With increasing numbers of children born with CHD surviving into adulthood, identification and management of late sequelae are important clinical issues. Aortic coarctation is a congenital cardiac condition characterised by narrowing within the distal aortic arch.Reference Brickner, Hillis and Lange 1 Aortic coarctation is typically associated with upper limb and cerebral vessel hypertension with inadequate perfusion to the abdominal, renal, and lower limb vasculature. Survival into adult life is excellent; treatment with surgery and catheter-based techniques offers relief of obstruction; however, a number of important late sequelae are recognised. This suggests that aortic coarctation may not simply reflect mechanical obstruction of the aorta; however, more likely represent a widespread abnormality of vascular homoeostasis.Reference Celermajer and Greaves 2 , Reference de Divitiis, Rubba and Calabro 3 In particular, a tendency towards hypertension is noted,Reference Brickner, Hillis and Lange 1 particularly with exercise,Reference Hauser 4 potentially related to disturbed renal perfusion during development. Additional important late complications include intracerebral aneurysm formation, with an increased frequency noted in the 3rd and 4th decades of life.Reference Connolly, Huston, Brown, Warnes, Ammash and Tajik 5 , Reference Rosenthal 6 Such cerebral aneurysms are potentially lethal, with the underlying mechanism of aneurysm formation unclear. Furthermore, an increased incidence of stroke in patients with a background of CHD is recognised,Reference Lanz, Brophy, Therrien, Kaouache, Guo and Marelli 7 with increased carotid artery stiffness, a recognised risk factor for stroke in the absence of aortic stiffness,Reference van Sloten, Sedaghat and Laurent 8 as is increased pulse pressure in large registry series.Reference Selvaraj, Steg and Elbez 9 Improved understanding of abnormalities in cerebral blood flow in patients with aortic coarctation may offer insight into the mechanism of both stroke and cerebral aneurysm formation.
We hypothesised that patients with a background of aortic coarctation may have abnormalities of cerebral vascular function. We compared the cerebral blood flow velocity in the middle and posterior cerebral arteries under basal and dynamic conditions and their pulsatility indices using transcranial Doppler ultrasound of patients with aortic coarctation with age- and sex-matched controls.
Materials and methods
In this cross-sectional study, 14 patients above the age of 18 years with a background of aortic coarctation and 13 age- and sex-matched controls residing in the Hunter region of New South Wales were invited for assessment at the University of Newcastle, Clinical Nutrition Research Centre. Eligible participants were non-smokers and had no history of cerebrovascular events, including transient ischaemic attacks or uncontrolled hypertension (>160/100 mmHg assessed on site). The study was conducted according to the International Conference on Harmonization guidelines for Good Clinical Practice and approved by Hunter New England Human Research Ethics Committee and the University of Newcastle Human Research Ethics Committee and was registered (ACTRN12615000418572). All study participants provided their written informed consent.
Patients’ clinical details were obtained by review of medical records maintained at the John Hunter Hospital, and, where relevant, private cardiology practices. Additional collated data included results of 24-hour ambulatory blood pressure monitoring and cerebral CT where available. Patients were considered to have mild hypertension if the mean systolic blood pressure was between 135 and 145 mmHg on 24-hour blood pressure monitor assessment. Transthoracic echocardiography was used for assessing left ventricular ejection fraction, presence of left ventricular hypertrophy, and to document residual gradient across the aortic coarctation site in coarctation patients. Anthropometric measurements of height, weight, body mass index, and waist circumference for all participants were obtained at the research centre. Control patients did not undergo baseline echocardiography.
Potentially eligible participants attended the one-hour visit after having refrained for at least two hours from medication, food, or beverages other than water. Height, weight, and waist circumference were first measured followed by seated clinic blood pressure to determine eligibility. Participants rested for 10 minutes in a seated position. Blood pressure and arterial compliance measurements were obtained using a cardiovascular profiler (HDI Cardiovascular Profiler CR2000; Hypertension Diagnostics; Minnesota, United States of America) in accordance with international guidelines.Reference Chobanian, Bakris and Black 10 A total of four consecutive readings of blood pressure were taken at two-minute intervals: the first was discarded and the remaining values were averaged to obtain resting blood pressure – systolic, diastolic, and mean arterial pressures – and heart rate for analysis. Measurements were taken by automated oscillometry using an appropriately sized blood pressure cuff placed over the brachial artery of the non-dominant hand. To assess large and small artery elasticity indices, a tonometer – for pulse wave analysis of arterial compliance – was also positioned perpendicularly over the right radial artery with a relative signal strength of at least 20%. After discarding the first reading, an average of the remaining measurements was recorded for analysis. If the average blood pressure was greater than 160/100 mmHg, the participants were excluded and referred to their healthcare professional for further evaluation.
Cerebral blood flow velocity was assessed using transcranial Doppler ultrasound. A transcranial Doppler ultrasound headpiece (Doppler-Box X, Singen, Germany) was used, and the middle cerebral artery and the posterior cerebral artery on both the left and right sides were isolated using the transtemporal window as this provides the least interference during insonation. If the investigator was unable to obtain a satisfactory signal in both middle cerebral arteries, the participant was excluded from the study. The depths of insonation for the middle cerebral artery and the posterior cerebral artery were between 45 and 60 mm and between 65 and 80 mm, respectively.Reference Tegeler, Crutchfield and Katsnelson 11
A 30-sec continuous recording of basal blood flow velocity, with eyes opened, in the middle cerebral artery and the posterior cerebral artery – maximum, minimum, and mean blood flow velocity – was obtained before any physiological challenge. The Gosling pulsatility index and the Pourcelot resistive index reflecting intracranial vessel stiffness as well as the basal mean blood flow velocity were determined by averaging the last 10 seconds of a 30-second basal recording. Pulsatility index and resistive index were calculated as follows: pulsatility index=(maximum blood flow velocity−minimum blood flow velocity)/mean blood flow velocity; resistive index=(maximum blood flow velocity–minimum blood flow velocity)/maximum blood flow velocity. Although the pulsatility index and resistive index are linearly correlated and reflect intracranial vascular resistance,Reference Nagai, Moritake and Takaya 12 resistive index is arguably a better reflection of resistance as it combines vascular compliance in the arterial waveform, which is modifiable by blood pressure, age, and medication use.Reference Bude and Rubin 13 The pulsatility index/mean blood flow velocity ratio – multiplied by 100 for ease of reporting – was also determined as it is a recognised index of cerebral microvascular disease.Reference Wijnhoud, Koudstaal and Dippel 14
Increases in blood flow velocity in the middle cerebral artery reflect the extent of endothelial dilation in downstream arteriolar vessels, providing a measure of global cerebrovascular responsiveness.Reference Tegeler, Crutchfield and Katsnelson 11 To assess cerebrovascular responsiveness to hypercapnia in the middle cerebral artery, participants inhaled a carbogen gas mixture (5% CO2, 95% O2) through a two-way non-rebreathing mouthpiece for 180 seconds, resulting in an acute increase in blood flow velocity. The transcranial Doppler ultrasound recorded bilateral beat-to-beat mean blood flow velocity during the hypercapnic provocation. Our group has shown excellent reproducibility with this technique and is sufficiently sensitive to detect intervention improvements, and thus the procedure was repeated once for reliability with a two-minute interval for washout.Reference Wong, Nealon, Scholey and Howe 15 Cerebrovascular responsiveness to hypercapnia is calculated as the peak increase in mean blood flow velocity, expressed as a percentage of the mean blood flow velocity recorded under basal conditions; this per cent increase was also expressed as a ratio of their arterial stiffness – that is, pulsatility index/mean blood flow velocity ratio.
We also assessed neurovascular coupling capacity as endothelial function is crucial for facilitating cerebral blood flow during neuronal activation. Transcranial Doppler ultrasound recordings of mean blood flow velocity change in the posterior cerebral artery in response to photic stimuli were used as a surrogate measure of neurovascular coupling.Reference Spelsberg, Bohning, Kompf and Kessler 16 Participants were instructed to alternate between keeping their eyes closed for 60 seconds and opening their eyes to a white-coloured image or a multi-coloured image presented on a computer monitor for 20 seconds. The increase in mean blood flow velocity in response to the photic stimuli was determined by the trapezoidal method of calculating area under the curve. This protocol was repeated twice with a five-minute interval, and the results of the two measurements were averaged.
The cerebrovascular haemodynamics on the left and right sides of the middle cerebral artery and the posterior cerebral artery were averaged and used in the statistical analysis. Using age as a covariate, one-way analysis of variance was used to determine the differences between groups for all outcome measures (IBM SPSS Statistics 22). Simple linear regression analysis was performed to determine whether the presence of hypertension and age at surgery were associated with abnormalities of cerebral blood flow expressed as a continuous variable. As this study was exploratory in nature, no corrections were applied for multiple comparisons. Results are presented as mean±SEM; statistical significance was set at p<0.05.
Results
A total of 14 patients with a background of aortic coarctation underwent initial assessment; one patient was excluded as an adequate cerebral artery signal was not obtained. The clinical characteristics of the 13 patients undergoing assessment are outlined in Table 1; none of the patients in the control group had underlying documented hypertension or diabetes mellitus.
Table 1 Baseline clinical characteristics.

CoA=coarctation of aorta
In patients who underwent cerebral imaging, there was no evidence of aneurysm formation.
Transcranial Doppler ultrasound assessment was carried out on 13 patients and 13 age- and sex-matched controls. Relevant clinical characteristics of the study and control groups are outlined in Table 2. Although both groups were normotensive at the time of assessment and had comparable systematic arterial elasticity at the time of baseline assessment, the pulse pressure was significantly greater in the patient group. Of the 13 patients, 11 underwent ambulatory blood pressure assessment with two patients having mild hypertension only.
Table 2 Demographic characteristics, clinic blood pressure, arterial compliance, and baseline cerebral haemodynamics in control and patient groups.

BFV=blood flow velocity; BP=blood pressure; MCA=middle cerebral artery; PCA=posterior cerebral artery; PI=pulsatility index
*Significant using t-test
Table 2 additionally shows the cerebral haemodynamics under basal conditions. The combined average of the left and right side of the middle cerebral artery and the posterior cerebral artery revealed a significant increase in vessel stiffness, denoted by the pulsatility and resistive indices. Pulsatility index/mean blood flow velocity ratio in the posterior cerebral artery was also significantly higher in the patient group.
Compared with the control group, cerebrovascular responsiveness to hypercapnia in the patient group was 23% lower (Control: 48.2±3.7%, patient: 37.3±3.9%, p=0.058) and was significantly diminished after controlling for basal pulsatility index/mean blood flow velocity (Fig 1). The time taken to reach the peak mean blood flow velocity was similar in both groups.
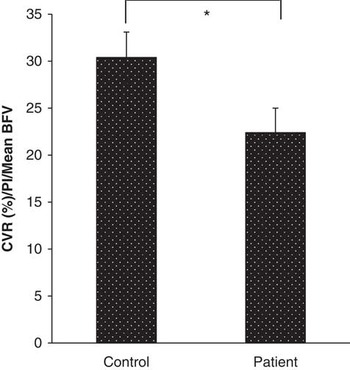
Figure 1 Cerebrovascular responsiveness to hypercapnia after adjustments for arterial stiffness and mean blood flow velocity under basal conditions. Data are presented as mean±standard error of mean. *Analysis of variance; p=0.045.
Figure 2 shows the area under the curve of mean blood flow velocity responses in the posterior cerebral artery to white- and multi-coloured images. The multi-coloured image elicited a smaller response than the white-coloured image in both groups. In response to 20 seconds of exposure to the white-coloured image, the area under the curve of mean blood flow velocity in the patient group was −66.0±46.1 cm2 and was 53.7±46.1 cm2 in the control group (p=0.080). For the multi-coloured image, the area under the curve was −0.3±16.7 cm2 and 15.7±16.7 cm2 (p=0.506) for patients and controls, respectively. Although the patient group exhibited diminished rise in mean blood flow velocity under photic stimulation for both images, there was no significant difference between the groups.

Figure 2 Area under curve of mean blood flow velocity responses in the posterior cerebral artery to white- and multi-coloured images. Data are presented as mean±standard error of mean.
Using combined cerebrovascular responsiveness to hypercapnia as the outcome variable of interest, we assessed the relationship between age at the time of surgery and cerebrovascular function. There was no evidence of any association between combined cerebrovascular responsiveness to hypercapnia and age at the time of coarctation repair (Coefficient −0.018, 95% CI −0.19 to 0.16, p=0.816). We also assessed the relationship between presence of hypertension and combined cerebrovascular responsiveness to hypercapnia. Patients with hypertension had higher mean combined cerebrovascular responsiveness to hypercapnia (mean difference=5.53%); however, this result was not statistically significant (p=0.596). The mean age of patients with hypertension was 12 years higher than those without, suggesting that age may be a potential confounder. When age was held constant, the mean difference between hypertensive and non-hypertensive subjects regarding cerebrovascular responsiveness to hypercapnia was 0.24% (p=0.984).
Discussion
The aim of the present study was to compare cerebrovascular function in patients with a background of aortic coarctation with age- and sex-matched controls. Cerebral haemodynamics in such a patient cohort have not been previously reported. Specifically, stiffness in the anterior and posterior cerebral vessels was evident, which was accompanied by diminished responsiveness to vasodilator stimuli. There was also a trend towards impaired neurovascular coupling in the patient group. If confirmed in a larger cohort, this would have potential implications for cognitive function later in life. Patients with aortic coarctation were more likely to be receiving therapy for hypertension and also noted to have a wider pulse pressure when compared with controls.
Patients with a background of aortic coarctation are at risk of late remote sequelae after relief of obstruction. As mentioned, survival into adult life is excellent; however, complications such as exercise-induced hypertensionReference Hauser 4 and cerebral aneurysmReference Connolly, Huston, Brown, Warnes, Ammash and Tajik 5 are noted with increasing age. Long-term follow-up documents late risks of coronary artery disease and stroke.Reference Cohen, Fuster, Steele, Driscoll and McGoon 17 It is believed that these complications may in part reflect abnormal long-term abnormalities in vascular function, occurring initially during development and persisting despite relief of aortic obstruction.Reference LaDisa, Dholakia and Figueroa 18 The development of intracranial aneurysm appears unrelated to hypertension alone, with endothelial dysfunction and subsequent inflammation complicating vascular and genetic abnormalities being likely contributory.Reference Chalouhi, Hoh and Hasan 19 , Reference Schievink, Mokri, Piepgras and Gittenberger-de Groot 20
More recently, an increased incidence of stroke was noted in an adult cohort with different forms of CHD.Reference Lanz, Brophy, Therrien, Kaouache, Guo and Marelli 7 Although the potential aetiology of stroke in this group may reflect in part the propensity of these patients to develop arrhythmia with subsequent thromboembolic stroke, aortic coarctation is not typically associated with an increased risk of arrhythmia during long-term follow-up.Reference Cohen, Fuster, Steele, Driscoll and McGoon 17 Therefore, alternate aetiologies of stroke should be considered. Abnormalities of vascular function and premature ageing of the vascular tree are strong potential contributory factors. Our findings provide potential mechanistic insights into this increased risk.
A recent meta-analysis has shown that impaired cerebrovascular responsiveness to hypercapnia in the middle cerebral artery is a predictor of future ischaemic stroke or transient ischaemic attack, independent of other risk factors such as diabetes, hypertension, or smoking. An absolute reduction of 10% in cerebrovascular responsiveness translates to a 64% increased risk of a cerebrovascular event.Reference Reinhard, Schwarzer and Briel 21 In the present study, the absolute cerebrovascular responsiveness difference between groups was 10.9%, suggesting a potential increased risk of future cerebrovascular events. The presence of increased carotid artery stiffness has been noted to be an important risk factor for the development of stroke, independent of traditional cardiovascular risk factors.Reference van Sloten, Sedaghat and Laurent 8 Although cerebral artery stiffness measures vascular function in a different vascular bed, these abnormalities are likely within the spectrum of adverse vascular re-modelling and may predispose to stroke in this population. Similar abnormalities in cerebral blood flow and intracranial vessel stiffness, using identical methodology, have been identified in older patients with diabetes mellitus (mean age of 68.5 years), a group with well-established vascular morbidity.Reference Wong, Nealon, Scholey and Howe 15 In fact, cerebrovascular responsiveness to hypercapnia in older diabetic adults was marginally better than our younger group of patients with a background of aortic coarctation. Previous investigations in such patients have documented abnormalities in vascular reactivity based on impaired endothelial-dependent and endothelial-independent vasodilator functions and increased vascular resistance after vasoconstrictor stimuli in peripheral vascular beds that are proximal to the aortic coarctation site.Reference Gardiner, Celermajer and Sorensen 22 – Reference de Divitiis, Pilla and Kattenhorn 25 These abnormalities, in turn, are associated with late-onset hypertension, left ventricular hypertrophy,Reference Rinnstrom, Dellborg and Thilen 26 and subsequent cardiovascular risk.Reference de Divitiis, Pilla and Kattenhorn 24 The effects of aortic coarctation, even after correction, on the cerebral vasculature are not well defined. Taken together, our findings confirm the potential adverse implications for our patients with history of aortic coarctation in terms of stroke risk and, potentially, cerebral aneurysm formation. The differences noted in cerebral blood flow in this cohort may reflect intrinsic abnormalities of cerebral vascular function in these patients, the influence of systemic hypertension, or the possible effects of residual aortic coarctation.
Poor cerebral perfusion has been associated with cognitive impairment later in life.Reference Silvestrini, Pasqualetti and Baruffaldi 27 Although the presence of cognitive impairment is recognised in patients with CHD compared with the general population, the underlying mechanisms remain unclear.Reference Gaynor, Stopp and Wypij 28 , Reference Li, Yin and Fang 29 Impaired executive function has been attributed to the effects of surgery and the underlying cardiac lesions on neurodevelopmentReference Hsia and Gruber 30 ; on the basis of these findings, abnormal cerebral perfusion may potentially contribute to this aspect. Neuronal activation promotes the endothelium to release nitric oxide, resulting in dilatation of local arterioles, which is reflected in increased blood flow in the larger vessels.Reference Dormanns, Brown and David 31 Impaired endothelial function may reduce the normal perfusion increase during neuronal activation, potentially contributing to poor cognitive performance. We observed a trend towards a blunted mean blood flow velocity increase in response to photic stimuli in the patient group compared with controls; however, we did not assess cognitive function in this study. Nonetheless, further investigation is warranted in older adults with a history of aortic coarctation to determine whether there are associations between abnormal cerebral perfusion and cognitive function.
We were unable to demonstrate any association between abnormal cerebral blood responses to stimuli and the presence of hypertension and age of surgery; this may reflect the small number of patients in the study cohort, as previous studies have demonstrated impairment of vasodilatation in patients with risk factors for vascular disease.Reference Wong, Nealon, Scholey and Howe 15
An important observation in this cohort was the increased pulse pressure noted in patients with a background of aortic coarctation compared with controls. A recent, large, registry series identified an increase in pulse pressure to be associated with an increased risk of adverse cardiovascular outcomes.Reference Selvaraj, Steg and Elbez 9 Our cohort was younger compared with the reported registry series, yet an increase in pulse pressure was already evident. This may be one of the factors contributing to premature vascular risk seen in patients with aortic coarctation.
No intracerebral aneurysms were documented in those undergoing imaging; this may reflect the young age of patients included, noting that risk increases with age,Reference Cook, Hickey and Maul 32 and the small sample size included. Similarly, we were unable to demonstrate abnormalities of systemic vascular resistance at rest, which has been previously reportedReference Gardiner, Celermajer and Sorensen 22 ; this may also reflect the cohort size. Longitudinal assessments to document the development of intracranial aneurysm, the onset of hypertension, presence of abnormal systemic vascular function, and episodes of stroke and transient cerebral ischaemia are required.
Although this study documented important initial, novel observations of cerebral blood flow characteristics in this patient cohort, a number of important limitations are recognised. As mentioned previously, the small sample size limits interpretation of data, particularly in terms of differentiating the effects of blood pressure on vascular responsiveness. A larger study with aortic coarctation subjects divided according to the presence and absence of hypertension would provide additional mechanistic insights. Irrespective of the underlying mechanism, however, this cohort developed functional abnormalities at a relatively young age, despite adequate therapy based on baseline and ambulatory blood pressure assessment; this highlights the need to aggressively address vascular risk in these patients. Blood pressure was typically recorded in the non-dominant limb; in patients with aortic arch hypoplasia, which may accompany aortic coarctation, blood pressure may therefore be underestimated. Ideally, larger trials addressing the potential confounding influence of age, hypertension, and additional cardiovascular risk factors are important in determining the underlying mechanism for adverse cardiovascular events in this cohort.
Conclusions
Adult patients with a background of aortic coarctation are known to be at risk for developing hypertension and, with increasing age, a higher incidence of intracerebral aneurysm. Stroke and cognitive impairment are also increasingly recognised as late sequelae in patients with adult CHD. Assessment of cerebral blood flow in a young cohort of patients with aortic coarctation using transcranial ultrasonography demonstrated increased intracranial vessel stiffness, widened pulse pressure, impaired vasoreactivity to CO2, and a trend towards impaired vasodilator response to visual stimuli. These abnormalities in cerebral vascular function may be associated with the increased risk of vascular events. Further investigations documenting impaired cerebral perfusion may offer additional mechanistic insights into adverse vascular function and cognitive outcomes in this growing population.
Acknowledgements
The authors acknowledge Natasha Baker for her assistance in preparing the ethics submission
Financial Support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The protocol was conducted according to International Conference on Harmonization guidelines for Good Clinical Practice and approved by Hunter New England Human Research Ethics Committee and the University of Newcastle Human Research Ethics Committee and registered (ACTRN12615000418572). All study participants provided written informed consent.







