INTRODUCTION
With the widest range of mammalian hosts, including humans, Toxoplasma gondii is the most unrestricted parasite on Earth, infecting one third of the global human population (Torrey and Yolken, Reference Torrey and Yolken2013). The organism has a complex life cycle that can involve all mammals and birds as intermediate hosts (by clonal division) and members of the Felidae family as definitive hosts (by reproduction). In immunocompetent individuals, T. gondii generally causes mild and self-limited infection. However, in categories of the population with a compromised immune system, such as foetuses and immunosuppressed individuals, the parasite may cause life-long infection of the nervous system and eyes or life-threatening disease.
Human infection occurs through the ingestion of tissue cysts, present in contaminated meat or oocysts (excreted by felids), via contaminated water, fruits and vegetables. A recent study has predicted the prevalence of T. gondii at 27% among pregnant French women until 2020 (Nogareda et al. Reference Nogareda, Le Strat, Villena, De Valk and Goulet2014). Along with sheep, pigs are the species mostly associated with human infection (Esteban-Redondo et al. Reference Esteban-Redondo, Maley, Thomson, Nicoll, Wright, Buxton and Innes1999). Consumption of infected undercooked meat or meat products is considered a major risk factor, especially in Europe, which has accounted for 30–63% of infections (Bobic et al. Reference Bobic, Jevremovic, Marinkovic, Sibalic and Djurkovic-Djakovic1998; Cook et al. Reference Cook, Gilbert, Buffolano, Zufferey, Petersen, Jenum, Foulon, Semprini and Dunn2000; Tenter et al. Reference Tenter, Heckeroth and Weiss2000). Pigs have traditionally been an important source of meat in many developing countries, and are the only species shown to regularly harbour the parasite (Hill and Dubey, Reference Hill and Dubey2013). Bradyzoites in tissue cysts have been experimentally shown to survive in meat stored at temperatures below 0 °C for 7 days (Hill et al. Reference Hill, Benedetto, Coss, McCrary, Fournet and Dubey2006), and at +58 °C for only 9·5 min (Dubey et al. Reference Dubey, Kotula, Sharar, Andrews and Lindsay1990), as well as at pressures below 300–400 Mega Pascal (Lindsay et al. Reference Lindsay, Collins, Holliman, Flick and Dubey2006). Chemical processing of pork in 2% sodium chloride and 1·4% potassium or sodium lactate inactivates parasites within 8 h (Hill et al. Reference Hill, Benedetto, Coss, McCrary, Fournet and Dubey2006) by reducing the quantity of free water (water activity index – a w ). Thus, inappropriate and insufficient use of these methods allows the parasite to stay infective in meat products, or even after preparation of pork for direct consumption.
A higher prevalence of T. gondii infection were recorded in pigs from organic and outdoor farming systems, while new sanitary conditions at intensive breeding farms have consistently resulted in a decrease in parasitic infection (van der Giessen et al. Reference van der Giessen, Fonville, Bouwknegt, Langelaar and Vollema2007). In pigs, T. gondii infection usually goes unnoticed, but can sometimes cause clinical signs such as fever, lymphadenopathy, lack of appetite (and weight loss), miscarriage, stillbirth and fetal malformation, which also lead to economic losses (Li et al. Reference Li, Wang, Yu, Li and Zhang2010).
Although in France almost 23 million (22 933 667) pigs are slaughtered every year (Association BD PORC, 2013), and pork, at 34·7%, represents a major meat consumed in France (AFSSA, 2005), no nation-wide surveillance for T. gondii in pigs has yet been conducted. One explanation is that pork is usually eaten well-cooked or roasted, but with the influence of different cultures and new culinary habits, the choice is left to the consumer regarding how long and how intensively the meat will be cooked. Therefore, in 2013, following the objectives and methods of previous nation-wide T. gondii surveillances in sheep (Halos et al. Reference Halos, Thebault, Aubert, Thomas, Perret, Geers, Alliot, Escotte-Binet, Ajzenberg, Darde, Durand, Boireau and Villena2010) and beef (data not published), a large-scale national cross-sectional survey of pigs born, raised and slaughtered in France was undertaken in order to assess the risk of T. gondii infection in pork, and its transmission to humans. The aim of this study was to investigate the prevalence of the parasite in pig meat from animals born and raised under two different breeding systems (intensive and outdoor pig farms), to analyse the geographical, age-related and breeding-system variations of this prevalence, as well as to isolate T. gondii strains present amongst pigs.
MATERIALS AND METHODS
Sampling strategy
In the Netherlands in 2007 different pig farming types were studied independently and the prevalence of 1% on intensive and 5% on outdoor farms was showed (van der Giessen et al. Reference van der Giessen, Fonville, Bouwknegt, Langelaar and Vollema2007). Based on the breeding practices reported in the Netherlands and France, the estimation was that T. gondii prevalence in France could correspond to that of the Netherlands. According to the annual pork production report (Association BD PORC, 2012) the number of samples originating from intensive farms was set at 1500, with 300 samples coming from outdoor farms. A stratified sampling strategy was devised. Based on the national report of pork production in France for 2012 (Association BD PORC, 2012), eight regions were chosen (Fig. 1), representing 92% of the annual pork production in intensive (six regions) and in outdoor farms (seven regions). In order to obtain the same precision of prevalence estimates on a national level, the same number of samples was collected from each region; 215 from intensive and 43 from outdoor farms, respectively. Next, two levels of stratification were formed: two production types and three age categories. The three age categories were defined as piglets (up to 25 kg or 2 months of age), fattening pigs (100–110 kg or 8 months of age) and older, breeding pigs-sows. From intensive farms, 115 fattening pigs, 50 piglets and 50 sow samples were collected per region. From outdoor farms, 23 fattening pigs, ten piglets and ten sow samples were obtained from each region.

Fig. 1. French pork production in numbers of animals slaughtered from A-intensive, B-outdoor pig farms and C-regions chosen for sampling. Shades of blue (intensive pig farms) and green (outdoor pig farms) represent scale of regional pork production in France. Eight regions, coloured on map C in blue, green and red, were selected for study rendering 92% of all animals slaughtered in 2012.
From all eight regions included in the study, 26 slaughterhouses were chosen in relation to the annual number of animals slaughtered. From each abattoir, samples were collected randomly, at least on two different occasions, in order to avoid overrepresentation of samples from the same farm.
Sampling protocol
Heart samples were collected, as described by Villena et al. (Reference Villena, Durand, Aubert, Blaga, Geers, Thomas, Perret, Alliot, Escotte-Binet, Thebault, Boireau and Halos2012). All samples were collected from February to December 2013. In agreement with the Ministry of Agriculture (DGAL office – The Directorate for food), all selected slaughterhouses were informed about mandatory participation in this survey. In order to avoid bias and collection errors, the slaughtering dynamics of each abattoir were analysed during the 2012 year. Based on these results, exact dates (periods of intensive production), numbers of samples, and animal categories were determined for sampling in each slaughterhouse.
On the slaughter line, the abattoir veterinarians collected heart samples (minimum 200 g) in sterile plastic containers labelled with unique bar codes. Samples were stored at +4 °C until the arrival of the transport vehicle, and were then taken (in cooled boxes at +4 °C within 12 h of slaughtering) to the laboratories of the Institute for Animal Health at Maisons-Alfort or the National Reference Centre for toxoplasmosis in Reims. Once in the laboratory, the hearts were stored at +4 °C and cardiac fluids were collected for serology analysis (Forbes et al. Reference Forbes, Parker and Gajadhar2012). Within the following 24 h, heart digestion was performed on all serology-positive animals, and randomly chosen negative ones.
Serology test
Detection of T. gondii antibodies was performed on cardiac fluids by the modified agglutination test (MAT), as described by Villena et al. (Reference Villena, Durand, Aubert, Blaga, Geers, Thomas, Perret, Alliot, Escotte-Binet, Thebault, Boireau and Halos2012). This is a species-independent serological test, considered to be the gold standard for the detection of T. gondii antibodies in animals and meat (Klun et al. Reference Klun, Djurkovic-Djakovic, Katic-Radivojevic and Nikolic2006). The antigen was provided by the National Reference Centre for toxoplasmosis in Reims, France. The starting dilution was 1:6, in accordance with a previous study in sheep (Halos et al. Reference Halos, Thebault, Aubert, Thomas, Perret, Geers, Alliot, Escotte-Binet, Ajzenberg, Darde, Durand, Boireau and Villena2010). Six further twofold dilutions were made, up to 1:200. All samples reactive at ⩾1:6 were considered positive.
Bioassay in mice and qPCR
Hearts were digested using trypsin, as described by Dubey et al. (Reference Dubey1998) and modified by Halos et al. (Reference Halos, Thebault, Aubert, Thomas, Perret, Geers, Alliot, Escotte-Binet, Ajzenberg, Darde, Durand, Boireau and Villena2010). Briefly, each whole heart was cut and ground slightly. Two-hundred grams were measured and incubated with trypsin at 37 °C for 90 min (final concentration 0·25%). The suspension was then filtered, centrifuged, and the pellet was washed twice in saline solution. From the total quantity of the pellet, 300 µL were used for DNA extraction and 2 mL were suspended in antibiotics for intra peritoneal inoculation into two Swiss-Webster mice (CD-1) in bioassay (1 mL per mouse. Ethics committee licence no: 21/01/13–2). Mice were bled after 6 weeks from the retro orbital sinus and tested by MAT for T. gondii antibodies. All seropositive mice were euthanized and brains were collected aseptically. Samples of whole brain homogenates were microscopically examined for T. gondii cysts. Of the brain homogenates, 300 µL were used for DNA extraction. All DNA extractions were done using QIAmp mini kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's instructions. Quantitative Polymerase Chain Reaction (qPCR), targeting the 529 bp, repetitive element (GeneBank accession number AF146527) was performed as previously described by Vujanic et al. (Reference Vujanic, Ivovic, Kataranovski, Nikolic, Bobic, Klun, Villena, Kataranovski and Djurkovic-Djakovic2011).
Genotyping of T. gondii isolates
All parasite isolates were genotyped by the PCR-Restriction Fragment Length Polymorphism (PCR-RLFP) method, using 11 genetic markers, including SAG1, 5′- and 3′-SAG2, altSAG2, SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1 and Apico (Su et al. Reference Su, Shwab, Zhou, Zhu and Dubey2010).
Definition of cases
A sample that was tissue-fluid positive in MAT (dilution ⩾1:6), and/or from which bio-assays live parasites/parasitic DNA were isolated, was considered as positive.
Statistical analysis
All statistics were performed using the R Core Team (2010). R is a language and environment for statistical computing made by R Foundation for Statistical Computing, Vienna, Austria (http://www.R-project.org/). For the calculation of national-level seroprevalence, the number of animals slaughtered in France in 2013, for each analysed category (Association BD PORC, 2013), was used to adjust the region- and category-specific rates. Prevalence according to the age, breeding system and region of slaughter (CATEG: ordinal scale) were analysed by generalized linear model (logistic link) (R. 3·0·3 MASS and lme4 package). Breeding system was treated as a qualitative variable (intensive or outdoor farm, reference intensive farm), as well as age (piglet, fattening pig or sow, reference: fattening pig) (Table 1). For the categorical variable – region of slaughtering (eight modalities) – the reference was the first region from the list (Aquitaine – Table 1) The correlation between age and prevalence enabled the determination of the age category in which animals are more frequently infected, and relationship prevalence – the breeding system enabled the identification of the farming method that posed a higher risk of infection. Variables associated with positivity at P ⩽ 0·1 at the 95% confidence interval (95% CI) level were tested for co-linearity and included in a multiple logistic regression model. Overall fit of the logistic regression model was assessed by the Hosmer–Lemeshow goodness-of-fit statistics. To calculate concordance, taking into account concordance by chance (between serology and bioassay, serology and qPCR and bioassay and qPCR), Kohen's kappa test with 95% CI was used (R package fmsb).
Table 1. Toxoplasma gondii seroprevalence and strain isolation from pigs in France.

All collected samples are arranged per production type, age and region of slaughtering. In every sub-category, the number of positive, total number of samples and prevalence are calculated. According to age sub-classes, calculations were possible only for fattening pigs. Finally, for all sub-categories of production and age, seroprevalence was adjusted according to total number of animals slaughtered in France, from each sub-class. No strains were isolated from piglets, 27 from fattening pigs and 14 from sows. All strains were genotyped by RFLP method as type II.
RESULTS
Toxoplasma gondii seroprevalence and strain presence in pork produced in France was analysed in eight out of 22 administrative regions based on the annual numbers of slaughtered pigs from intensive and outdoor farms.
Collected samples
The study was conducted on a total of 1549 pigs (Table 1), from 752 farms. Of the 1549, 1342 (86·7%) were intensively bred animals, and 207 (13·3%) were outdoor pigs, which is proportional to the number of animals slaughtered from each rearing system in France. According to age, samples were collected from 133 (8·6%) piglets, 1158 (74·7%) fattening (market weight) pigs and 258 (16·7%) sows (Table 1), which matches with the number of pigs slaughtered in each of these age categories in the country. The number of samples from individual farms varied between one and 25 animals, and depended on the production type, age category and size of the originating farm. Since the study was based on the numbers of animals slaughtered per region, the seroprevalence results were further analysed according to the region of slaughtering.
Seroprevalence analysis
Overall, adjusted seroprevalence among fattening pigs from both indoor and outdoor breeding systems was 2·9% (95% CI: 0·9–5·0%) (Fig. 2).

Fig. 2. Toxoplasma gondii adjusted seroprevalence and strain isolations according to the production and age categories in analysed regions. Adjusted Seroprevalence (calculated in percentage) is presented according to: 1. Production categories: outdoor pig farming – B (green scale) and intensive pig farming – A, C, D, E (blue scale); 2. Age categories: A – piglets, B and C – fattening pigs, E – sows. Numbers of strains isolated from each category per region are written in red. For intensively bred pigs the sum of all age classes is calculated on map D. Comparison was made only between B and C where there is a noticeably higher prevalence in pigs from outdoor than indoor farms. The highest number of strains originates from fattening pigs bred in outdoor farms.
Seroprevalence in pigs from intensive farms
The adjusted seroprevalence of T. gondii infection in pigs raised in intensive farm systems was 2·7% (95% CI: 0·0–7·0%). The highest seroprevalence was in the Bretagne region (6·3%, 95% CI: 3·3–9·3%) and the lowest in Basse – Normandie (1·6%, 95% CI: 1·0–5·0%) (Fig. 2). The highest adjusted seroprevalence was found in sows (13·4%, 95% CI: 6·8–19·9%), followed by fattening pigs (2·9%, 95% CI: 0·8–4·9%), while it was the lowest in piglets (2·6%, 95% CI: 0·0–6·9%) (Table 1; Fig. 2).
Seroprevalence in pigs from outdoor farms
Due to the small number of piglet and sow samples (five and seven, respectively) collected from outdoor farms, the seroprevalence was calculated only for fattening pigs. The adjusted seroprevalence was 6·3% (95% CI: 2·7–9·9%), the highest in Midi-Pyrénées (17·2% 95% CI: 5–33%) and the lowest in Poitou-Charentes (2·6%, 95% CI: 0·1–14%); in Bretagne, only three samples were tested and all were negative (Fig. 2).
Live parasite isolation
Out of all 1549 samples, parasite isolation was attempted on 160 samples (69 positive samples and 91 randomly selected negatives). In total, 41 parasite strains were isolated (Table 1), of which only one originated from an indoor fattening pig that was seronegative. All 41 strains belonged to type II, as in most European countries. The region with the highest number of the T. gondii isolates (10) was Midi-Pyrenees and only one strain originated from Poitou – Charentes (Table 1).
Bio-assays were performed on 11 piglet samples, but no strain was identified. Various T. gondii isolates were found in 27 out of 98 fattening pigs (27·55%) and 14 out of 51 sows (27·45%). From the 27 strains found in fattening pigs, 12 originated from intensive and 15 from outdoor farms. The majority of strains isolated from sows, 13 of them, originated from intensively bred animals (but 49 samples were tested), and only one came from an outdoor animal (only two samples were tested) (Table 1).
When adjusted according to the total number of animals in each age category, the strain prevalence in intensive fattening pigs was 0·9% (95% CI: 0·0–1·9%) and 8·8% in sows (95% CI: 3·2–14·3%). In outdoor fattening animals the overall strain presence was 10·2% (95% CI: 0·0–22·1%).
Concordance between serology and parasite isolation
Out of 160 bio-assays, 41 strains were found. Substantial concordance between serology and the presence of infective parasites was calculated (Kohen's kappa 0·66, 95% CI: 0·52–0·80). All digested hearts were tested in bio-assay and examined for T. gondii specific DNA by qPCR. Thirty-four out of 160 found positive (between 20th and 35th cycle), indicating moderate concordance between serology and PCR (Kohen's kappa 0·58, 95% CI: 0·43–0·72). Moderate accord was also obtained between two direct detection methods: bioassay and PCR (Kohens's kappa 0·58, 95% CI: 0·43–0·72).
Risk-factor analysis
Risk-factor analysis was performed for all 1549 animals. Univariate analysis shows that age and breeding methods both correlated with T. gondii seropositivity (Table 2). The region of slaughtering was not associated with seropositivity, which excluded possibility that certain slaughterhouses collect animals from more contaminated farms.
Table 2. Analysis of risk factors associated with T. gondii contamination of pork.

Univariate models show that age (P < 0·001) and production ( P = 0·004) but not the region of slaughtering ( P = 0·259), have influence on seroprevalence of T. gondii specific antibodies in pork. In multiple analyses, all three variables were tested and the final model showed that pigs from outdoor farms are eight times more likely to be infected with T. gondii than animals raised in intensive farms. Sows are almost five times more likely to be parasite carriers than fattening animals, and there is no statistical difference between fattening pigs and piglets ( P = 0·96). Region of slaughtering has no influence on the prevalence of T. gondii infection in pigs ( P = 0·319).
The final multiple model included age and breeding, with a 95% (0·968) rate of correct prediction in Hosmer–Lemeshow goodness-of-fit test. The results in Table 2 display a risk of in infection that is almost four times (3·62) greater for pigs raised in outdoor farms then those reared in intensive farming (P = 0·004, 97·5% CI: 1·94–6·59). Concerning the age of the animals, compared to fattening pigs, sows were at an almost fivefold (4·63) higher risk of infection (P < 0·001, 97·5% CI: 2·65–8·09), and there was no statistically significant difference between fattening animals and piglets (P = 0·96) (Table 2).
DISCUSSION
There are numerous data on the seroprevalence of T. gondii infection in pigs throughout the world, with values ranging from 0·9% in sows from Austria (Edelhofer, Reference Edelhofer1994) to 0% in pork from the Netherlands (Kijlstra et al. Reference Kijlstra, Eissen, Cornelissen, Munniksma, Eijck and Kortbeek2004), to as high as 45·3% in organic farms from Veracruz, Mexico (Alvarado-Esquivel et al. Reference Alvarado-Esquivel, Romero-Salas, Garcia-Vazquez, Crivelli-Diaz, Barrientos-Morales, Lopez-de-Buen and Dubey2014) and 37·8% in Argentina (Venturini et al. Reference Venturini, Bacigalupe, Venturini, Rambeaud, Basso, Unzaga and Perfumo2004) (Table 3). The live parasites are isolated from pigs in all parts of the world with types II and III predominant in Europe and North America (Mondragon et al. Reference Mondragon, Howe, Dubey and Sibley1998; Klun et al. Reference Klun, Vujanic, Yera, Nikolic, Ivovic, Bobic, Bradonjic, Dupouy-Camet and Djurkovic-Djakovic2011; Dubey et al. Reference Dubey, Hill, Rozeboom, Rajendran, Choudhary, Ferreira, Kwok and Su2012; Turcekova et al. Reference Turcekova, Antolova, Reiterova and Spisak2013), and atypical strains more characteristic for South America and Asia (Zakimi et al. Reference Zakimi, Kyan, Oshiro, Sugimoto, Xuenan and Fujisaki2006; Belfort-Neto et al. Reference Belfort-Neto, Nussenblatt, Rizzo, Muccioli, Silveira, Nussenblatt, Khan, Sibley and Belfort2007; Zhou et al. Reference Zhou, Nie, Zhang, Wang, Yin, Su, Zhu and Zhao2010b ; Bezerra et al. Reference Bezerra, Carvalho, Guimaraes, Rocha, Maciel, Wenceslau, Lopes and Albuquerque2012).
Table 3. Review of seroprevalences in pigs per country/continent according to pig production systems.
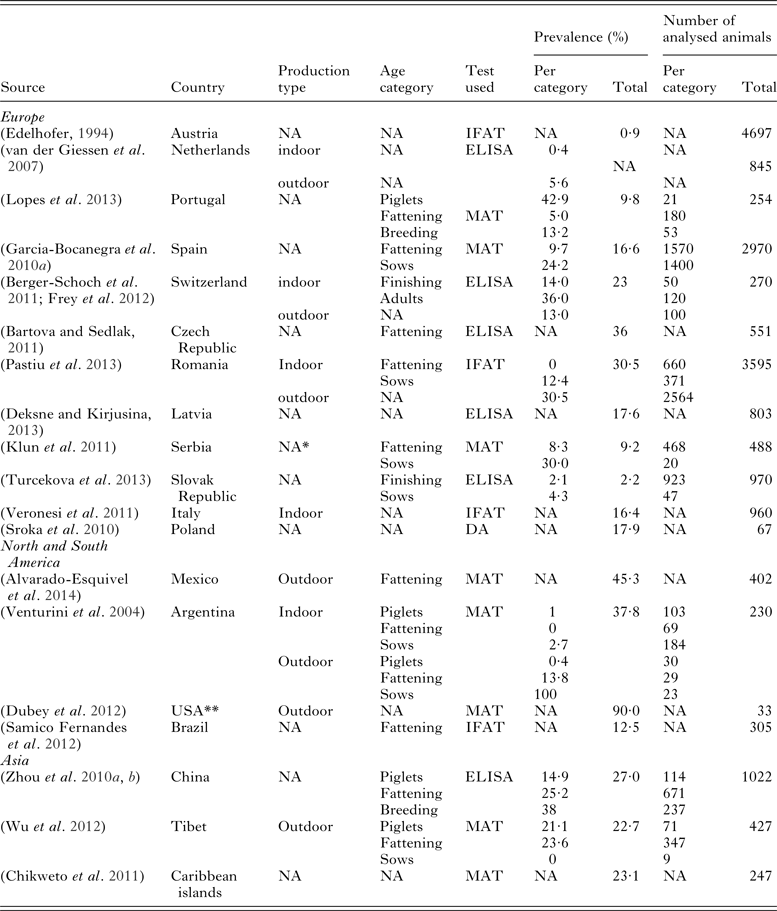
The table shows the number of animals included in each study serological tests used and obtained seroprevalence. The highest seroprevalence was recorded in Mexico (45.3%) by Alvarado-Esquivel et al. from 2014 when MAT was used for serology. In Europe, the highest seroprevalences were found in the Czech Republic and Romania, 36% (by ELISA) and 30.5% [by Immunofluorescence Antibody Test (IFAT)], respectively. In all countries except Switzerland it was found that T. gondii is more prevalent in outdoor pigs. NA, not analysed; *, production was analysed as a risk factor but the exact number of animals per production and age category cannot be determined; **, data from two farms are published.
One of the benefits of intensive pig farming is that sanitary and technical control measures are at higher levels. Keeping the animals in containment does little for their natural welfare but provides higher sanitary and animal health standards. Findings of parasitic, bacterial and virus species in these conditions are proof that these environments still have not met satisfactory levels of containment. At the same time, although organic and backdoor farming provide greater animal welfare, they represent constant reservoirs of different diseases for pigs and a greater threat to public health.
This is the first nation-wide study in France that uses pork production from both systems (indoor and outdoor) and consumption data to construct the sampling strategy. The results of this cross-sectional survey of T. gondii infection in pigs from France show adjusted prevalence values of 3·0% at intensive and 6·3% at outdoor farms. Studies in Spain and Portugal, using the same test (MAT), showed a prevalence of 16·6 and 9·8%, respectively (Garcia-Bocanegra et al. Reference Garcia-Bocanegra, Simon-Grife, Sibila, Dubey, Cabezon, Martin and Almeria2010b ; Lopes et al. Reference Lopes, Dubey, Neto, Rodrigues, Martins, Rodrigues and Cardoso2013) while in Switzerland, using an Enzyme-linked immunosorbent assay (ELISA), Berger-Schoch et al. (Reference Berger-Schoch, Herrmann, Schares, Muller, Bernet, Gottstein and Frey2011) showed prevalence of 23% (Table 3). The highest prevalence in Europe was recently reported in the Czech Republic – 36% among intensive breeding pigs (Bartova and Sedlak, Reference Bartova and Sedlak2011), and Romania – 30·5% in outdoor animals (Pastiu et al. Reference Pastiu, Gyorke, Blaga, Mircean, Rosenthal and Cozma2013).
In risk-factor analysis we found that age and breeding type associated with T. gondii positivity. Age is often considered a major risk factor for T. gondii infection in pigs, as older animals have a greater chance of acquiring an infection in a longer time frame (Villari et al. Reference Villari, Vesco, Petersen, Crispo and Buffolano2009; Halos et al. Reference Halos, Thebault, Aubert, Thomas, Perret, Geers, Alliot, Escotte-Binet, Ajzenberg, Darde, Durand, Boireau and Villena2010; Klun et al. Reference Klun, Vujanic, Yera, Nikolic, Ivovic, Bobic, Bradonjic, Dupouy-Camet and Djurkovic-Djakovic2011; Halova et al. Reference Halova, Mulcahy, Rafter, Turcekova, Grant and de Waal2013; de Sousa et al. Reference de Sousa, Lemos, Farias, Lopes and Dos Santos2014). Current study also indicates that younger age categories (piglets and fattening pigs) are more protected than breeding animals. Piglets from intensive farms are slaughtered very young (up to 45–60 days of life −25 kg), a period when maternal antibodies still can be identified (Garcia-Bocanegra et al. Reference Garcia-Bocanegra, Simon-Grife, Sibila, Dubey, Cabezon, Martin and Almeria2010b ). Infected piglets cannot be detected by current methods because animals are either in prepatent period or the parasitic infection is stopped by maternal antibodies. Moreover, in our study, out of 11 piglets from which parasitic isolation was tried, no strains were isolated; from 98 fattening pigs in 27·55% and from 51 sows in 27·45%, isolation was successful. Many piglet-producing farms have outside pens for the young, but not for sows, making these facilities more vulnerable to cat and rodent access (AFSSA, 2005) and increasing the chance of infection through contact with oocysts or infected mice and rats. Therefore, piglets may import infective oocysts that during the mother's grooming can infect the sow, or cause infection in late weaning and fattening period, after maturation of the digestive system (one oocyst is sufficient to produce infection in pigs (Dubey et al. Reference Dubey, Lunney, Shen, Kwok, Ashford and Thulliez1996)).
High prevalence among outdoor pigs is common, if we take into account that outdoor pigs are more exposed to environmental contamination (cat feces, rodents and contaminated nutrients), as well as potential contaminants of indoor farms. In Europe, the prevalence ranges from 5·6% in the Netherlands (van der Giessen et al. Reference van der Giessen, Fonville, Bouwknegt, Langelaar and Vollema2007), 17·6% in Latvia (Deksne and Kirjusina, Reference Deksne and Kirjusina2013), and 23% in Switzerland (Frey et al. Reference Frey, Berger-Schoch, Herrmann, Schares, Muller, Bernet, Doherr and Gottstein2012) (Table 3). On other continents prevalence rises, reaching 27% in South China (Zhou et al. Reference Zhou, Liang, Yin, Zhao, Yuan, Lin, Song and Zhu2010a ), 37·8% in Argentina (Venturini et al. Reference Venturini, Bacigalupe, Venturini, Rambeaud, Basso, Unzaga and Perfumo2004), 45·3% in Mexico (Alvarado-Esquivel et al. Reference Alvarado-Esquivel, Romero-Salas, Garcia-Vazquez, Crivelli-Diaz, Barrientos-Morales, Lopez-de-Buen and Dubey2014), and the Northern USA where Dubey et al. found 90% prevalence on two organic farms (33 animals) (2012). Only Berger-Schoch et al. in Switzerland showed no statistically significant difference between indoor and outdoor breeding systems (Berger-Schoch et al. Reference Berger-Schoch, Herrmann, Schares, Muller, Bernet, Gottstein and Frey2011). This study once again showed an increased risk of infection in outdoor pigs which may also be explained by the greater possibility of environmental contamination by T. gondii oocysts (Jiang et al. Reference Jiang, Gong, Ma, Nie, Zhou, Yao and Zhao2008; Feitosa et al. Reference Feitosa, Vilela, de Melo, de Almeida Neto, Souto, de Morais, Athayde, Azevedo and Pena2014) or, if there are no cats, higher incidence of pigs having contact with rodents, especially if rodent-control measures are absent (Garcia-Bocanegra et al. Reference Garcia-Bocanegra, Simon-Grife, Dubey, Casal, Martin, Cabezon, Perea and Almeria2010a , Reference Garcia-Bocanegra, Simon-Grife, Sibila, Dubey, Cabezon, Martin and Almeria b ; Piassa et al. Reference Piassa, de Araujo, da Rosa, Mattei, da Silva, Langoni and da Silva2010).
Between serology results and parasite isolation by bioassay, concordance was substantial (0·66), which suggests that the heart can be recommended as the sample of choice for epidemiological studies (Dubey, Reference Dubey1988; Halos et al. Reference Halos, Thebault, Aubert, Thomas, Perret, Geers, Alliot, Escotte-Binet, Ajzenberg, Darde, Durand, Boireau and Villena2010; Wang et al. Reference Wang, Wang, Luo, Huo, Wang, Liu, Xu, Wang, Lu, Lun, Yu and Shen2012). Worryingly, one strain has been isolated from a seronegative animal. Similar results were reported from Portugal, Ireland and Slovakia (Halova et al. Reference Halova, Mulcahy, Rafter, Turcekova, Grant and de Waal2013; Turcekova et al. Reference Turcekova, Antolova, Reiterova and Spisak2013; Esteves et al. Reference Esteves, Aguiar, Rosado, Costa, de Sousa, Antunes and Matos2014) and even the USA (Lehmann et al. Reference Lehmann, Graham, Dahl, Sreekumar, Launer, Corn, Gamble and Dubey2003; Dubey et al. Reference Dubey, Hill, Rozeboom, Rajendran, Choudhary, Ferreira, Kwok and Su2012). At the opposite, 13 positive qPCRs were obtained from seronegative animals, but without strain isolation. This can be interpreted as a lack of sensitivity of available detection methods. Considering the fast fattening period, the time between pig infection and the development of an immunological reaction could be quite limited and undetectable using existing methods. Thus, new diagnostic tools are needed in order to detect early infections in pigs and allow for more accurate risk-assessment studies. RFLP genotyping showed that type II strains are 100% prevalent among pigs in France, which corresponds with the majority of strains found in sheep (Halos et al. Reference Halos, Thebault, Aubert, Thomas, Perret, Geers, Alliot, Escotte-Binet, Ajzenberg, Darde, Durand, Boireau and Villena2010) and bovines slaughtered in France (unpublished data). Type II was also the most prevalent in other European countries: pigs from Portugal and Slovakia (de Sousa et al. Reference de Sousa, Ajzenberg, Canada, Freire, da Costa, Darde, Thulliez and Dubey2006; Turcekova et al. Reference Turcekova, Antolova, Reiterova and Spisak2013), sheep from Switzerland and Serbia (Frey et al. Reference Frey, Berger-Schoch, Herrmann, Schares, Muller, Bernet, Doherr and Gottstein2012; Markovic et al. Reference Markovic, Ivovic, Stajner, Djokic, Klun, Bobic, Nikolic and Djurkovic-Djakovic2014), but also cats from Germany (Schares et al. Reference Schares, Vrhovec, Pantchev, Herrmann and Conraths2008).
In summary, the described prevalence of T. gondii infection in pigs from France provides further evidence of the extensive infection of this parasite regardless of breeding systems, and thus innovative prevention measures are needed to lower the parasite burden in pigs, and consequently the zoonotic risk. Although more desirable from a market point of view, outdoor pigs are still more exposed to environmental contamination. Still, parasite isolation from pigs originating from closed, intensive farms goes to show that zootechnical containment measures are not sufficient in either of these conditions to allow the labelling of Toxoplasma-free pork on the market. Until more detailed risk-factor analysis can be performed, recommendations to farmers may include the introduction of more strict regulations at animal facilities.
ACKNOWLEDGEMENTS
Authors thank Professor Olin Taft for his help in improving the use of English in the manuscript.
FINANCIAL SUPPORT
This work was supported by DGAL (General Direction of Agriculture) from the Ministry of Agriculture of France (to Laboratoire de santé animale de Maisons-Alfort: R. B., C. P., T. D., I. V. & P. B.; and Centre National de Référence de la Toxoplasmose: D. A., R. G., A. M & I. V.) and co-tutelle scholarship for Ph.D. studies (to V. D.) from the French government.








