Introduction
The monoamine hypothesis of major depressive disorder (MDD) proposes that MDD is due to a deficiency in brain neurotransmitters such as serotonin (5-hydroxytryptamine or 5-HT) (Hindmarch, Reference Hindmarch2002). This hypothesized pathophysiology is supported by the mechanism of action of antidepressants, as agents that elevate the levels of these neurotransmitters in the brain have been shown to be effective in improving depressive symptoms (Prins et al. Reference Prins, Olivier and Korte2011). Recently, we reported that the 5-HT1A receptor gene was significantly associated with MDD in a meta-analysis (3119 MDD patients and 4380 healthy controls) (Kishi et al. Reference Kishi, Yoshimura, Fukuo, Okochi, Matsunaga, Umene-Nakano, Nakamura, Serretti, Correll, Kane and Iwata2013). The 5-HT1A receptors regulate 5-HT neuronal firing, and other 5-HT auto- and heteroreceptors mediate serotonin effects in various brain regions. The alteration of the brainstem 5-HT1A receptor expressions in the raphe nuclei that occurs with antidepressant treatment mediates greater 5-HT neurotransmission to the forebrain regions (Kato, Reference Kato2007; Savitz et al. Reference Savitz, Lucki and Drevets2009; Pompili et al. Reference Pompili, Serafini, Innamorati, Moller-Leimkuhler, Giupponi, Girardi, Tatarelli and Lester2010; Drago et al. Reference Drago, Crisafulli, Sidoti and Serretti2011; Pinto et al. Reference Pinto, Souza, Lioult, Semeralul, Kennedy, Warsh, Wong and Luca2011). The 5-HT1A receptor contributes to the modulation of serotonergic neural transmission activity impacting various functions, such as cognition and emotion (Aznar et al. Reference Aznar, Qian, Shah, Rahbek and Knudsen2003; Le Francois et al. Reference Le Francois, Czesak, Steubl and Albert2008; Savitz et al. Reference Savitz, Lucki and Drevets2009). In addition, the 5-HT1A receptor is considered to play a major role in neuronal migration, neurite outgrowth and synapse formation inherent to the neurodevelopmental process (Whitaker-Azmitia et al. Reference Whitaker-Azmitia, Druse, Walker and Lauder1996; Savitz et al. Reference Savitz, Lucki and Drevets2009).
The azapirone anxiolytic drugs (buspirone, gepirone, ipsapirone, tandospirone and zalospirone), which function as 5-HT1A receptor partial agonists, are frequently used as adjunctive treatments for MDD patients with inadequate response to first-line antidepressant drugs (Matheson et al. Reference Matheson, Pfeifer, Weiberg and Michel1994; Newman-Tancredi & Kleven, Reference Newman-Tancredi and Kleven2011). We identified 15 randomized controlled trials (RCTs) comparing 5-HT1A agonists with placebo: four studies with buspirone (Fabre, Reference Fabre1990; Rickels et al. Reference Rickels, Amsterdam, Clary, Hassman, London, Puzzuoli and Schweizer1990; Schweizer et al. Reference Schweizer, Rickels, Hassman and Garcia-Espana1998; Fabre et al. Reference Fabre, Clayton, Smith, Goldstein and Derogatis2012), seven with gepirone (Jenkins et al. Reference Jenkins, Robinson, Fabre, Andary, Messina and Reich1990; Rausch et al. Reference Rausch, Ruegg and Moeller1990; McGrath et al. Reference McGrath, Stewart, Quitkin, Wager, Jenkins, Archibald, Stringfellow and Robinson1994; Feiger, Reference Feiger1996; Wilcox et al. Reference Wilcox, Ferguson, Dale and Heiser1996; Feiger et al. Reference Feiger, Heiser, Shrivastava, Weiss, Smith, Sitsen and Gibertini2003; Bielski et al. Reference Bielski, Cunningham, Horrigan, Londborg, Smith and Weiss2008), three with ipsapirone (Heller et al. Reference Heller, Beneke, Kuemmel, Spencer and Kurtz1990; Lapierre et al. Reference Lapierre, Silverstone, Reesal, Saxena, Turner, Bakish, Plamondon, Vincent, Remick, Kroft, Payeur, Rosales, Lam and Bologa1998; Stahl et al. Reference Stahl, Kaiser, Roeschen, Keppel Hesselink and Orazem1998) and one with zalospirone (Rickels et al. Reference Rickels, Derivan, Kunz, Pallay and Schweizer1996). Four 5-HT1A agonist augmentation studies were identified: three with buspirone (Landen et al. Reference Landen, Bjorling, Agren and Fahlen1998; Appelberg et al. Reference Appelberg, Syvalahti, Koskinen, Mehtonen, Muhonen and Naukkarinen2001; Onder & Tural, Reference Onder and Tural2003) and one with tandospirone (Yamada et al. Reference Yamada, Yagi and Kanba2003). However, as shown in Table 1, the data showing efficacy of 5-HT1A agonists in MDD were inconsistent. These discrepant results may be due to the inclusion of small sample trials and different outcome measures. A meta-analysis can increase the statistical power for group comparisons and can overcome the limitation of sample size in underpowered studies (Cohn & Becker, Reference Cohn and Becker2003). To our knowledge, no comprehensive meta-analysis addressing the efficacy and effectiveness of 5-HT1A agonists in MDD has been published to date. To bridge this gap and synthesize the available trial evidence, we carried out a systematic review and meta-analysis of RCTs of 5-HT1A agonists for the treatment of MDD.
Table 1. Study, patient and treatment characteristics of included double-blind randomized controlled trials
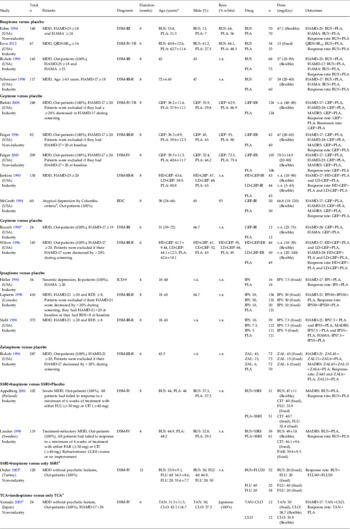
BUS, Buspirone; CLO, clomipramine; CGI-I, Clinical Global Impression – Improvement scale; CIT, citalopram; GEP, gepirone; ER, extended release; FLU, fluoxetine; HAMA, Hamilton Rating Scale for Anxiety; HAMD, Hamilton Rating Scale for Depression; HD, high dose; IPS, ipsapirone; LD, low dose; MADRS, Montgomery–Asberg Depression Rating Scale; MDD, major depressive disorder; n, number of patients; n.r., not reported; PAR, paroxetine; PLA, placebo; QIDS-SR16, 16-item Quick Inventory of Depressive Symptoms – Self-Rated scale; RDC, Research Diagnostic Criteria; s.d., standard deviation; SSRI, selective serotonin reuptake inhibitor; TAN, tandospirone; TCA, tricyclic antidepressant; ZAL, zalospirone.
a Age is given as mean±standard deviation or range.
b The criteria for atypical depression required that the patients maintain mood reactivity while depressed (the capacity to experience a mood fit in response to favorable events) and have at least one of the associated features of overeating, oversleeping, extreme anergy or pathological sensitivity to interpersonal rejection.
c Single-blind study.
d No placebo-controlled trial.
e Open trial.
Method
This meta-analysis was performed according to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 (Moher et al. Reference Moher, Liberati, Tetzlaff and Altman2009).
Inclusion criteria, search strategy, data extraction and outcome measures
Included in this study were RCTs of 5-HT1A agonists used for patients with MDD. To identify relevant studies, PubMed, Cochrane Library databases and PsycINFO citations without language restrictions were searched up to 12 October 2013, using the keywords ‘alnespirone’, ‘binospirone’, ‘buspirone’, ‘enilospirone’, ‘eptapirone’, ‘gepirone’, ‘ipsapirone’, ‘revospirone’, ‘tandospirone’ OR ‘zalospirone’ AND ‘major depressive disorder’ OR ‘major depression’. Two of the authors of this review (T.K. and Y.M.) scrutinized the inclusion and exclusion criteria of the studies identified. When the data required for the meta-analysis were missing, the first and/or corresponding authors of the papers were contacted for additional information (including endpoint scores). Two authors of this study (T.K. and Y.M.) independently extracted, checked and entered the data into Review Manager version 5.0 (Cochrane Collaboration, http://ims.cochrane.org/revman).
Data synthesis
We included outcome measures of at least two studies for each outcome measure. The primary outcome measure for efficacy was the response rate (the definition of responder based on the original study). For the 5-HT1A agonist versus placebo trials, this was: ⩾50% reduction in the total score on the 17-item Hamilton Rating Scale for Depression (HAMD-17; Hamilton, Reference Hamilton1960) from one study (Bielski et al. Reference Bielski, Cunningham, Horrigan, Londborg, Smith and Weiss2008), ⩾50% reduction in the HAMD-21 total score from one study (Lapierre et al. Reference Lapierre, Silverstone, Reesal, Saxena, Turner, Bakish, Plamondon, Vincent, Remick, Kroft, Payeur, Rosales, Lam and Bologa1998), and much improved or very much improved on the Clinical Global Impression – Improvement scale (CGI-I; Guy & Bonato, Reference Guy and Bonato1970) from 10 studies (Fabre, Reference Fabre1990; Jenkins et al. Reference Jenkins, Robinson, Fabre, Andary, Messina and Reich1990; Rickels et al. Reference Rickels, Amsterdam, Clary, Hassman, London, Puzzuoli and Schweizer1990, Reference Rickels, Derivan, Kunz, Pallay and Schweizer1996; McGrath et al. Reference McGrath, Stewart, Quitkin, Wager, Jenkins, Archibald, Stringfellow and Robinson1994; Feiger, Reference Feiger1996; Wilcox et al. Reference Wilcox, Ferguson, Dale and Heiser1996; Schweizer et al. Reference Schweizer, Rickels, Hassman and Garcia-Espana1998; Feiger et al. Reference Feiger, Heiser, Shrivastava, Weiss, Smith, Sitsen and Gibertini2003; Fava et al. Reference Fava, Tarqum, Nierenberg, Bleicher, Carter, Wedel, Hen, Gage and Barlow2012). For the 5-HT1A agonist augmentation trials, this was: ⩾50% reduction in the HAMD-17 total score from two studies (Onder & Tural, Reference Onder and Tural2003; Yamada et al. Reference Yamada, Yagi and Kanba2003), much improved or very much improved in CGI-I from one study (Landen et al. Reference Landen, Bjorling, Agren and Fahlen1998), and at least a two-point reduction on the CGI – Severity scale (CGI-S) from one study (Appelberg et al. Reference Appelberg, Syvalahti, Koskinen, Mehtonen, Muhonen and Naukkarinen2001). The secondary outcome measures also included discontinuation for all-cause, discontinuation due to adverse events, and discontinuation due to inefficacy. In addition, we pooled the data for side-effects.
Statistical analysis
We based the analyses on intent-to-treat (ITT) or modified ITT data (i.e. at least one dose or at least one follow-up assessment). However, the data from the completer analysis were not excluded to obtain as much information as possible; i.e. response rates from the studies of Rickels et al. (Reference Rickels, Derivan, Kunz, Pallay and Schweizer1996) and Onder & Tural (Reference Onder and Tural2003). The meta-analysis was performed using Review Manager version 5.1 for Windows. To combine studies, the random effects model of DerSimonian & Laird (Reference DerSimonian and Laird1986), which is conservative, was used for all cases because the underlying effect possibly differed across studies and populations, which are typically heterogeneous. The relative risk (RR) was estimated along with its 95% confidence interval (CI). In this study, when the random effects model showed significant differences between groups, the number needed to treat (NNT)/number needed to harm (NNH) was calculated. Then, NNT/NNH values were derived from the risk differences (RD) using the formula NNH = 1/RD, with the 95% CIs of NNH being the inverse of the upper and lower limits of the 95% CI of the RD. For continuous data, the standardized mean difference (SMD) was used, combining the effect size (Hedges' g) data.
There are three tests for heterogeneity: (1) Cochrane Q, (2) I 2 and (3) tau2. The I 2 statistic describes the percentage of variability due to heterogeneity rather than chance (0–100%). Because I 2 can be compared directly between meta-analyses with different numbers of studies and different types of outcome data and is preferable to a test for heterogeneity in judging consistency of evidence, we selected I 2 as a test for heterogeneity (considering values of ⩾50% to reflect considerable heterogeneity; Higgins et al. Reference Higgins, Thompson, Deeks and Altman2003). Funnel plots were inspected visually to assess the possibility of publication bias. We also assessed the methodological quality of the articles included in the meta-analysis based on the Cochrane risk of bias criteria (Cochrane Collaboration, www.cochrane.org/).
Subgroup meta-analysis
In cases of I 2 values ⩾50% for the primary outcome measures, we planned to conduct sensitivity analyses to determine the reasons for the heterogeneity. No significant heterogeneity in primary outcome measure was found. However, we performed the following four subgroup meta-analyses. (1) Atypical depression and neurotic depression have different symptoms from other types of depression. Although there were no data from a neurotic depression study (Heller et al. Reference Heller, Beneke, Kuemmel, Spencer and Kurtz1990) in primary outcome, we performed a subgroup analysis with the exception of an atypical depression study (McGrath et al. Reference McGrath, Stewart, Quitkin, Wager, Jenkins, Archibald, Stringfellow and Robinson1994). (2) We found significant subgroup differences when we subdivided types of 5-HT1A agonists (p = 0.06, I 2 = 59.2%). Therefore, we tried to find the reasons for the difference (influence of completer analysis or types of drugs). (3) We conducted another subgroup meta-analysis of clinically significant response as defined by the original studies. If the study reported plural data of response rate with difference definition, we used all available data in this subgroup meta-analysis. (4) Because 5-HT1A agonists had a higher discontinuation rate due to side-effects, the US Food and Drug Administration (FDA) rejected approval of gepirone immediate-release (IR) for the treatment of MDD and generalized anxiety disorder (GAD). The reason was that the short half-lives of 5-HT1A agonists necessitated frequent administration, and high peak plasma drug concentrations have often led to dose-limiting side-effects (Robinson et al. Reference Robinson, Sitsen and Gibertini2003). Thus, an extended-release (ER) form of gepirone was developed to allow once-daily dosing and administration of a larger single dose, with the intent of maintaining relatively low peak concentrations, thereby improving tolerability relative to earlier IR formulations (Robinson et al. Reference Robinson, Sitsen and Gibertini2003). Therefore, we conducted a subgroup meta-analysis divided by gepirone-IR versus gepirone-ER.
Results
Study characteristics
A search using the keywords mentioned earlier yielded 201 references. Seventy-five references were excluded based on the title and review of the abstracts, and 49 references were excluded based on the full text (see Fig. S1 in the online Supplementary Material). We identified 15 RCTs comparing 5-HT1A agonists with placebo (total n = 2469): four buspirone (Fabre, Reference Fabre1990; Rickels et al. Reference Rickels, Amsterdam, Clary, Hassman, London, Puzzuoli and Schweizer1990; Schweizer et al. Reference Schweizer, Rickels, Hassman and Garcia-Espana1998; Fava et al. Reference Fava, Tarqum, Nierenberg, Bleicher, Carter, Wedel, Hen, Gage and Barlow2012), three gepirone-IR (Jenkins et al. Reference Jenkins, Robinson, Fabre, Andary, Messina and Reich1990; Rausch et al. Reference Rausch, Ruegg and Moeller1990; McGrath et al. Reference McGrath, Stewart, Quitkin, Wager, Jenkins, Archibald, Stringfellow and Robinson1994), four gepirone-ER (Feiger, Reference Feiger1996; Wilcox et al. Reference Wilcox, Ferguson, Dale and Heiser1996; Feiger et al. Reference Feiger, Heiser, Shrivastava, Weiss, Smith, Sitsen and Gibertini2003; Bielski et al. Reference Bielski, Cunningham, Horrigan, Londborg, Smith and Weiss2008), three ipsapirone studies (Heller et al. Reference Heller, Beneke, Kuemmel, Spencer and Kurtz1990; Lapierre et al. Reference Lapierre, Silverstone, Reesal, Saxena, Turner, Bakish, Plamondon, Vincent, Remick, Kroft, Payeur, Rosales, Lam and Bologa1998; Stahl et al. Reference Stahl, Kaiser, Roeschen, Keppel Hesselink and Orazem1998), and one zalospirone study (Rickels et al. Reference Rickels, Derivan, Kunz, Pallay and Schweizer1996). We also identified four 5-HT1A agonist augmentation studies (total n = 365): three with buspirone (Landen et al. Reference Landen, Bjorling, Agren and Fahlen1998; Appelberg et al. Reference Appelberg, Syvalahti, Koskinen, Mehtonen, Muhonen and Naukkarinen2001; Onder & Tural, Reference Onder and Tural2003) and one with tandospirone (Yamada et al. Reference Yamada, Yagi and Kanba2003) (Table 1). For RCTs comparing 5-HT1A agonists with placebo, the mean study duration was 7.2 weeks, with one study lasting 4 weeks, four studies lasting 6 weeks and 10 studies lasting 8 weeks. Fourteen of the 15 studies were of high methodological quality based on the Cochrane risk of bias criteria, as these 14 were double-blind, RCTs, and mentioned the required details of the study design. One study was a single-blind, placebo-controlled RCT (Rausch et al. Reference Rausch, Ruegg and Moeller1990) (Supplementary Fig. S2). We based the analyses on ITT or modified ITT data (i.e. at least one dose or at least one follow-up assessment). However, the data from the completer analysis were not excluded to obtain as much information as possible (i.e. response rate from the Rickels et al. Reference Rickels, Derivan, Kunz, Pallay and Schweizer1996 study). Sample sizes ranged from 12 to 210 patients. The mean age of the study population was 43.4 years. Only one study (Schweizer et al. Reference Schweizer, Rickels, Hassman and Garcia-Espana1998) included elderly MDD patients (mean age = 72 years). No studies of adolescents/young adults were included in the meta-analysis. Twelve studies were sponsored by the pharmaceutical industry, and all studies were published in English. For 5-HT1A agonist augmentation studies, the mean study duration was 7.5 weeks, with one study lasting 4 weeks, one lasting 6 weeks, one lasting 8 weeks and one lasting 12 weeks. Two of the four studies were double-blinded RCTs (Landen et al. Reference Landen, Bjorling, Agren and Fahlen1998; Appelberg et al. Reference Appelberg, Syvalahti, Koskinen, Mehtonen, Muhonen and Naukkarinen2001) (Supplementary Fig. S2). The data from the completer analysis (i.e. response rate from the Onder & Tural, Reference Onder and Tural2003 study) were also used. Sample sizes ranged from 12 to 61 patients. The mean age of the study population was 38.7 years. Two of the four studies were sponsored by the pharmaceutical industry, and all studies were published in English. The characteristics of the studies included in our analysis are shown in Table 1.
Meta-analysis results
5-HT1A agonist versus placebo
Pooled studies of 5-HT1A agonists showed significant superiority of 5-HT1A agonists relative to placebo in responder rate (RR 0.74, 95% CI 0.65–0.83, p < 0.00001, I 2 = 48, NNT = 5, p < 0.00001; 12 trials, n = 1816) (Table 2 and Fig. 1). All of the 5-HT1A agonists, except for ipsapirone (only one study), were individually superior to placebo in response rate (buspirone: RR 0.71, 95% CI 0.54–0.92, p = 0.01, NNT = 5, four trials, n = 444; gepirone-IR+gepirone-ER: RR 0.74, 95% CI 0.65–0.85, p < 0.0001, NNT = 6, six trials, n = 826; zalospirone: RR 0.65, 95% CI 0.46–0.91, p = 0.01, NNT = 5, one trial, n = 168). Visual inspection of the funnel plot for primary outcome in 5-HT1A agonists versus placebo did not suggest the presence of publication bias (Supplementary Fig. S3), but their sensitivity was limited by the small number of studies (only 11).

Fig. 1. Forest plot of response rate.
Table 2. 5-HT1A agonist versus placebo and 5-HT1A agonist augmentation treatment: results of response rate and discontinuation rate
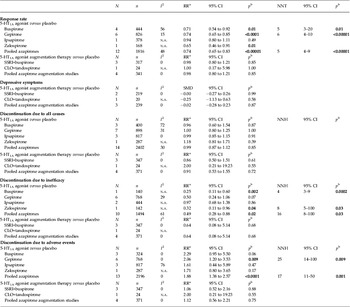
CLO, Clomipramine; N, number of studies included in the meta-analysis for each outcome; n, number of patients included in the meta-analysis for each outcome; CI, confidence interval; RR, risk ratio; NNH, numbers needed to harm; NNT, numbers needed to treat; n.a., not applicable; SMD, standardized mean difference; SSRI, selective serotonin reuptake inhibitor.
a RR < 1 favors azapirone; RR > 1 favors placebo.
b p values < 0.05 are in bold.
Neither pooled nor individual 5-HT1A agonists outperformed placebo regarding all-cause discontinuation (pooled 5-HT1A agonists: RR 0.99, p = 0.85, 14 trials, n = 2402) (Table 2 and Supplementary Fig. S4). However, whereas pooled studies of 5-HT1A agonists showed 5-HT1A agonist superiority to placebo in discontinuation due to inefficacy (RR 0.49, p = 0.02, NNH = 16, p = 0.03, 10 trials, n = 1494) (Table 2 and Fig. 2), pooled studies of 5-HT1A agonists showed 5-HT1A agonist inferiority to placebo in discontinuation due to side-effects (RR 1.88, p < 0.0001, NNH = 17, p = 0.001, 13 trials, n = 2196) (Table 2 and Fig. 3). Individually, buspirone and zalospirone were superior to placebo in discontinuation due to inefficacy (buspirone: RR 0.25, p = 0.002, NNH = 4, p = 0.0002, one trial, n = 140; zalospirone: RR 0.32, p = 0.04, NNH = 8, p = 0.03, one trial, n = 142) (Table 2 and Fig. 2). Conversely, gepirone-IR+gepirone-ER was inferior to placebo in discontinuation due to side-effects (RR 2.06, p = 0.009, NNH = 25, p = 0.009, six trials, n = 768) (Table 2 and Fig. 3). As shown in Supplementary Table S1, at least one of the side-effects, gastric distress/dyspepsia, constipation, dizziness, nausea, vomiting, insomnia, palpitation, paresthesia and sweating, occurred significantly more often with pooled studies of 5-HT1A agonists than placebo (p < 0.00001 to 0.03). Conversely, there were no significant differences in diarrhea, fatigue, drowsiness/somnolence/lightheadedness, dry mouth, headache, nervousness/asthenia and serious/severe side-effects between both treatment groups. Buspirone was associated with higher risk for at least one of the side-effects, gastric distress/dyspepsia, constipation, dizziness, nausea and sweating, compared with placebo. Gepirone was inferior to placebo in at least one of the side-effects, dizziness, nausea, insomnia, nervousness/asthenia and paresthesia. Ipsapirone and zalospirone were associated with higher risk of dizziness and nausea compared with placebo (Supplementary Table S1).
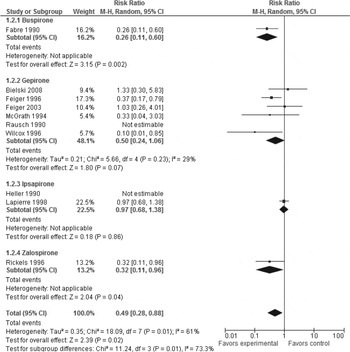
Fig. 2. Forest plot of discontinuation due to inefficacy.
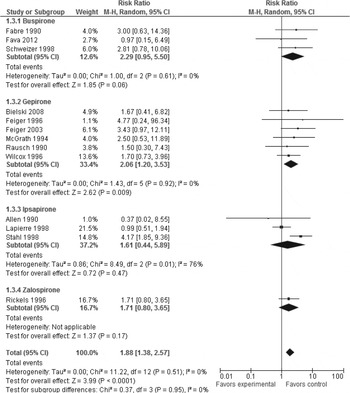
Fig. 3. Forest plot of discontinuation due to side-effects.
Subgroup meta-analysis
When excluding an atypical depression study (McGrath et al. Reference McGrath, Stewart, Quitkin, Wager, Jenkins, Archibald, Stringfellow and Robinson1994) from the meta-analysis of primary outcome, the overall result did not change (RR 0.71, 95% CI 0.64–0.80, p < 0.00001, I 2 = 26, NNT = 5, p < 0.00001, 11 trials, n = 1438).
We found significant subgroup differences in the primary outcome when we subdivided types of 5-HT1A agonists (p = 0.06, I 2 = 59.2%). When excluding an ipsapirone study (Lapierre et al. Reference Lapierre, Silverstone, Reesal, Saxena, Turner, Bakish, Plamondon, Vincent, Remick, Kroft, Payeur, Rosales, Lam and Bologa1998), the significant subgroup differences disappeared (p = 0.75, I 2 = 0%). Although response rates in completer subsets tend to be elevated for all treatments, relative to response rates in ITT subsets of patients, the data of the completer analysis were not excluded to obtain as much information as possible in the study. However, when excluding the data of the completer analysis (Rickels et al. Reference Rickels, Derivan, Kunz, Pallay and Schweizer1996) from the primary outcome, the significant superiority of 5-HT1A agonists remained (RR 0.75, 95% CI 0.66–0.85, p < 0.00001, NNT = 5, p < 0.00001), but the significant subgroup differences also remained (p = 0.05, I 2 = 66.4%).
We also conducted a subgroup meta-analysis of clinically significant response as defined by the original studies. The studies that were defined as ‘at least 50% HAMD-17 total score reduction from baseline’, ‘at least 50% HAMD-21 total score reduction from baseline’, ‘at least 50% HAMD-25 total score reduction from baseline’, ‘at least 50% Montgomery–Asberg Depression Rating Scale (MADRS; Montgomery & Asberg, Reference Montgomery and Asberg1979) total score reduction from baseline’ and ‘CGI-I (much improved or very much improved)’ as responder numbered three, one, two, one and 10 studies respectively (Supplementary Fig. S5). In all definitions of clinically significant response, except for ‘at least 50% HAMD-21 total score reduction from baseline’, 5-HT1A agonists were superior to placebo in response rate (Supplementary Fig. S5). Although there was significance for subgroup differences (I 2 = 55.7%) in this subgroup meta-analysis, when the ‘at least 50% HAMD-21 total score reduction from baseline’ group (only one ipsapirone study; Lapierre et al. Reference Lapierre, Silverstone, Reesal, Saxena, Turner, Bakish, Plamondon, Vincent, Remick, Kroft, Payeur, Rosales, Lam and Bologa1998) was excluded from the analysis, the significant subgroup difference disappeared (I 2 = 0%). When we also excluded the data of completer analysis (Rickels et al. Reference Rickels, Derivan, Kunz, Pallay and Schweizer1996) from the subgroup of ‘CGI-I (much improved or very much improved)’, the significant superiority of 5-HT1A agonists to placebo remained (RR 0.71, 95% CI 0.61–0.82, p < 0.00001, NNT = 5, p < 0.00001).
Moreover, we conducted a subgroup meta-analysis divided by gepirone-IR versus gepirone-ER. As shown in Supplementary Table S2, gepirone-ER was superior to placebo in response rate (RR 0.78, 95% CI 0.68–0.89, p = 0.0002, I 2 = 0, NNT = 7, p < 0.0001, four trials, n = 646). Although discontinuation due to all-cause (RR 0.97, p = 0.87) and inefficacy (RR 0.53, p = 0.17) was similar in both groups, gepirone-ER was inferior to placebo in discontinuation due to side-effects (RR 2.09, p = 0.02, NNH = 25, p = 0.02). On the contrary, gepirone-IR was superior to placebo in response rate (RR 0.60, 95% CI 0.41–0.88, p = 0.01, I 2 = 37, NNT = 3, p = 0.02; two trials, n = 180). Discontinuation due to all-cause (RR 1.00, p = 0.99), inefficacy (RR 0.33, p = 0.33) and side-effects (RR 1.95, p = 0.24) was similar in both groups (Supplementary Table S2).
5-HT1A agonist augmentation study
Neither pooled nor individual 5-HT1A agonists outperformed placebo regarding response rate (RR 0.98, p = 0.85), depressive symptoms (SMD = −0.02, p = 0.87) and discontinuation due to all-cause (RR 0.91, p = 0.72), inefficacy (RR 0.64, p = 0.68) and side-effects (RR 1.12, p = 0.75) (Table 2).
Discussion
To our knowledge, this is the first comprehensive meta-analysis on the effectiveness and tolerability of 5-HT1A agonists as anxiolytic drugs for the treatment of MDD and 5-HT1A agonist augmentation therapy used as an adjunct to antidepressant medications in the treatment of MDD. For this meta-analysis, we examined 19 RCTs involving 2834 patients. Our study suggests significant clinical benefits of 5-HT1A agonists for response rate (RR 0.74) and discontinuation due to inefficacy (RR 0.49). The effect sizes of response rate and discontinuation due to inefficacy were moderate (NNT: response rate = 5, discontinuation due to inefficacy = 16). However, 5-HT1A agonists were inferior to placebo in discontinuation due to side-effects (RR 1.88, NNH = 17) and 5-HT1A agonists caused several side-effects, such as gastrointestinal problems, dizziness and insomnia, more often than placebo. Although it has been reported that gepirone-ER formulations have significant antidepressant effects and better tolerability than gepirone-IR (Robinson et al. Reference Robinson, Sitsen and Gibertini2003), we found no significant difference in discontinuation due to side-effects between gepirone-IR and placebo (RR 1.95, p = 0.24) whereas gepirone-ER was significantly inferior to placebo in this outcome (RR 2.09, p = 0.02, NNH = 25, p = 0.02). In addition, although buspirone was superior to placebo in response rate, buspirone was inferior to placebo in discontinuation due to side-effects. Finally, although there was only one study on the meta-analysis of zalospirone, neither ipsapirone not zalospirone was inferior to placebo in discontinuation due to side-effects.
5-HT1A agonist augmentation therapies did not demonstrate greater efficacy than controls for the treatment of depressive symptoms. However, two of four studies were aimed at patients with treatment-refractory or severe MDD, and one of four studies was using the antidepressant clomipramine, which is a tricyclic antidepressant that tends to have more unpleasant side-effects than the newer antidepressants, such as selective serotonin reuptake inhibitors (SSRIs). In addition, we included completer analysis data. Thus, study, patient and treatment characteristics included in the meta-analysis differed greatly. Therefore, it is necessary to confirm these findings by performing a double-blind, randomized, placebo-controlled, large-sample trial of 5-HT1A agonist augmentation therapy for the treatment of MDD.
Patients with depression were reported to be two to three times more likely to rate co-morbidity of sexual dysfunction than a normal population (Angst, Reference Angst1998; Bonierbale et al. Reference Bonierbale, Lancon and Tignol2003); however, antidepressant medication can sometimes worsen or even cause sexual problems (Moll & Brown, Reference Moll and Brown2011). The impact of sexual dysfunction is substantial and negatively affects quality of life, self-esteem, mood and relationships with sexual partners. Fabre et al. (Reference Fabre, Brown, Smith and Derogatis2011a ,Reference Fabre, Smith and DeRogatis b , Reference Fabre, Clayton, Smith, Goldstein and Derogatis2012) reported that gepirone-ER improves sexual dysfunction in depressed men and women. 5-HT1A agonists seem to be better antidepressants for the treatment of MDD co-morbid patients with sexual dysfunction.
We found the following differences in intrinsic activity at 5-HT1A sites of four azapirones: buspirone ∼65%, ipsapirone ∼49%, tandospirone ∼100% and zalospirone ∼ 47% (we did not find any data for the intrinsic activity of gepirone) (Tanaka et al. Reference Tanaka, Tatsuno, Shimizu, Hirose, Kumasaka and Nakamura1995; Newman-Tancredi et al. Reference Newman-Tancredi, Gavaudan, Conte, Chaput, Touzard, Verriele, Audinot and Millan1998; Zuideveld et al. Reference Zuideveld, Rusic-Pavletic, Maas, Peletier, Van der Graaf and Danhof2002). Although tandospirone's intrinsic activity is the largest, there has been no RCT comparing tandospirone with placebo in MDD. However, Tsutsui et al. (Reference Tsutsui, Saito and Katsura1992) reported that tandospirone was significantly superior to placebo in improving disease severity scores in patients with neurosis (total n = 130, anxiety neurosis = 62, depressive neurosis = 38, obsessional neurosis = 7, phobia = 1, hysteria = 11 and hypochondria = 11). Moreover, only one patient in the tandospirone group was reported to show drowsiness (Tsutsui et al. Reference Tsutsui, Saito and Katsura1992). Tandospirone has a more beneficial effect on disease severity scores in patients with neurosis than placebo, and this treatment seemed to be well tolerated. The differences in the intrinsic activity at 5-HT1A sites of the four azapirones may influence response rates or side-effects.
The main limitation of this study is that all trials included in the meta-analysis were of short duration (⩽12 weeks). Additional long-term efficacy and safety data are needed. The second limitation is that 12 of the 15 studies of 5-HT1A agonist versus placebo were sponsored by the pharmaceutical industry. All ipsapirone and zalospirone studies were industry sponsored. Three of the four buspirone studies and five of the seven gepirone studies were also industry sponsored. Leucht et al. (Reference Leucht, Corves, Arbter, Engel, Li and Davis2009a ,Reference Leucht, Komossa, Rummel-Kluge, Corves, Hunger, Schmid, Asenjo Lobos, Schwarz and Davis b ) showed that the results from industry-sponsored studies sometimes differed from those not from industry-sponsored studies (sponsorship bias). Third, there were only six studies that excluded placebo responders in 15 RCTs of 5-HT1A agonists. There was also no study excluding the placebo responders in the 5-HT1A agonist augmentation therapy studies. Fourth, the number of 5-HT1A agonist augmentation therapy studies in the meta-analysis was low, at only four. Fifth, all studies, except for the study of Schweizer et al. (Reference Schweizer, Rickels, Hassman and Garcia-Espana1998), included adult MDD patients. Therefore, we did not evaluate the efficacy and safety of azapirone for the treatment of adolescents/young adults, or in elderly MDD patients. Although SSRIs have been reported to be associated with worsening of suicidal ideation, and there was only one paper that stated the number of patients that had suicidal ideation in adolescents and young adults, we did not perform a meta-analysis of this outcome. Moreover, because there were few studies that reported mean change and/or endpoint scores of depressive symptoms scales such as the HAMD, we did not perform a meta-analysis of this outcome. Thus, the results of all outcomes regarding efficacy and safety (discontinuation rate and individual side-effects) did not include the data from all studies included in our analysis. In addition, we did not include all azapirone studies of MDD in our study (gepirone-ER trials: 134004, 134 006 and 134 017).
Conclusions
The results of this meta-analysis demonstrate that 5-HT1A agonists are effective for the treatment of MDD, although they are reported to produce side-effects. 5-HT1A agonist augmentation therapy does not seem to be beneficial for the treatment of MDD. However, the sample size was small, and therefore it is necessary to confirm these findings by performing a double-blind, randomized, placebo-controlled, large-sample trial 5-HT1A of agonist augmentation therapy for the treatment of MDD.
Supplementary material
For supplementary material accompanying this paper, please visit http://dx.doi.org/10.1017/S0033291713002857.
Acknowledgments
We thank Dr L. F. Fabre (Fabre-Kramer Pharmaceuticals) for providing important information on recent gepirone-ER trials (134 004, 134 006 and 134 017) that have not yet been published. Dr H. Y. Meltzer is the recipient of a research grant from Dainippon Sumitomo; his work on this manuscript received no financial compensation.
Declaration of Interest
Dr T. Kishi has received speaker's honoraria from Abbott, Astellas, Daiichi Sankyo, Dainippon Sumitomo, Janssen, Eli Lilly, GlaxoSmithKline, Yoshitomi, Otsuka, Meiji, Shionogi, Tsumura, Tanabe-Mitsubishi, Novartis and Pfizer. Dr H. Y. Meltzer receives grant support from Dainippon Sumitomo, En Vivo, Otsuka and Janssen. Dr N. Iwata has received speaker's honoraria from Astellas, Dainippon Sumitomo, Eli Lilly, GlaxoSmithKline, Janssen, Yoshitomi, Otsuka, Meiji, Shionogi, Novartis and Pfizer.







