas affected by chronic pain to delineate the regions of interest (ROIs) relevant for subsequent analyses.
To assess whether differences between chronic pain patients at T1 and the controls were reduced at T2, we performed ROI-based paired-sample t tests by comparing the ICN connectivity strength within the networks at T1 and T2 in chronic pain patients. Significance was set at the uncorrected voxel level of p = 0.005, followed by the family-wise error (FWE)-corrected cluster level of p = 0.05. We also performed a two-sample t test to compare ICN connectivity strength of patients at T2 with ICN connectivity strength of the controls, with an inclusive mask generated by the comparison of ICN connectivity strength in patients at T1 and controls. An in-depth description has been previously reported (Kaichi et al. Reference Kaichi, Okada, Takamura, Toki, Akiyama and Higaki2016).
Furthermore, to explore the potential relationships of the pre- to post-treatment changes (score at T1 minus score at T2) between treatment outcome variables such as SF-MPQ, BDI-II, STAI and PCS, and ICN connectivity strength in ROIs related to treatment changes, we used the Pearson correlation coefficient test. For statistical significance testing, we used the permutation test (Anderson & Robinson, Reference Anderson and Robinson2001). The number of permutations was set to 10 000 and the threshold for significance was set at p < 0.05.
To determine whether the ICN connectivity strength at T1 was predictive of the treatment outcome, we assessed the correlations between T1 ICN connectivity strength and improvements on clinical assessments scores, including the SF-MPQ, BDI-II, STAI, and PCS (score at T1 minus score at T2) using the Pearson correlation coefficient test. For statistical significance testing, we used the permutation test (Anderson & Robinson, Reference Anderson and Robinson2001). The number of permutations was set to 10 000 and the threshold for significance was set at p < 0.05. The ROIs for the analyses were identified by comparing the findings at T1 for the patients and controls.
Results
Clinical characteristics
Detailed demographic and clinical characteristics of the participants are presented in Table 1. After CBT, patients showed significant improvements on all clinical measures. A summary of all clinical change scores is given in Table 1.
fMRI data
Difference in T1 ICN connectivity strength between chronic pain patients and controls
Two-sample t tests revealed ICN connectivity strength differences within the DAN, DMN, and SMN between the two groups (Fig. 1 and online Supplementary Table S2; uncorrected p < 0.005 and an extent threshold of 10 voxels). There were no ICN connectivity strength differences within the CEN.

Fig. 1. This figure shows differences between patients at pretreatment (T1) and controls. Regions where the ICN connectivity strength was significantly higher or lower in patients with chronic pain than the controls (uncorrected p < 0.005 and an extent threshold of 10 voxels). CP; chronic pain, HC; healthy control, IPL; inferior parietal lobule, PCC; posterior cingulate cortex, MTG; medial temporal gyrus, OFC; orbitofrontal cortex, STG; superior temporal gyrus, IFG; inferior frontal gyrus, PCL; paracentral lobule and MPFC; medial prefrontal cortex.
Difference in ICN connectivity strength for chronic pain patients at T1 and T2 and ICN connectivity strength for controls and chronic pain patients at T2
We restricted our assessment of treatment-related ICN connectivity changes to 15 ROIs that showed a difference between controls and patients at T1 (Fig. 1 and online Supplementary Table S2). After CBT treatment, ICN connectivity strength was increased (i.e. T2 > T1) in the right OFC (x = 15, y = 33, z = −18, size = 11, t value = 4.28) within the DAN, and ICN connectivity strength was decreased (i.e. T1 > T2) in the right inferior parietal lobule (IPL) (x = 36, y = −42, z = 36, size = 10, t value = 3.80) within the DAN and in the left PCL (x = −18, y = −18, z = 69, size = 13, t value = 4.08) within the SMN (uncorrected p < 0.005 and FWE-corrected cluster level p = 0.05; Fig. 2 and online Supplementary Table S3). Figure 3 shows that for ROIs related to treatment changes, ICN connectivity strength in patients normalized after CBT. At T2, the abnormalities in ICN connectivity strength persisted in the medial temporal gyrus and thalamus within the DAN, the lingual, superior temporal gyrus, inferior frontal gyrus, and MPFC within the DMN, and the MPFC within the SMN of chronic pain patients (online Supplementary Fig. S2; uncorrected p < 0.005 and an extent threshold of 10 voxels).
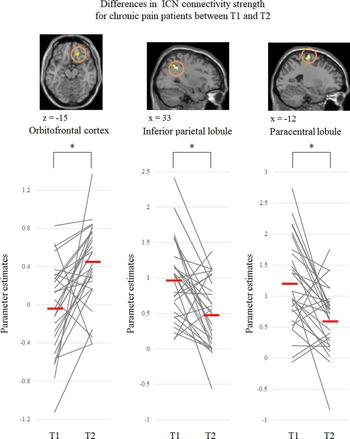
Fig. 2. Three brain regions where the intrinsic functional connectivity (ICN) connectivity strength was significantly increased (orbitofrontal cortex) or decreased (inferior parietal lobule and paracentral lobule) at post-treatment from pre-treatment (*uncorrected p < 0.005 and FWE-corrected cluster level p = 0.05). T1; pretreatment, T2; post-treatment.

Fig. 3. This figure shows comparison between patients at post-treatment (T2) and controls. These networks in patients with chronic pain normalized after CBT. ICN; intrinsic connectivity network, CP; chronic pain, HC; healthy control.
Furthermore, we found that pre–post changes in VAS scores were negatively correlated with changes in ICN connectivity strength in the right OFC within the DAN (r = −0.38, permutation test p = 0.041). As VAS scores decreased after CBT, ICN connectivity strength in the OFC increased. This analysis revealed no relationships between other brain regions and the other clinical assessments, including the BDI-II, STAI, and PCS scores.
Predictors of a treatment response
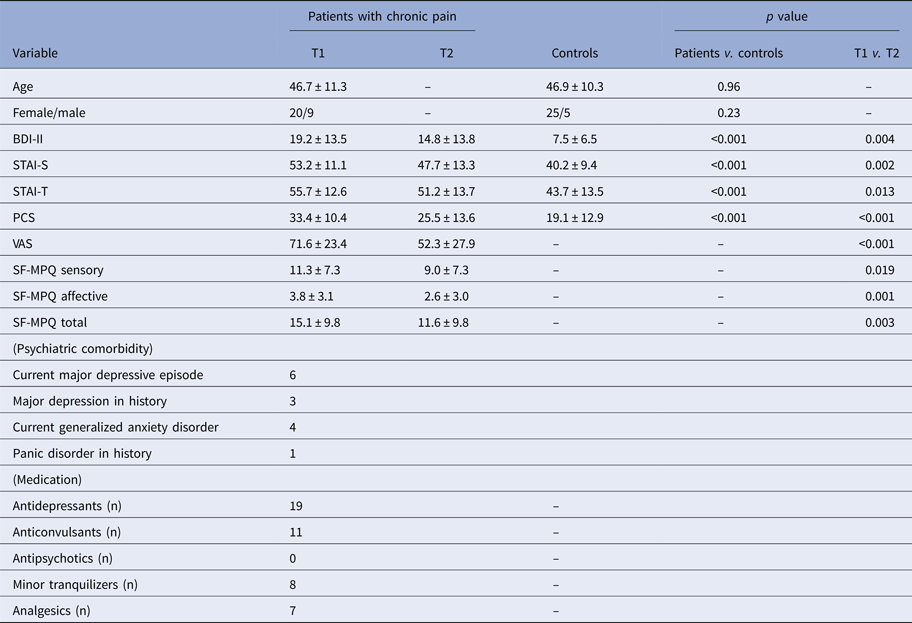
Our assessment was again restricted to 15 ROIs that at T1 showed an ICN connectivity difference between chronic pain patients and controls. There were negative correlations between T1 ICN connectivity strength in the dorsal PCC within the DAN and improvement on VAS (r = −0.39, permutation test p = 0.038), PCS (r = −0.47, permutation test p = 0.010), STAI-S (r = −0.43, permutation test p = 0.018), and STAI-T (r = −0.40, permutation test p = 0.031) scores in patients with chronic pain (Fig. 4). There were no other brain regions that reached the required level of significance.
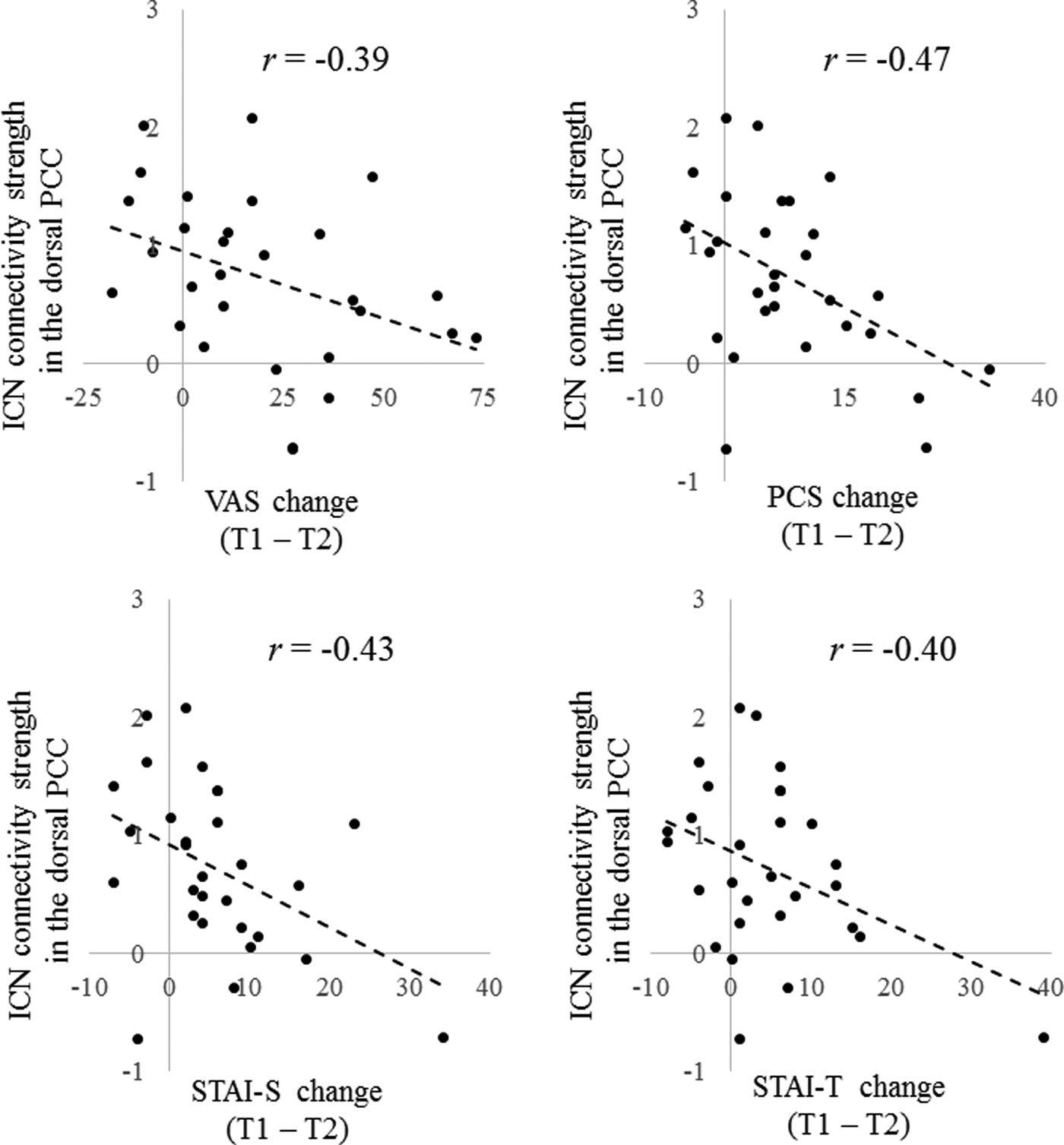
Fig. 4. There were negative correlations between pretreatment (T1) intrinsic connectivity network (ICN) connectivity strength in the dorsal posterior cingulate cortex (PCC) within the dorsal attention network (DAN) and improvement on visual analog scale (VAS), Pain Catastrophizing Scale (PCS), State trait anxiety inventory-state (STAI-S) and State trait anxiety inventory-trait (STAI-T) scores in patients with chronic pain (p < 0.05 corrected for multiple comparisons with permutation test). T2; post-treatment.
Discussion
This study was conducted to examine neural mechanisms underlying the effects of a CBT program for patients with chronic pain who were diagnosed with somatoform pain disorder, according to the DSM-IV (APA, 1994). Abnormal ICN connectivity of the OFC and IPL within the DAN and of the PCL within the SMN normalized in chronic pain patients after CBT treatment, and there were negative correlations between pre–post changes in VAS scores and ICN connectivity strength in the OFC. Furthermore, ICN connectivity strength in the dorsal PCC within the DAN at T1 negatively correlated with CBT-related clinical improvements, including pain intensity and anxiety.
Previous neuroimaging studies of CBT for chronic pain have reported changes in the OFC (Jensen et al. Reference Jensen, Kosek, Wicksell, Kemani, Olsson and Merle2012; Seminowicz et al. Reference Seminowicz, Shpaner, Keaser, Krauthamer, Mantegna and Dumas2013; Shpaner et al. Reference Shpaner, Kelly, Lieberman, Perelman, Davis and Keefe2014), and we also found that a similar alteration in the OFC was associated with CBT treatment. The OFC is crucial for the cognitive processing of pain perception, including decision-making and pain modulation (Neugebauer et al. Reference Neugebauer, Galhardo, Maione and Mackey2009). The DAN is associated with goal-directed processing and maintenance or replacement of attention (Crowther et al. Reference Crowther, Smoski, Minkel, Moore, Gibbs and Petty2015; Mason et al. Reference Mason, Peters and Kumari2016), and appears to be an important network for emotional, visceral, and autonomic responses (Sheline et al. Reference Sheline, Price, Yan and Mintun2010). Previous studies found that connectivity between the prefrontal and parietal cortices plays a key role in pain modulation (Wager et al. Reference Wager, Atlas, Leotti and Rilling2011). Crowther et al. showed hypoconnectivity between the OFC and DAN in patients with major depressive disorder and discovered that such connectivity was an important predictive factor of treatment effects (BDI-II) in Behavioral Activation Treatment (Crowther et al. Reference Crowther, Smoski, Minkel, Moore, Gibbs and Petty2015). Interestingly, they reported that this connectivity was particularly important for somatic elements of BDI-II scores. Our present study revealed significant activity changes toward normalization in the OFC via CBT, and a negative correlation between CBT-induced changes in VAS scores and ICN connectivity strength in the OFC. Based on these findings, CBT may reinforce the top–down aspects of these neural mechanisms and lead to increased OFC function, which we suggest the association of CBT modifying dysfunctional processing of pain perception.
The results of the present study showed that increased ICN connectivity at the IPL region within the DAN in patients with chronic pain was significantly reduced by CBT treatment. We did not expect to find this change in IPL activation associated with CBT for chronic pain. However, increased IPL activity has been previously reported in chronic pain patients (Loggia et al. Reference Loggia, Kim, Gollub, Vangel, Kirsch and Kong2013). The IPL has been associated with attention and self-awareness, including of our own emotional and somatic sensations (Spreng et al. Reference Spreng, Mar and Kim2009), and thus CBT may make a positive contribution to self-awareness disturbance in chronic pain patients. However, it is impossible to support this speculation using the present results, and further studies are needed to further assess this possibility.
Our study also found normalization of the PCL (sensorimotor areas) by CBT treatment. A significant increase in PCL activity in chronic pain patients before treatment has been previously observed (Yoshino et al. Reference Yoshino, Okamoto, Kunisato, Yoshimura, Jinnin and Hayashi2014; Chou et al. Reference Chou, Yang, Fuh, Kuo, Wang and Lirng2016). The PCL, which belongs to the sensorimotor areas, is associated mainly with the action-execution or processing and perception of pain (Smith et al. Reference Smith, Fox, Miller, Glahn, Fox and Mackay2009). Patients with chronic pain show distorted recognition or sensory pain assessment accompanied by abnormal activities of the PCL (Juottonen et al. Reference Juottonen, Gockel, Silén, Hurri, Hari and Forss2002; Catley et al. Reference Catley, O'Connell, Berryman, Ayhan and Moseley2014), and altered PCL activity has also been associated with pain chronicity (Juottonen et al. Reference Juottonen, Gockel, Silén, Hurri, Hari and Forss2002; Chou et al. Reference Chou, Yang, Fuh, Kuo, Wang and Lirng2016). Furthermore, it has been reported that transcranial magnetic stimulation and brain–machine interface training for sensorimotor areas can lead to improvement of various chronic pain syndromes (Tamura et al. Reference Tamura, Okabe, Ohnishi, Saito, Arai and Mochio2004; Yanagisawa et al. Reference Yanagisawa, Fukuma, Seymour, Hosomi, Kishima and Shimizu2016). Based on these points, we speculate that changes in this region in patients with chronic pain may be related to improvement in pain perception and that self-monitoring techniques in CBT might lead to correction of distorted self-awareness related to pain perception.
As for prediction of treatment response, we found that the reduced dorsal PCC activity within the DAN at T1 was related to greater improvements in pain intensity, pain-related cognition, and anxiety. The dorsal PCC plays an important role as a hub in connecting other brain regions including the PFC, which is particularly involved in cognitive control and sensory areas for information processing, and is also involved in attention to changes of various internal or external circumstances and to adjust accordingly by examining and integrating past self-experiences (Leech & Sharp, Reference Leech and Sharp2014). A previous CBT study of depression demonstrated a relationship between rumination, an internal thought pattern that involves repetitively and passively focusing on negative feelings, and PCC activity (Jacobs et al. Reference Jacobs, Watkins, Peters, Feldhaus, Barba and Carbray2016). The connectivity of the dorsal PCC and DAN are also important for modulating the balance between internal and external attentional focus (Leech & Sharp, Reference Leech and Sharp2014). The activity of the dorsal PCC is relatively reduced during cognitive processes such as working memory and attentional choice (Leech & Sharp, Reference Leech and Sharp2014). Our study showed higher ICN connectivity strength in the dorsal PCC within the DAN in chronic pain patients at T1 compared with healthy controls. Impaired functioning of the dorsal PCC seems to contribute to various psychiatric disorders due to a dysregulated balance between internal v. external attention (Leech & Sharp, Reference Leech and Sharp2014). Chronic pain patients (particularly those with somatoform pain disorder) are often characterized by a tendency to pay attention to internal somatic symptoms in response to various external stressors (Lipowski, Reference Lipowski1988), and our results for the dorsal PCC might be related to these mechanisms of persistence.
In the present study, lower ICN connectivity strength in the dorsal PCC in chronic pain patients at T1 (near the state of healthy controls) was also linked to greater improvement in various psychiatric symptoms at T2. For chronic pain patients, deficits in attentional functioning are reported to affect treatment response (Baker et al. Reference Baker, Georgiou-Karistianis, Gibson and Giummarra2017), and our treatment response pattern might be related to dorsal PCC activity through the above-mentioned attentional processing. From these previous studies, the role of the dorsal PCC in the prediction of CBT treatment responses such as pain intensity, anxiety, and pain catastrophizing in the present study might affect the ability to switch continuous attention from pain perception to considering and inspecting thoughts, although further research is needed. Thus, CBT treatment effects are likely to involve reorganization of the functional networks supporting these various processes.
Limitation
Our exclusion criteria for participants did not include all possible treatment effects that might influence pain perceptions of patients, such as the use of antidepressants. Furthermore, we did not establish a therapy control group, and therefore we cannot rule out the potential influence of various factors as a psychotherapy placebo, such as expectancy to receive treatment and contact with others in pain. We did not assess behavioral indices (e.g. attentional function) to examine their influence on the effectiveness or prediction of treatment response. Further study is needed to advance the results found here, including investigations of potentially more effective techniques and treatment mechanisms.
Conclusions
We investigated how CBT treatment modulates neural system mechanisms in chronic pain patients. The results showed that CBT for chronic pain normalized various aberrant neural patterns such as those associated with the OFC, IPL, and PCL. The present study has also revealed that improvement of pain intensity scores after CBT was associated with greater ICN connectivity strength in the OFC. Furthermore, we found that there were negative correlations between dorsal PCC activity at baseline and CBT-related clinical improvements such as pain intensity and anxiety. We consider that the OFC primarily modulates the cognitive process of pain perception and that the dorsal PCC is an important region as a predictor of CBT effects for chronic pain. These brain regions may potentially play an important role in treatment mechanisms.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291717002598.
Acknowledgements
This work was supported by a Grant-in-Aid for ‘Integrated Research on Depression, Dementia and Development Disorders (16dm0107093h0001)’ and ‘Development of BMI technologies for clinical application (16dm0107050h0003)’ carried out under the Strategic Research Program for Brain Sciences by AMED, the Brain Mapping by Integrated Neurotechnologies for Disease Studies (16dm0207012h0002) by AMED, and KAKENHI (Grant numbers 15K19730) from the JSPS.
Declaration of Interest
Atsuo Yoshino has received support in other research from Eli Lily. There are no other disclosures to report.