Introduction
The past two decades have seen the publication of a growing number of studies on patients with first-episode psychosis (FEP), which work on the assumption that FEP is comparatively more treatment responsive than multi-episode psychosis, and that intensive phase-specific treatment may result in both short- and medium-term improvements of outcome (Edwards & McGorry, Reference Edwards and McGorry2002). Understanding and improving the outcome of psychosis remains, however, a major challenge for clinical research (Emsley et al. Reference Emsley, Chiliza and Schoeman2008). Although focusing on FEP populations has enabled researchers to provide useful information regarding differential effects of treatment on outcome (McGlashan et al. Reference McGlashan, Carpenter and Bartko1988; Ram et al. Reference Ram, Bromet, Eaton, Pato and Schwartz1992), generalization of the findings has been hampered by a number of methodological problems, such as sample selection bias (i.e. exclusion of patients with lower socio-economic background or of patients who are difficult to engage or who fail to collaborate), poor definition of the catchment areas from which samples are drawn, unsystematic attention to environmental and contextual factors and lack of information on interventions provided (Friis et al. Reference Friis, Larsen, Melle, Opjordsmoen, Johannessen, Haahr, Simonsen, Rund, Vaglum and McGlashan2003).
Moreover, most FEP research has been conducted in experimental or academic services (Malla & Norman, Reference Malla and Norman2006). Naturalistic follow-up studies on large samples of patients receiving care in ‘real world’ services (both academic and non-academic, research and routine) are still lacking. Studies providing information on the outcome of patients treated in routine conditions are extremely useful as a basis for healthcare planning. Psychiatric service delivery has undergone significant organizational changes over the past 20 years in many western countries, while facing a dramatic reduction in structural and personnel resources. This represents a major challenge for mental health service planning and delivery: a sound basis of evidence is therefore needed to develop more effective and efficient strategies for treating persons with psychosis. Therefore, large naturalistic long-term studies performed in routine services may provide a positive feedback loop from ‘real world’ health services research into clinical practice (Lasalvia & Ruggeri, Reference Lasalvia and Ruggeri2007).
Another important issue in FEP research is the diagnostic boundary of patients under scrutiny. Most studies have restricted their focus to first-episode schizophrenia, excluding other non-schizophrenic psychoses or affective psychoses (AP). Although this reflects the understandable wish to obtain as homogeneous a sample of subjects as possible, it nevertheless creates a number of difficulties. Diagnosis, which is often made on the basis of cross-sectional interviews, may be subject to change as the clinical picture develops over the initial 6–12 months after presentation (Addington et al. Reference Addington, Chaves and Addington2006; Salvatore et al. Reference Salvatore, Baldessarini, Tohen, Khalsa, Sanchez-Toledo, Zarate, Vieta and Maggini2009). Therefore, FEP research should adopt as broad a concept of psychosis as possible. Studies on FEP populations, in fact, have the advantage of examining large samples of patients who, while diagnostically heterogeneous, share some common elements of psychopathology. Although major distinctions in diagnosis can be made relatively early on between non-affective psychoses (NAP) and AP, some degree of overlap becomes apparent only over time (Malla & Payne, Reference Malla and Payne2005).
Conflicting findings have also been reported about the clinical presentation of FEP patients: it is unclear whether patients experiencing a first episode of psychosis display a specific profile of psychopathological symptoms. Some studies reported a relatively low prevalence of negative symptoms in FEP patients (Malla et al. Reference Malla, Takhar, Norman, Manchanda, Cortese, Haricharan, Verdi and Ahmed2002; Harris et al. Reference Harris, Brennan, Anderson, Taylor, Sanbrook, Fitzgerald, Lucas, Redoblado-Hodge, Gomes and Gordon2005) and an increasing frequency of negative symptoms with a longer duration of illness (Bottlender et al. Reference Bottlender, Jaeger, Groll, Strauss and Moeller2001). Other studies failed to identify marked differences between first episode and chronic schizophrenic disorders with respect to psychopathological symptoms (Moritz et al. Reference Moritz, Lambert, Andresen, Bothern, Naber and Krausz2001), neuropsychological function (Moritz et al. Reference Moritz, Andresen, Perro, Schickel, Kraus and Naber2002) or social deficits (Grant et al. Reference Grant, Addington, Addington and Konnert2001). More research is therefore needed to gain a clear-cut picture of the clinical presentation of FEP, in order to define more focused and early treatment strategies.
Although symptom severity and remission are important measures of outcome, researchers have increasingly focused their attention on various aspects of psychosocial functioning to gain a more comprehensive measure of outcome in FEP (Ruggeri et al. Reference Ruggeri, Lasalvia, Tansella, Bonetto, Abate, Thornicroft, Allevi and Ognibene2004). It has been argued that functional dimensions of outcome are relatively independent from symptom reduction and may be more reliably predicted by pre-morbid adjustment (Larsen et al. Reference Larsen, Friis, Haahr, Johannessen, Melle, Opjordsmoen, Rund, Simonsen, Vaglum and McGlashan2004). Assessing psychosocial functioning in FEP patients allows one to understand the impact of psychosis on the patient's general well being, role functioning and community integration (Malla & Payne, Reference Malla and Payne2005). Moreover, with the shift in treatment of schizophrenic patients from long-term hospitalization to an outpatient community service, research on psychosocial adjustment has become increasingly important. This is a major area of interest when planning intervention and evaluating treatment outcome in a recovery perspective (Wunderink et al. Reference Wunderink, Sytema, Nienhuis and Wiersma2009) and should therefore be systematically addressed.
FEP patients show heterogeneous outcomes (McGorry et al. Reference McGorry, Krstev and Harrigan2000) and despite major advances in their treatment a significant percentage may have a poor outcome (Emsley et al. Reference Emsley, Chiliza and Schoeman2008; van Os & Kapur, Reference van Os and Kapur2009). Reasons for these variations are still inadequately understood. The identification of consistent and reliable prognostic indicators has proved to be a challenge. The last systematic review on the topic (Menezes et al. Reference Menezes, Arenovich and Zipursky2006) provided rather disappointing findings (i.e. being recruited from non-representative samples, living in a developing country and being treatment-naive at study entry were the only consistent predictors of a good outcome, whereas use of typical antipsychotics at study entry was a predictor of poor outcome). These inconclusive results probably reflect inherent methodological limitations of the published studies, which included a lack of baseline standardized measures, the variation in definitions of ‘outcome’, and the limited length of follow-up periods (which, on average, hardly exceeded 2 years) (Menezes et al. Reference Menezes, Arenovich and Zipursky2006). To better understand the predictors of outcomes in FEP patients, future longitudinal research should incorporate standard design features that include: prospective follow-up of more than 2 years' duration; use of baseline standardized measures; confirmation of diagnosis at least 1 year later; a large epidemiologically representative sample including both in- and outpatients; multi-dimensional models of outcome incorporating symptomatic, functional and personal variables measured at multiple time points; use of standard and reliable scales for measuring outcome; inclusion of potential determinants of outcome such as treatment adherence, substance use, co-morbidity, pre-morbid functioning, cognitive status, etc.); recording of all interventions provided, both pharmacologic and psychosocial (Menezes et al. Reference Menezes, Arenovich and Zipursky2006).
Future research should also pay specific attention to the mediating processes involved in the complex relationships that exist between predictors and trajectories of outcome. Problems in integrating findings from multiple methods of investigation (e.g. epidemiological, genetic and brain-imaging) to explain variations in trajectories of outcome still remain major challenges (Malla & Payne, Reference Malla and Payne2005). A further important limitation of outcome research on FEP is that most studies do not systematically consider the role of biological variables, among which genetic factors and abnormalities in brain morphology and functioning play a crucial role. As far as we know, the only population-based research that considered clinical, environmental and biological predictors in FEP patients is the ÆSOP study (Fearon et al. Reference Fearon, Kirkbride, Morgan, Dazzan, Morgan, Lloyd, Hutchinson, Tarrant, Fung, Holloway, Mallett, Harrison, Leff, Jones and Murray2006; Morgan et al. Reference Morgan, Dazzan, Morgan, Jones, Harrison, Leff, Murray and Fearon2006), which, however, did not include the genetic profile of patients among possible predictors.
In recent years, an increasing number of studies have reported on possible susceptibility genes involved in psychosis. None of them, however, have been unambiguously linked to dysfunctions leading to psychosis. A thousand association studies involving over 700 candidate genes supported the role of some genes [i.e. neuregulin 1 (NRG1), dysbindin (DTNBP1), dopamine receptors D1–4 (DRD1–4) and disrupted-in-schizophrenia-1 (DISC1)] in the development of psychosis (Allen et al. Reference Allen, Bagade, McQueen, Ioannidis, Kavvoura, Khoury, Tanzi and Bertram2008). However, even for these promising genes, there has been a remarkable failure to replicate exactly the same markers and haplotypes across studies and a lack of consistency in implicating particular alleles in the development of psychosis (Alkelai et al. Reference Alkelai, Baum, Carless, Crowley, Dasbanerjee, Dempster, Docherty, Hare, Galsworthy, Grover, Glubb, Karlsson, Mill, Sen, Quinones, Vallender, Verma, Vijayan, Villafuerte, Voineskos, Volk, Yu, Zimmermann and DeLisi2008; Sanders et al. Reference Sanders, Duan, Levinson, Shi, He, Hou, Burrell, Rice, Nertney, Olincy, Rozic, Vinogradov, Buccola, Mowry, Freedman, Amin, Black, Silverman, Byerley, Crowe, Cloninger, Martinez and Gejman2008; Sullivan, Reference Sullivan2008). Moreover, none of the genome-wide association study (GWAS) on schizophrenia or bipolar disorder so far implicated any of the previously involved candidate genes (Shi et al. Reference Shi, Li, Xu, Wang, Li, Shen, Zhang, Chen, Zhou, Ji, Li, Xu, Liu, Wang, Yang, Liu, Sun, Wan, Qin, He, Steinberg, Cichon, Werge, Sigurdsson, Tosato, Palotie, Nöthen, Rietschel, Ophoff, Collier, Rujescu, Clair, Stefansson, Stefansson, Ji, Wang, Li, Zheng, Zhang, Feng and He2011; Bergen & Petryshen, Reference Bergen and Petryshen2012). These inconclusive results seem to suggest that phenotype characterization might be particularly important when identifying true and valid candidate genes and that several genes might interact to determine a particular phenotype.
To overcome the difficulties that are inherent in research on multifactorial phenotypes, such as psychosis, an approach based on phenotypic dissection has been proposed (Rietkerk et al. Reference Rietkerk, Boks, Sommer, Liddle, Ophoff and Kahn2008). This approach deconstructs schizophrenia and bipolar disorder into phenotypes based on symptoms, and then correlates particular phenotypes with genetic variants (Jablensky, Reference Jablensky2006). The prospective of a dimensional, symptom-based approach focused on an individual and subsyndromal phenotype is attractive since it may provide a model for studying the heterogeneity of schizophrenia and the underlying pathophysiology of the disorder (Carpenter et al. Reference Carpenter, Buchanan, Kirkpatrick, Tamminga and Wood1993). To date, relatively limited work has been done to identify genetic variants associated with specific clinical phenotypes. Gene–symptom relationships have emerged primarily from follow-up studies of putative schizophrenia risk genes, with only a handful of replicated findings (DeRosse et al. Reference DeRosse, Funke, Burdick, Lencz, Ekholm, Kane, Kucherlapati and Malhotra2006; Tosato et al. Reference Tosato, Ruggeri, Bonetto, Bertani, Marrella, Lasalvia, Cristofalo, Aprili, Tansella, Dazzan, Diforti, Murray and Collier2007). This approach will make it possible to define persistent aspects of the schizophrenic profile which are more likely to represent an underlying biological pathogenesis as opposed to fluctuating, possibly environmentally mediated symptoms (Tosato & Lasalvia, Reference Tosato and Lasalvia2009). Although genetic research has achieved some encouraging findings (Cook & Scherer, Reference Cook and Scherer2008; Maier, Reference Maier2008), the specific genotype–phenotype relation of psychosis still remains unclear.
The integration of clinical and genetic assessment with brain imaging techniques has also been unsystematic. Numerous imaging studies have revealed structural brain abnormalities in schizophrenia and related NAP, with the most consistent findings being enlarged lateral ventricles and reduced medial temporal and prefrontal lobe volumes (Shenton et al. Reference Shenton, Dickey, Frumin and McCarley2001; Liddle & Pantelis, Reference Liddle, Pantelis, Hirsch and Weinberger2003). Although such abnormalities are likely to be subtle (Weinberger, Reference Weinberger1995), the nature, timing and course of the associated neurobiological changes have proved difficult to elucidate (Harrison & Lewis, Reference Harrison, Lewis, Hirsch and Weinberger2003). There is evidence that these brain abnormalities are already present prior to illness onset or at onset (Pantelis et al. Reference Pantelis, Yücel, Wood, Velakoulis, Sun, Berger, Stuart, Yung, Phillips and McGorry2005; Arango et al. Reference Arango, Moreno, Martínez, Parellada, Desco, Moreno, Fraguas, Gogtay, James and Rapoport2008) and that progressive changes in a number of brain regions occur over time (Gogtay et al. Reference Gogtay, Vyas, Testa, Wood and Pantelis2011); their associations, however, with clinical and functional outcomes have so far proved to be inconsistent (Cahn et al. Reference Cahn, Hulshoff Pol, Lems, van Haren, Schnack, van der Linden, Schothorst, van Engeland and Kahn2002; Ho et al. Reference Ho, Andreasen, Nopoulos, Arndt, Magnotta and Flaum2003; DeLisi et al. Reference DeLisi, Sakuma, Maurizio, Relja and Hoff2004; DeLisi & Hoff, Reference DeLisi and Hoff2005; Price et al. 2006). Moreover, most recent MRI longitudinal studies on FEP suffer from some methodological flaws, including the fact that many so-called first-episode studies included patients who had already been ill for a number of years, the relatively small sample sizes (average number of patients per study: 32, s.d. = 26.9) and the sample selection procedure (i.e. convenience groups of selected patients) (Steen et al. Reference Steen, Mull, McClure, Hamer and Lieberman2006). Finally, longitudinal investigations are needed to take into account the interplay of various likely aetiological factors (both environmental and biological) in understanding the evolution of brain structural as well as functional deficits in FEP (Pantelis et al. Reference Pantelis, Yücel, Wood, Velakoulis, Sun, Berger, Stuart, Yung, Phillips and McGorry2005).
To fill these gaps a research project was undertaken – Psychosis Incident Cohort Outcome Study (PICOS) – aiming at integrating clinical, psychosocial and biological perspectives into research on FEP to better understand possible mechanisms underlying treatment outcomes. PICOS is a large multisite naturalistic research that aimed at examining the relative role of clinical, social, genetic and morpho-functional brain factors in predicting symptomatic and functional outcomes in a large cohort of FEP patients receiving care from public mental health services located in a broad area of the Veneto region (north-eastern Italy). Specifically, PICOS is aimed at: (a) characterizing new cases of psychosis at onset, in terms of clinical presentation and social functioning; (b) determining symptomatic and functional outcomes of both affective and non-affective FEP patients treated in routine non-experimental settings; (c) exploring to what extent clinical, psychosocial and biological factors (i.e. genetics and brain functional/structural characteristics) influence the outcome of FEP patients and examine their mutual interactions; (d) developing a comprehensive predictive model of outcome for FEP and identifying predictors of ‘good’ and ‘poor’ outcomes that might be useful for both clinical and research purposes. In this paper, we aim at providing an overview of methodology and design of PICOS and to give some initial baseline findings.
Methods
Project overview
In order to achieve its aims, PICOS was designed with a modular structure.
Module 1 – Clinical and social evaluations
It includes the assessment of a number of patients' clinical and social characteristics, such as pre-morbid IQ, pre-morbid social adjustment, stressful life events, psychopathology, social disability, insight of illness, subjective quality of life, needs for care and service satisfaction. This information was collected by using a set of well-known international standardized measures (see below). In addition, the perceptions of relatives (or informal caregivers) were also assessed using a set of standardized measures, with specific regard to burden of care, psychological distress and service satisfaction (see below). This module also includes the quantification of structural and human resources of mental health facilities located in PICOS participating sites (Lasalvia et al. Reference Lasalvia, Gentile and Ruggeri2007). A thorough assessment was also made of the emotional and organizational well-being of the staff working at the participating sites (Lasalvia et al. Reference Lasalvia, Bonetto, Bertani, Bissoli, Cristofalo, Marrella, Ceccato, Cremonese, De Rossi, Lazzarotto, Marangon, Morandin, Zucchetto, Tansella and Ruggeri2009). The assumption behind this data collection is that, along with patients' personal and clinical characteristics, contextual factors (e.g. services' structural characteristics and resources, emotional atmosphere of the therapeutic milieu and degree of staff burnout) play a crucial role in explaining treatment outcomes.
Module 2 – Genetics
It focuses on the assessment of family history of psychiatric disorders and genetic liability to psychoses. This module includes the reconstruction of probands' family trees for psychotic disorders and the assessment of Neurological Soft Signs. Moreover, for each subject recruited to the study (both patients and their first-degree biological relatives), venous blood samples (15 ml) were collected in EDTA-containing tubes. DNA was extracted from blood leukocytes and it was stored. To perform a case-control study, controls, selected from a population ethnically similar to the patients, were recruited from repeat blood donors via the Blood Transfusion Service from the same area of Verona. The policy of the Blood Transfusion Centre is not to collect blood from individuals who are on medication. The absence of a personal or family history of psychotic disorders was ascertained using the SCID-NP and the Family Interview for Genetics Study (FIGS; Maxwell, Reference Maxwell1992). The place of birth of both parents and grandparents was ascertained in order to match controls by ethnicity. DNA from patients, relatives and controls was used to genotype the SNPs belonging to the different candidate genes. The SNPs were selected using PLINK software, in order to identify for each gene in the study the minimal number of SNPs necessary for the identification of the maximal haplotypic variability in Caucasian population. All genotyping analyses were performed blind to status. The quality control criteria were: (i) genotypes form three distinct clusters; (ii) water controls are negative; (iii) number of genotypes callable is >90% and (iv) minor allele frequency is greater than 2%. In addition, inter-plate and intra-plate duplicate testing of known DNAs was performed.
Module 3 – Brain imaging
This module includes the evaluation of brain features using MRI scans and a series of neuropsychological tests, with the aim of exploring brain structure and cognitive dimensions. All patients recruited to Module 1 who agreed to undergo MRI were enrolled in Module 3 and contacted by clinical research psychologists by phone to arrange an appointment and to check the absence of MRI counter-indications (i.e. pregnancy and metallic prosthesis). With respect to the research exclusion criteria adopted by Module 1, further criteria were applied in Module 3: history of traumatic head injury with loss of consciousness, major medical diseases, alcohol or substance abuse in the 6 months preceding MRI. All the 1.5 T MRI scans (Magnetom Symphony Maestro class syngo MR 2002B – Siemens) were performed in the Section of Radiology at the Verona University Hospital and included 3D sequences, perfusion-weighted and diffusion-weighted imaging acquisitions (for the evaluation of structural, cerebral blood and white matter microstructure organization, respectively). In order to minimize any anxiety symptoms, clinical research psychologists carefully provided full information on MRI and personally accompanied research subjects to the MRI centre, waiting for them until the end of the session. On the same day, patients received a full neuropsychological assessment, including the following tasks: Iowa Gambling Task (Bechara et al. Reference Bechara, Damasio, Damasio and Anderson1994), Continuous Performance Task (Nuechterlein, Reference Nuechterlein, Seinhauer, Gruzelier and Zubin1991), Wisconsin Card Sorting Test (Heaton, Reference Heaton1981), Span of Apprehension Test (Estes & Taylor, Reference Estes and Taylor1964) and N-back Test (Kirchner, Reference Kirchner1958) for the evaluation of decision making, sustained attention, executive functions and working memory; narrative/conversational task and syntactic comprehension task for the assessment of linguistic production and comprehension. Also, a visual-motor task (Poffenberger paradigm) (Marzi, Reference Marzi1999; Bellani et al. Reference Bellani, Marzi, Savazzi, Perlini, Cerruti, Ferro, Marinelli, Sponda, Rambaldelli, Tansella and Brambilla2010) was administered in order to explore inter-hemispheric communication. Finally, Papagno's test (Papagno et al. Reference Papagno, Cappa, Capitani, Forelli, Garavaglia, Laiacona, Capitani and Vallar1995) for the investigation of concrete thought was administered and the Mini Mental State Examination (Folstein et al. Reference Folstein, Folstein and McHugh1975), while Raven's Progressive Matrices (Raven et al. Reference Raven, Raven and Court2003) were used to measure overall cognitive functioning.
Study design
PICOS is a naturalistic study, conducted with a prospective longitudinal design. Evaluations of both patients and relatives were carried out at baseline and at 1, 2 and 5 years (currently underway).
The geographical context
The Veneto region has a population of approximately 4.6 million inhabitants (Census data, 2001), representing 8% of Italy's total population. Nearly 2.5 million of these inhabitants are aged 15–54 years and are thus considered a population at risk for psychosis. The region's population structure is in line with the national average in terms of older inhabitants (>65 years = 7%), whereas the number of younger people is slightly below the national average (<25 years = 23.8%, v. 25.8%). The vast majority of residents are of Caucasian background, making up an ethnically homogeneous population. Over the last 10 years, however, the proportion of foreign immigrants with respect to the total population increased from 6.76% in 2001 (Census data, 2001) to 9.30% in 2008 (Administrative data, Veneto region). The urban structure of the region is polycentric, with only a few large-scale cities (i.e. Venice, Padua and Verona, which exceed 200 000 inhabitants) and many mid- and smaller-scale cities. An entrepreneurial system of small- and mid-size businesses located throughout the region makes the economic system very competitive, which has led Veneto to become one of Italy's most affluent regions.
Participating sites
Overall, 25 collaborating sites took part in PICOS, covering a catchment area of nearly 3.3 million inhabitants, corresponding to 76% of the inhabitants living in the entire Veneto. PICOS was coordinated by the Section of Psychiatry and Clinical Psychology at the Department of Public Health and Community Medicine, University of Verona. The collaborating sites were homogeneously distributed across the regional territory and included either whole Departments of Mental Health (DMHs) (n = 9) or single departmental units (n = 16). In addition, two private psychiatric inpatient facilities took part in the study (Lasalvia et al. Reference Lasalvia, Gentile and Ruggeri2007). For a detailed list of participating sites, together with the respective local research teams see the Appendix.
The care context
PICOS participating sites were routine public community-based mental health services, established according to the 1978 psychiatric legislation reform and which operate in the Italian National Health Service (NHS) context. Psychiatric care in the Veneto region is delivered by the NHS through its DMHs, each of which has its own geographically defined catchment area (Tansella et al. Reference Tansella, Amaddeo, Burti, Lasalvia and Ruggeri2006). Multi-disciplinary teams operating these DMHs provide a wide range of comprehensive and integrated programmes for the local adult population, including inpatient care, day care, rehabilitation, outpatient care, home visits, 24-h emergency services and residential facilities for long-term patients (Tello et al. Reference Tello, Mazzi, Tansella, Bonizzato, Jones and Amaddeo2005). Standard care for FEP patients generally consists of personalized outpatient psychopharmacological treatment, combined with non-specific supportive clinical management at the Community Mental Health Centre level or – when required – in patients' homes (Lasalvia et al. Reference Lasalvia, Gentile and Ruggeri2007). When necessary, brief hospital stays can also be arranged in small inpatient psychiatric units located in public general hospitals (Lasalvia & Tansella, Reference Lasalvia and Tansella2010).
Subjects
All psychiatric facilities located in the area covered by PICOS were asked to refer to the local research teams all potential cases of psychosis at first service contact during the index period (1st January 2005–31st December 2007). There were no formal diagnostic criteria for entry into the study (only psychopathological criteria were used). Based on the over-inclusive screening methodology adopted in the WHO ten-country study (Screening Schedule for Psychosis; Jablensky et al. Reference Jablensky, Sartorius, Ernberg, Anker, Korten, Cooper, Day and Bertelsen1992), the inclusion criteria were: (1) age 15–54 years; (2) residence in the Veneto region; (3) presence of (a) at least one of the following symptoms: hallucinations, delusions, qualitative speech disorder, qualitative psychomotor disorder, bizarre or grossly inappropriate behaviour, or (b) at least two of the following symptoms: loss of interest, initiative and drive, social withdrawal, episodic severe excitement, purposeless destructiveness, overwhelming fear, marked self-neglect; (4) first lifetime contact with any mental health service located in PICOS area during the study period occasioned by symptoms enumerated in (3). The exclusion criteria were: (1) prior treatment with an antipsychotic agent for more than 3 months; (2) mental disorders due to a general medical condition; (3) moderate to severe mental retardation.
The screening instrument was administered to all potentially eligible patients as soon as possible after their first service contact (and in all cases within 30 days of first contact). The instrument was completed by a face-to-face interview with the patient and/or using case notes and information provided by the treating staff. Each patient who met the inclusion criteria was approached and invited to undertake standardized assessments (see below). Patients' interviews were carried out by local mental health staff trained in the use of study instruments. The assessment would take place only after having gained written informed consent, as approved by both the Ethics Committee of the coordinating centre and the local Ethics Committees of participating sites. All subjects provided written informed consent for study procedures and for anonymous and aggregate reporting of clinical findings. The participants were informed that they might withdraw consent to the assessments at any time. The eligible patients were also asked for consent to involve a key family member in the assessments. If the patient or the family member did not agree to be assessed, the local research staff would briefly record their reasons for not agreeing, whenever possible. The patients and family members who refused to participate in the study were re-contacted at monthly intervals up to three more times.
Case ascertainment
Routine case ascertainment was conducted through ongoing liaison between the local PICOS research teams at each study site and local mental health services. The clinical staff were encouraged to refer all people who met the initial screening criteria to the study offices, using a variety of agreed routes including telephone, 24-h answering services, postal pro-forma and dedicated fax returns. There was regular phone or face-to-face contact between study teams and both the in-patient and community mental health teams serving the populations at risk. Regular training events for clinical teams ensured that all staff knew about PICOS, regardless of staff turnover. Promotional materials were made available in all clinical settings to ensure awareness and continuation of referrals and presentations were made to user and carer groups within the relevant areas. A ‘leakage study’, based on the method described by Fearon et al. (Reference Fearon, Kirkbride, Morgan, Dazzan, Morgan, Lloyd, Hutchinson, Tarrant, Fung, Holloway, Mallett, Harrison, Leff, Jones and Murray2006), was also undertaken at 14 PICOS sites, in order to further assess the accuracy of the recruitment procedure and to identify any cases missed through the routine procedures. All electronic and paper information systems were carefully scrutinized for any cases aged 15–54 years, presenting to the services for the first time during the index period, with ICD-10 diagnostic codes of psychosis (F1x.4; F1x.5; F1x.7; F20-29; F30.2, F31.2, F31.5, F31.6, F32.3, F33.3). This information was compared with case records to confirm eligibility.
Diagnostic ascertainment
The formal best-estimate research diagnosis was made six months after inception using the Item Group Checklist (IGC) of the Schedule for Clinical Assessment in Neuropsychiatry (SCAN; World Health Organization, 1992a). At that time, two psychiatrists, one who had reviewed the subject initially and one who had not, independently reviewed the relevant baseline and follow-up information and formulated the ICD-10 diagnosis. In the cases where a consensus was not reached, the opinion of a third psychiatrist was solicited to clarify diagnostic problems. Only patients with a confirmed ICD-10 diagnosis of psychosis, either non-affective or affective (F1x.4; F1x.5; F1x.7; F20–29; F30.2, F31.2, F31.5, F31.6, F32.3, F33.3), were suitable for re-assessment at the later follow-up stages.
Clinical and psychosocial assessment
A comprehensive set of well-known standardized measures was used to collect patients' clinical and psychosocial information. Face-to-face interviews were conducted at baseline (during or shortly after discharge from the hospital for the most part) and at 1- and 2- and 5-year follow-up. Table 1 presents an overview of the instruments administered at each of these interviews.
Table 1. Assessment instruments and measures used in PICOS
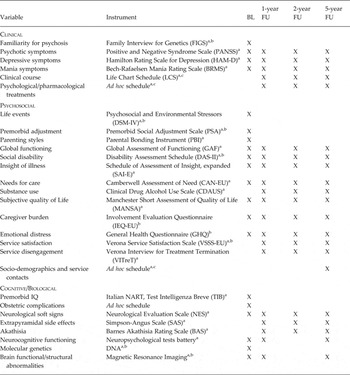
Used to collect data from: a patients; b relatives; c case records.
FIGS (Maxwell, Reference Maxwell1992), PANSS (Kay et al. Reference Kay, Fiszbein and Opler1987), HAM-D (Hamilton (Reference Hamilton1960), BRMRS (Bech et al. Reference Bech, Rafaelsen, Kramp and Bolwig1978), LCS (World Health Organization, 1992b; Lasalvia et al. Reference Lasalvia, Bonetto, De Santi and Ruggeri2004), PSA (Foerster et al. Reference Foerster, Lewis, Owen and Murray1991), PBI (Parker et al. Reference Parker, Tupling and Brown1979), GAF (APA, 1994), DAS-II (World Health Organization, 1988), SAI-E (David et al. Reference David, Buchanan, Reed and Almeida1992), CAN-EU (McCrone et al. Reference McCrone, Leese, Thornicroft, Schene, Knudsen, Vazquez Barquero, Lasalvia, Padfield, White and Griffiths2000), MANSA (Priebe et al. Reference Priebe, Huxley, Knight and Evans1999), CDAUS (Mueser et al. Reference Mueser, Nishith, Tracy, DeGirolamo and Molinaro1995), IEQ-EU (van Wijngaarden et al. Reference van Wijngaarden, Schene, Koeter, Vázquez-Barquero, Knudsen, Lasalvia and McCrone2000), GHQ (Goldberg & Williams, Reference Goldberg and Williams1988), VSSS-EU (Ruggeri et al. Reference Ruggeri, Lasalvia, Dall'Agnola, Wijngaarden van, Knudsen, Leese, Gaite and Tansella2000), VITreT (Ruggeri et al. Reference Ruggeri, Salvi, Bonetto, Lasalvia, Allevi, Parabiaghi, Bertani and Tansella2007), TIB (Nelson, Reference Nelson1982; Colombo et al. Reference Colombo, Sartori and Brivio2002), SAS (Simpson & Angus, Reference Simpson and Angus1970), BARS (Barnes, Reference Barnes1989), NES (Buchanan & Heinrichs, Reference Buchanan and Heinrichs1989).
The duration of untreated psychosis (DUP) was also established for each patient by reviewing relevant information in the case notes and questioning the patient and relatives and/or caregiver and was operationally defined as the time from onset of psychotic symptoms to first treatment with antipsychotic medication (Norman & Malla, Reference Norman and Malla2001).
The patients were also asked for consent to involve their key family members in the assessment, and when given, the family members who provided written informed consent were also assessed at baseline and re-assessed at subsequent follow-up points.
Interviewer training
Within each participating site a small multi-disciplinary local research team was established, composed of routine mental health staff (e.g. psychiatrists, clinical psychologists, community nurses and occupational therapists), who were preliminarily trained in the use of the study instruments. In fact, prior to the recruitment of the patients, all local mental health service staff involved in the standardized evaluations (n = 101) received a specific 3-day training in administering the study's instruments. The training of the interviewers included sessions for discussion of all standardized assessment schedules used in the study and interview of patients with psychosis by each interviewer, watched by all remaining interviewers and coordinators of the study, followed by a discussion. There was constant supervision of the interviewers during the study, with a discussion of the difficulties and doubts in any of the schedules of the study protocol. In order to determine the training effectiveness and to test the consistency of the evaluations among the raters, an inter-rater reliability exercise was carried out on the clinical measures (such as the PANSS) by involving the local staff trained in the use of the study's instruments (see Results).
Statistics
Sample size and power calculation
For power calculation, we considered the difference between total PANSS scores of two time points to be the outcome measure, specifically the first and second follow-up because this appears to be the most conservative approach due to reduced sample sizes. At the first follow up, 209 patients were assessed using the PANSS and the mean total score was 53.32 (s.d. 19.59); at the second follow up, 190 patients were assessed, with a mean score of 48.43 (s.d. 17.93). The Pearson correlation coefficient between observations at first and second follow-up is 0.4. Assuming an alpha level of 0.05, these parameters made it possible to achieve 87% power.
Statistical analyses
Participants' evaluation was carried out at baseline and at 1, 2 and 5 years. Since there are many issues (such as correlation between repeated outcome measurements, mixture of non-time-varying and time-varying covariates and missing data) which make the analysis of longitudinal data complicated, it will be necessary to adopt specific statistical techniques to take these effects into account. Longitudinal data can be viewed as multilevel data, with repeated measures nested within individuals. In its simplest form, this leads to a two-level model, with the series of repeated measures at the lowest level and the individuals at the highest level. In order to take into account the within subject correlation in the presence of missing data, multilevel regression models will be estimated by linear regression equations, with different regression coefficients for different individuals (Hox, Reference Hox2002; Leyland & Goldestein, Reference Leyland and Goldestein2003). Using multilevel models to analyse repeated measures data will have several advantages. First, by modelling varying regression coefficients at the occasion level, we have growth curves that are different for each person. Second, the number of repeated measures may differ across persons. Third, the co-variances between the repeated measures can be modelled by specifying a definite structure for the variances and co-variances. Fourth, if we have balanced data and use RML estimation, the usual repeated measures analysis of variance can be derived from the multilevel regression results. Fifth, it is straightforward to include time varying or time constant explanatory variables in the model, which allow us to model both the average group development and the development of different individuals. Since multilevel regression models do not require balanced data, this will allow for the inclusion of data from patients with incomplete observations at follow ups. We will allow for the presence of missing outcome data under the assumption that the data are missing completely at random conditional on the covariates included in the models [i.e. missing at random, using the terminology of Little & Rubin (Reference Little and Rubin2002)].
Statistical significance is defined at two-sided p < 0.05. All analyses will be performed using STATA 9.0 for Windows.
Results
Reliability exercise
The level of agreement between the raters was tested by the Intra-class Correlation Coefficient (ICC). The agreement was considered to be high if ICC was equal to or greater than 0.75. Each rater independently coded three videotaped interviews of psychotic patients. High levels of agreement (mean percentage on the items of each scale) were reached between each coder and the clinician. In detail, 85% for positive scale, 70% for negative scale and 82% for general scale. The intra-class correlation coefficient reached a value of 0.81.
Recruitment and baseline evaluation
The flow diagram in Figure 1 gives an overview of patients' recruitment and baseline evaluations.
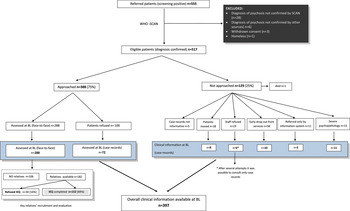
Fig. 1. Flow diagram of recruitment and baseline evaluation.
Of all the patients referred to PICOS research staff as potentially eligible cases, 517 had a confirmed ICD-10 diagnosis of psychosis. The majority (75%) were directly approached by the research staff and asked to be interviewed, while 25% could not be approached for the reasons detailed in Figure 1. However, every effort was made by the research staff to gain as much information as possible on the patients who were not approached: specifically, all available case records and/or all other clinical documentation were carefully scrutinized and the treating clinicians were interviewed by the research staff in order to allow the completion of the core set of assessment instruments (i.e. PANSS, HAM-D, BRMRS, DAS and GAF); using this procedure, clinical information was collected on a further 37 patients. Of the patients approached (n = 388), 288 were interviewed face-to face by the research staff with the full range of the study instruments, whereas 100 did not consent to meet local researchers for the assessment. It was possible, however, to complete the core study instruments for 72 of them on the basis of the clinical information drawn from the case records and/or from treating clinicians' interviews. Therefore, a total of 397 patients were assessed with the core set of study instruments, and they represent the baseline sample of this study. No significant differences, in terms of socio-demographic or diagnostic characteristics, were found between those who were assessed with the full range of study instruments and the others.
Follow-up assessments
Follow-up assessments were conducted within each participating site by the same research staff that had performed the baseline evaluations, and took place in chronological order of initial contact with psychiatric services. The patients currently in psychiatric care were approached through their treating clinicians or key-workers. The patients who had left the area of residence since the original intake were traced by contacting family members or their general practitioner. The patients living in the area covered by PICOS but no longer in contact with the services were contacted through their former treating clinicians and were asked to be approached for the follow-up evaluations (this was done following confirmation with their former treating clinicians that such an approach was appropriate). The follow-up assessments included face-to-face interviews with subjects, family members and the treating psychiatric teams and perusal of psychiatric case notes, general medical notes and community mental health team notes. Figure 2 shows the flow diagram of the 1-year and the 2-year follow-up assessments. Since PICOS was conducted in a large number of ‘real world’ mental health services spread across a broad geographical area and involved an unselected sample of patients reflecting the composition of routine patients on the caseloads of public services, the follow-up design is quite complex.
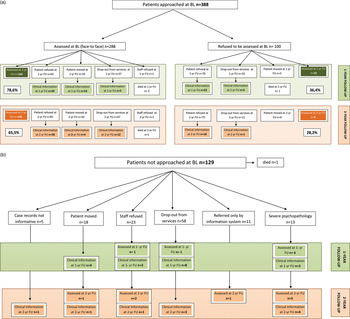
Fig. 2. (a) Flow diagram of 1-year and 2-year follow-up: patients approached. (b) Flow diagram of 1-year and 2-year follow-up: patients not approached.
Of the patients interviewed face-to-face at baseline (n = 288) (Fig. 2a, left side, upper part), three had died during the follow-up interval, 166 were approached at 1 year and re-evaluated face-to face with the full range of the study instruments, 67 were assessed at 1 year with the core set of clinical measures (PANSS, HAM-D, BRMS, DAS and GAF) on the basis of information drawn from case notes and/or after having interviewed treating clinicians. Overall, 224 patients were assessed at 1 year, resulting in a follow-up rate of nearly 79%. It should be noted that every effort was made by the local research staff and by the coordination centre to trace, approach and assess both patients who had refused to be interviewed at baseline and those who had not been approached at baseline. Of the patients who had refused to be interviewed face-to-face at baseline (Fig. 2a, right side, upper part), 10 consented to be interviewed face-to face at 1 year and 26 were assessed with the core set the study instrument on the basis of clinical information drawn from case records and/or from treating clinicians' interviews. Moreover, of those not approached at baseline (Fig. 2b, upper part), 26 additional patients were assessed at 1 year, either face-to face (n = 8) or on the basis of information drawn from case records or treating clinicians' interviews (n = 18), thus yielding a total of 286 patients with a 1-year follow-up assessment available. No significant differences in socio-demographic, diagnostic and clinical characteristics were found between those followed for 1 year and those lost to follow up.
At 2 years, of the patients interviewed face-to-face at baseline (Fig. 2a, left side, lower part) one had died during the follow-up interval, 135 were re-interviewed face-to-face with the full range of the study instruments and 51 were assessed with the core set of clinical measures (PANSS, HAM-D, BRMS, DAS and GAF) on the basis of information drawn from case records and/or treating clinicians' interviews. Overall, 186 patients were re-assessed at 2 years, resulting in a follow-up rate of 65.5%. It should be noted that also at 2 years, every effort was made to trace and approach both patients who had refused to be interviewed at baseline and those who had not been approached at baseline: from the former group (Fig. 2a, left side, lower part) 29 patients were assessed at 2 years either face-to face (n = 9) or on the basis of information drawn from case records or treating clinicians' interviews (n = 19), and from the latter group (Fig. 2b, lower part) 19 additional patients were assessed either face-to face (n = 9) or on the basis of information drawn from case records or treating clinicians' interviews (n = 10), thus yielding a total of 233 patients with 2-year follow-up assessment available. No significant differences were found in terms of socio-demographic or diagnostic characteristics between those followed for 2 years and those lost to follow-up. With respect to baseline clinical assessment, the groups did not differ for PANSS negative symptoms and DAS, whereas PANSS positive symptoms [3.27, s.d. 1.05 v 3.02, s.d. 1.01; p = 0.018 t-test], PANSS general symptoms [2.77, s.d. 0.75 v 2.59, s.d. 0.76; p = 0.021 t-test], PANSS total score [2.85, s.d. 0.74 v 2.66, s.d. 0.71; p = 0.011 t-test] and GAF [37.92, s.d. 10.15 v 40.40, s.d. 10.98; p = 0.021 t-test] were higher among patients assessed at 2 years than among those lost to follow-up.
Findings from the leakage study
On the basis of the existing literature, annual treated incidence for schizophrenia and related functional psychoses in the Veneto ranges from around 17/100 000 (de Salvia et al. Reference de Salvia, Barbato, Salvo and Zadro1993) to 11/100 000 (Tansella et al. Reference Tansella, Balestrieri, Meneghelli, Micciolo and Tansella1991). Some PICOS sites recruited a number of incident cases lower than expected. Specifically, among the 25 PICOS sites, the cases were lower than expected in 11 sites (n = 101; person-years: 1 505 739; incidence rate: 6.7/100 000). The 14 remaining sites recruited a number of cases (n = 426; person-years: 3 144 483; incidence rate: 13.5/100 000) that was substantially in line with the numbers reported in previous incidence studies.
Through the ‘leakage study’ procedure it was found that in seven sites the proportion of missed cases was negligible (missed cases n = 27; 12%; recruited cases n = 183; person-years: 1 311 493; incidence rate: 16/100 000), whereas in the remaining seven sites the proportion of missed cases was higher (missed cases n = 152; 39.9%; recruited cases n = 229; person-years: 1 832 990; incidence rate: 20.8/100 000). So a conservative assumption was made that the sub-sample recruited in the former seven sites was fully representative of new cases of psychosis living in their respective catchment areas. Overall, on the basis of the leakage study, the treated incidence rate of psychosis in the Veneto region is 18.8/100 000 a year (further details on incidence data in PICOS area will be given in future publications).
Clinical and social characteristics of patients
Of patients assessed at baseline (n = 397), 25.6% were diagnosed with ‘Acute and transient psychotic disorder’ (F23), 22.1% with ‘Schizophrenia’ (F20), 13.4% with ‘Psychosis NOS’ (F29), 12.1% with ‘Depressive episode, severe with psychotic symptoms’/‘Bipolar disorder, current episode depressed, severe, with psychotic features’ (F32.3, F31.5), 9.2% with ‘Delusional disorder’ (F22), 8.0% with ‘Manic episode, severe with psychotic symptoms/’Bipolar affective disorder, current episode manic with psychotic symptoms/‘Bipolar affective disorder, current episode mixed’ (F30.2, F31.2, F31.6), 7.4% with ‘Schizoaffective disorder’ (F25), 1.2% with ‘Schizotypal disorder’ (F21), 1.0% with ‘Substance-induced psychoses’ (F11–19). For the purpose of analysis, the specific ICD-10 categories were aggregated into three main diagnostic groups, ‘Schizophrenia’ (F20), ‘Non Affective Psychoses’ (NAP) (F11-19, F22, F23, F25, F29) and ‘Affective Psychoses’ (AP) (F32.3, F31.5, F30.2, F31.2, F31.6).
Table 2 shows the baseline patients' demographic information by diagnostic group.
Table 2. Baseline socio-demographic characteristics by main diagnostic category (n = 397)
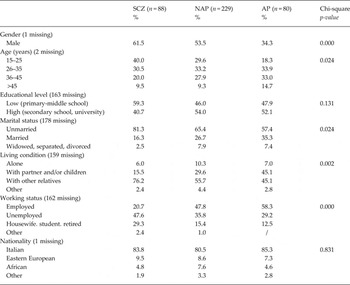
SCZ = schizophrenia, NAP = non affective psychoses, AP = affective psychoses
As expected, significant differences were found between the diagnostic groups in terms of age, gender, marital status, living conditions and employment status.
Table 3 shows comparisons between baseline ratings of the PANSS in the three diagnostic groups.
Table 3. Baseline mean scores and frequency distribution of symptom levels for the PANSS subscales (moderate/severe symptoms >3.5) and PANSS items (moderate/severe symptoms ≥4) (n = 397) (ANOVA and Chi-square)
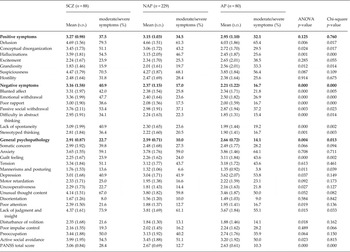
Significant differences were found in a number of psychopathological dimensions, with schizophrenic patients showing more severe delusions (ANOVA, p < 0.01), conceptual disorganization (ANOVA, p < 0.05), hallucinations (ANOVA, p < 0.01), mannerisms and posturing, uncooperativeness, attention deficit, lack of judgment and insight, disturbance of volition and active social avoidance (ANOVA, p < 0.05). On the other hand, patients with AP showed higher levels of grandiosity (ANOVA, p < 0.05), higher levels of guilt feelings (ANOVA, p < 0.001) and more severe depressed mood (ANOVA, p < 0.05).
Table 4 shows baseline levels of patients' disability in social roles in the three diagnostic groups.
Table 4. Baseline DAS mean scores and frequency distribution of disability levels (n = 334) (DAS items: ‘severe/very severe disability’: ≥3; DAS total score, ‘severe disability’: >3

Patients with schizophrenia also displayed poorer overall social functioning (ANOVA, p < 0.01). From item analyses it emerged that from one third to nearly one half of the patients (with non-significant differences between groups) displayed severe disability in occupational role, friction in social contact and participation in their households at illness onset. It is also interesting to note that for schizophrenic patients, over 80% of the items regarding relationship with partner and parental role were ‘not applicable’, as well as over 50% of items relating to occupation. It should be noted that among NAP and AP patients too, the items most frequently ‘not applicable’ were found in parental role and relationship with partner, though to a lesser degree than among patients with schizophrenia.
Genetic data
Of the patients assessed, 218 (55%) agreed to give a venous blood sample for DNA analysis. Regarding ethnicity, 198 patients were Caucasian (91% from Italy and 9% from Eastern Europe, mainly Romania), nine were Black Africans, six North Africans and five were of other origins (two Chinese, two Brazilians, one Indian). This sample included 47 (22%) patients with schizophrenia (F20), 127 (58%) with non schizophrenic NAP (F21–F29) and 44 (20%) with AP (F30–F32). The frequency of first-degree relatives with a history of psychosis was explored using the FIGS and compared in the three diagnostic groups. The FIGS was completed by 291 subjects (100% of the subjects from whom blood samples were obtained). The prevalence of first-degree relatives with a history of psychosis was 8.5% in the schizophrenic group, 15% in the non-schizophrenic NAP group and 13.6% in the AP group. The prevalence of first-degree relatives with a history of other psychiatric disorders was 36.2% in the schizophrenic group, 42.5% in non-schizophrenic NAP group and 43.2% in the AP group. Moreover, 142 first-degree relatives of patients assessed in PICOS (78% of relatives approached) also gave a blood sample for the DNA analysis: specifically, 76 were mothers, 56 fathers, 10 brothers or sisters; 50 trios were also available. In addition, 514 healthy control subjects selected from a population similar to the patients in ethnicity and area of residence were recruited from the Blood Transfusion Service at the Verona University Hospital and were genotyped.
A sub-sample of 116 subjects was approached for the neurological examination. Of these, 29 were excluded due to ethnicity and 19 refused the examination. Consequently, 68 subjects were assessed using the NES.
MRI and neuropsychological data
Eighty-three patients of those approached (mean age 31.02, s.d. 9.59; 36 females and 47 males) were enrolled in Module 3 at baseline. After at least 1 year, 39 patients (mean age 33.23, s.d. 9.30; 17 females, 22 males) repeated MRI and 34 (mean age 33.32, s.d. 9.47; 15 females, 19 males) of them also completed the battery of neuropsychological tests.
All the MRI and cognitive data were subsequently transferred to a PC workstation. Region of Interest (ROI) structural analyses, voxel-wise investigation of gray matter density and exploration of white matter microstructure will be implemented using specific post-processing techniques.
Discussion
This study gave an overview of the background and the methodology of PICOS, a large population-based epidemiological study of FEP patients receiving care from public mental health services located within a broad area of the Veneto region. This report also provided results of the recruitment process and of the 1- and 2-year follow-up evaluations and gave a preliminary account of baseline findings on the demographic, diagnostic and clinical characteristics of the study sample.
PICOS presents a number of strengths compared with previous research on FEP patients. First, it was conducted on the largest catchment area ever reported in the literature, covering 76% of the overall population of the Veneto region, corresponding to over 3 million inhabitants. No previous study has been performed on such a large population or has covered such a broad geographical area (over 12 000 square kilometres). Second, PICOS was conducted by examining a large epidemiologically-based cohort of FEP patients, composed of both AP and NAP, so as to reduce the probability of selection bias due to diagnostic sampling. Third, this study provided information on one of the largest epidemiological FEP samples to date; moreover, unlike clinical trials, the present study imposed no selection criteria and made no attempt to influence treatment (as such, the findings of PICOS provide a more accurate picture of routine treatment of out-patients than is possible from clinical trials). Fourth, this study aimed to monitor the course of illness over the short and medium term (i.e. 1, 2 and 5 years) in an area with relatively low mobility. Fifth, PICOS was carried out in ‘real world’ public mental health services which have been operational for several years – an approach that obviated the limitations of research programmes run in dedicated research centres. Sixth, this study included an extensive set of environmental, clinical, psychosocial and neurobiological variables, measured in both patients and their family members. Seventh, this study investigated the relationship between genetic patterns and clinical and social characteristics of FEP patients, both at the cross-sectional and longitudinal level and explored possible correlations with neurobiological data drawn from both structural and functional MRI. Eighth, an extensive and thorough assessment of service and treatment variables was also undertaken, with a specific emphasis on the contextual factors characterising each treatment setting (including staff burnout, staff quality of life and emotional atmosphere of mental health services) which are assumed to impact on patients' outcomes. Ninth, evaluations were conducted by local mental health service staff, specifically trained in the use of a set of well-known standardized assessment instruments, rather than by professional researchers: mental health staff who were systematically involved in the assessment process demonstrated that it is feasible to implement a carefully developed routine outcome assessment in mental health services by involving healthcare providers and at the same time to guarantee a satisfactory quality of data collected, provided that training in the correct use of the standardized instruments and regular checks on the quality of data are conducted.
This study also has some limitations. Specifically, the patients' DUP information was self-reported and not corroborated by ‘objective’ standardized evaluation. Moreover, it was not always possible to ask patients' family members about pre-morbid adjustment and this type of information was also frequently self-reported. In addition, only some of the PICOS sites provided a number of patients in line with the number of expected cases and could therefore be considered representative of all FEP patients treated in the overall PICOS catchment area. It is, however, noteworthy that analyses separately conducted on patients recruited in ‘non-representative sites’ yielded no patterns significantly different from those found in the overall study sample, which shows a good level of stability of this study's results (Bertani et al. Reference Bertani, Lasalvia, Bonetto, Tosato, Cristofalo, Bissoli, De Santi, Mazzoncini, Lazzarotto, Santi, Sale, Scalabrin, Abate, Tansella and Ruggeri2012). Finally, PICOS substantially recruited FEP patients who had been treated within the public sector (with the exception of two small private specialist hospitals located near Verona); it is therefore likely that the patients going to private clinicians or private facilities would have been excluded. However, this should not be considered a major problem, since previous research has shown that in the Veneto only a negligible fraction of psychotic patients are treated in private hospitals or in private practice alone and that it is standard practice for a GP to refer all psychosis cases to the public mental health services (Amaddeo et al. Reference Amaddeo, Zambello, Tansella and Thornicroft2001).
Preliminary findings indicate that: (1) a project of this type is feasible; (2) the participation rate is acceptable; (3) the demographic characteristics of the sample cover a broad spectrum, and (4) the clinical presentations are heterogeneous, both before and at the time of the first service contact.
This study has outlined the characteristics of a carefully characterized sample of young people presenting their first episode of psychosis to a range of treatment facilities in an epidemiologically-based catchment area. This paper serves as an introduction to a complex longitudinal project. Further longitudinal examination will help to confirm the diagnoses, check for change in diagnosis and provide far more detailed information about the variation of psychopathology and outcome. PICOS will also chart the course of illness and its predictors. Identification of specific predictors of course and outcome in FEP patients is expected to have considerable benefits in clinical practice. Early identification of poor responders to treatment would allow timely adjustments to management programmes. In addition, as some predictors are modifiable, they may provide specific treatment targets. Unfortunately, most of the predictors so far identified in the literature, such as DUP, pre-morbid functioning and family history are not ‘modifiable’ predictors for patients suffering from psychosis (Nasrallah et al. Reference Nasrallah, Tandon and Keshavan2011). Thus, the challenge is to identify significant individual predictors that can be modified. New psychotherapeutic interventions (i.e. cognitive behavioral therapy for early psychosis) and psycho-educational treatment programmes also show promise (particularly when conceptualized as long-term, sustained interventions) (Fowler et al. Reference Fowler, Rollinson and French2011; Jackson et al. Reference Jackson, Bernard and Birchwood2011; Onwumere et al. Reference Onwumere, Bebbington and Kuipers2011). In short, we would advocate that future outcome studies be designed more programmatically by including and testing potentially ‘modifiable’ risk factors that can subsequently be evaluated in experimental clinical research (Bromet et al. Reference Bromet, Naz, Fochtmann, Carlson and Tanenberg-Karant2005). PICOS was designed with the aim of making a substantial contribution to these important research and clinical questions. It is our hope that the research strategies adopted in PICOS will enhance the convergence of methodologies across ongoing and future studies on FEP patients.
Acknowledgements
This study was supported by the Ricerca Sanitaria Finalizzata 2004, Giunta Regionale del Veneto with a grant to Prof M. Ruggeri, Ricerca Sanitaria Finalizzata 2005, Giunta Regionale del Veneto with a grant to Dr A. Lasalvia, and by the Fondazione Cariverona, which provided a 3-year grant to the WHO Collaborating Centre for Research and Training in Mental Health and Service Organization at the University of Verona, directed by Prof M. Tansella. The grant (‘Promoting research to improve quality of care. The Verona WHO Centre for mental health research’) supports the main research projects of the following Units of the Verona WHO Centre: Psychiatric Case Register, Geographical Epidemiology and Mental Health Economics (Head: Prof F. Amaddeo); Clinical Psychopharmacology & Drug Epidemiology (Head: Dr C. Barbui); Environmental, Clinical and Genetic Determinants of Outcome of Mental Disorders (Head: Prof M. Ruggeri).
Declaration of interests
All authors declare that they have no conflicts of interest.
Appendix. The PICOS-VENETO group
Coordinating staff: M. Tansella M. Ruggeri, A. Lasalvia, M. Bertani, C. Bonetto, P. Brambilla, S. Tosato, D. Cristofalo, G. Marrella, S. Bissoli, M. Zanoni, C. Perlini, A. Ferro, S. Cerruti, M. Bellani, V. Marinelli, G. Rambaldelli.
Collaborating Sites: Bassano del Grappa: P. Tito, M. Lunardon, F. Gava, E. Borso, L. Grandina, M. Paliotto, L. Roggia. Thiene: A. Danieli, C. Poloni, M.R. Altiero, F. Piazza. Monteccchio M.: E. Ceccato, C. Busana, A. Campi, A. Zanconato. Vicenza 1 UO:P. Zamorani, R. Binotto, A. Caneva, E. Lazzarin, G. Zordan. Vicenza 2 UO:C. Dolce, G.B. Fanchin, C. Negro. Vicenza 3 UO:F. Gardellin, M. Crestale, L. Paiola, A. Sale. Pieve di Soligo: I. Morandin, E. Biondi, A. Cordella G. Favaretto, S. Geatti, P. Urbani. Treviso: M. De Rossi, G. Zanatta, J. Spessotto, R. Penelope, L. Grando, M. Sgnaolin, C. Tozzini, G. Visentin, L. Schiavon. Portogruaro:B. Gentile, M.G. Bolacchi, L. Marzotto, F. Moni, L. Rossi. San Donà di Piave: I. Amalric, C. Miceli, M.R. De Zordo, L. Ramon, S. Russo. Venezia:R. Rossi, G. Casagrande, V. De Nardo, A. Facchetti, F. Ramaciotti. Mirano: V. Marangon, G. Coppola, A. Marcolin, P. Meneghini, F. Sbraccia, C. Segato. Camposampiero:R. Riolo, L. Cappellari, M. Cutugno, L. Meneghetti, L. Longhin, B. Paoleschi. Cittadella:D. Scalabrin, L. Antonello, A. Purgato, G. Santucci, C. Tosin, R. Volpato, R. Zurlo. Padova 2 Serv.: M. Zucchetto, M. Pedron, S. Pinton, M. Benetazzo. Padova 3 Serv.:C. Cremonese, L. Pavan, M. Semenzin, L. Sifari, F. Zorzi. Rovigo: M.M. Martucci, N. Magno, G. Meloni, E. Toniolo. Adria: M. Pavanati, E. Destro, L. Finotti. Verona 1 Serv.: R. Fiorio, A. Marsilio, N. Pedrocco, P. Pollola. Verona 2 Serv.: L. Lazzarotto, F. Nosè, P. Rossin, V. Vivenza. Verona 3 Serv.: A. Lasalvia, M. Bertani, S. Bissoli, K. De Santi, G. Marrella, R. Mazzoncini, M. Ruggeri. Verona 4 Serv.: A. Urbani, L. Bianchi, G. Carcereri, L. Lunardi, G. Migliorini, G. Perdonà, C. Piazza. Legnago: D. Lamonaca, G. D'Agostini I. Boggian, G. Piccione, E. Saladini. Domegliara:F. Gomez, S. Frazzingaro. Isola della Scala: S. Nicolaou, L. Cordioli, G. Bertolazzi, V. Pagliuca. Villa Santa Chiara: M. Abate, M. Bortolomasi, M. Giacopuzzi, M. Segala. Villa Santa Giuliana: F. De Nardi, F. Basetto, C. Bernardis, A. Bezzetto, M. Santi.








