Introduction
Clozapine is the most effective antipsychotic agent available for treatment-resistant schizophrenia (McEvoy et al. Reference McEvoy, Lieberman, Stroup, Davis, Meltzer, Rosenheck, Swartz, Perkins, Keefe, Davis, Severe and Hsiao2006) and has beneficial effects not just for positive and negative symptoms but also for cognitive deficits (Burton, Reference Burton2006) and functioning (Wheeler et al. Reference Wheeler, Humberstone and Robinson2009). However, clozapine is associated with a wide array of adverse effects including agranulocytosis, orthostatic hypotension, seizures, myocarditis and cardiomyopathy (Lewis et al. Reference Lewis, Barnes, Davies, Murray, Dunn, Hayhurst, Markwick, Lloyd and Jones2006) that require constant monitoring and management to minimise morbidity and improve treatment adherence. However, there are several documented adverse effects that have been demonstrated to occur less frequently (Murphy et al. Reference Murphy, Gallagher, Sharma, Ali, Lewis, Murray and Hallahan2015). Angioedema is characterised by local dermis, subcutaneous, mucosal or submucosal swelling, caused by extravasation of fluid into intestinal tissues and can be life threatening when obstruction of the airway is caused by swelling of the larynx, tongue or upper airway. It is a well-documented adverse effect of angiotensin-converting enzyme (ACE) inhibitors, and has been demonstrated to occur even after several years of treatment, leading to a failure to recognise the medications as causative agents, thus increasing morbidity and mortality (Vleeming et al. Reference Vleeming, van Amsterdam, Stricker and de Wildt1998). To our knowledge, there are only three cases reported to date of angioedema secondary to clozapine treatment. In two of these cases, angioedema developed shortly after clozapine initiation with rapid resolution on discontinuation (Mishra et al. Reference Mishra, Sahoo, Sarkar and Akhtar2007) with the other reporting angioedema 5 years after clozapine initiation with a similar resolution post-discontinuation (Tatar et al. Reference Tatar, Oflaz and Baran2014). We describe in the case report below a case of angioedema 3 months after clozapine initiation.
Case report
We report the case of a 57-year-old, single, un-employed patient (AB), residing alone in independent accommodation, with a diagnosis of schizo-affective disorder since 1986. She had four previous psychiatric inpatient admissions in 1986, 1987, 1988 and 1996. Her most prominent symptoms at these times included delusions of a persecutory and referential nature with associated significant agitation and anxiety. Depressed mood with associated biological and cognitive symptoms of depression was also evident during these periods of inpatient admission. As an out-patient, she frequently displayed symptoms of psychosis albeit to a less intense level and attained support from her community mental health team. These psychotic symptoms predominantly consisted of persecutory delusions to the extent that she often feared for her own safety, resulting in limited engagement in activities except for attendance at a mental health service training centre (4 days per week). She displayed prominent negative symptoms including amotivation, alogia and social withdrawal.
AB had no past history of deliberate self-harm or suicide attempts, however, she has described several periods where she felt life was not worth living and had associated suicidal ideation. She had no past history of psychoactive substance misuse, and had abstained from alcohol since 1988 as she had used it harmfully in the past. AB was treated with a variety of psychotropic agents in the past including flupenthixol decanoate, pimozide, olanzapine, sulpiride, trifluoperazine, lofepramine, dosulepin and buspirone. In September 2014, due to ongoing delusions of a persecutory nature, negative symptoms, depressed mood and adverse effects of medications (Parkinsonism, dystonia) ABs antipsychotic agents were switched from olanzapine 20 mg nocte and flupenthixol decanoate 40 mg intramuscularly every 2 weeks to clozapine. Of note, there was no previous history of treatment non-adherence. The dose of clozapine was increased over the course of 6 weeks to 350 mg/day. Her persecutory delusions reduced significantly and after 10 weeks of treatment she was delusion free, displayed a warmer affect, was more engaging in conversations with others and denied depressive symptomotology. The antidepressant dosulepin 150 mg nocte was continued. AB reported feeling well, not distressed by any upsetting thoughts and having a greater ability to engage with other people socially. Collateral history from family members and mental health staff noted that her mental health status was better than at any stage over the previous 25 years.
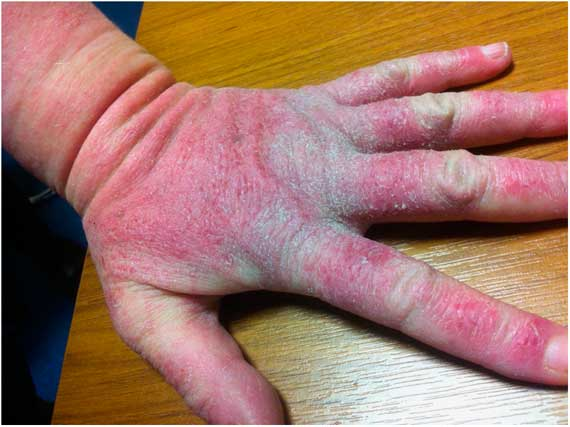
Three months after commencement of clozapine, the patient developed a generalised erthyematous pruritic rash on her trunk, arms (Figs 1 and 2) and legs with facial, periorbital, upper and lower limb oedema. There was no angioedema in or near the airway. At this time she was taking clozapine 350 mg daily and dosulepin 150 mg nocte. Plasma clozapine and norclozapine levels were normal. She was not taking any other prescribed or over the counter medications. Despite changing bed linen, detergent and out-ruling environmental causes, the rash and oedema continued to deteriorate and after 3 days her family contacted her General Practitioner (GP) and her nurse involved in administering clozapine to her. Her GP could not ascertain a medical cause for this allergic reaction which continued to deteriorate, despite the administration of an anti-histamine (Promethazine 50 mg bd). AB was significantly distressed by these symptoms. Six days after the onset of the rash, her clozapine was discontinued as her treating team believed that this was the probable cause of her symptoms. The anti-histamine was continued and over the course of 1 week the angioedema and rash completely resolved (Fig. 3).

Fig. 1 Angioedema with a generalised pruritic rash (day clozapine was discontinued).

Fig. 2 Angioedema with a generalised pruritic rash (day clozapine was discontinued).

Fig. 3 Almost complete resolution five days after discontinuation of clozapine.
No formal dermatology review was obtained, however, the case was discussed with a doctor working in the dermatology department and they were provided with a clinical description of her case including her blood results.
She was commenced on olanzapine initially, increasing to a dose of 25 mg nocte. Due to the re-emergence of psychotic symptoms (delusions of a persecutory nature) and increased social withdrawal 4 weeks after the initiation of olanzapine, this medication was augmented with aripiprazole to no effect. Subsequently aripiprazole was switched to amisulpride 400 mg bd and after the initiation of this agent over the course of 8 weeks her psychotic symptoms resolved and remained psychosis free. Some negative symptoms of psychosis have emerged (social withdrawal, alogia) but are not as prominent as prior to clozapine administration.
Of note there is no past history or family history of allergic reactions, however, no family member has previously been treated with clozapine. The causal relationship between clozapine and the adverse event was evaluated using the Naranjo Algorithm and scored 6, indicating a probable reaction to the drug (Naranjo et al. Reference Naranjo, Busto, Sellers, Sandor, Ruiz, Roberts, Janecek, Domecq and Greenblatt1981).
AB demonstrated no blood test abnormalities in relation to her liver (including alanine tramsaminase, aspartate transaminase, alkaline phosphatase and γ glutamyltransferase); thyroid or renal function. Her full blood count showed a mild eosinophilia of 0.7×10−9, with no other abnormalities (white cell count=4.8×10−9; neutrophils 2.8×10−9; lymphocytes 0.6×10−9; basophils 0.1× 10−9; leucocytes 0.1×10−9). Her eosinophil count both prior to and since commencing clozapine has been within the normal range when tested. Her clozapine level when established on 350 mg daily was 0.43 mg/l (reference range 0.35–0.5), with a norclozapine level at 0.21 mg/l. There was no concern from her family or mental health care staff regarding a period of non-compliance with prescribed treatment.
There has been no similar cutaneous or allergic reactions for the past 30 months. AB is now free from psychotic symptoms for 25 months. Her follow-up consists of out-patient clinics, attendance at a training centre and engagement with a psychologist for cognitive behaviour therapy which she previously did not feel able to engage with. This therapy will focus on understanding her illness and managing negative cognitions when her mood is depressed.
Discussion
To our knowledge, only three cases to date report angioedema secondary to clozapine treatment. Two of these cases developed angioedema shortly after clozapine initiation, with rapid resolution on discontinuation (Mishra et al. Reference Mishra, Sahoo, Sarkar and Akhtar2007). One previous report noted a significant allergic reaction and angioedema 5 years after clozapine initiation, with a similar reaction (albeit more acutely noted with olanzapine subsequently). A skin prick test revealed a sensitivity to clozapine, olanzapine and sulpiride, allowing the treating clinician to safely prescribe an alternate antipsychotic agent (Tatar et al. Reference Tatar, Oflaz and Baran2014). Significant allergic reactions with associated angioedema have also been documented in a number of case reports after treatment with other antipsychotic agents shortly after their commencement including olanzapine (Kaplan & Greaves, Reference Kaplan and Greaves2005), ziprasidone (Mohan & Dhillon, Reference Mohan and Dhillon2009) and risperidone (Cooney & Nagy, Reference Cooney and Nagy1995; Soumya et al. Reference Soumya, Grover, Dutt and Gaur2010), although we are unaware of other reported cases of allergic reactions with angioedema after medium- to long-term treatment of these antipsychotic agents. Re-challenge with risperidone after an allergic reaction was associated with a re-emergence of adverse effects to a greater severity (Soumya et al. Reference Soumya, Grover, Dutt and Gaur2010).
Angioedema can be mediated through mast cells (e.g. allergic reactions) and bradykinin (e.g. ACE inhibitor induced, hereditary angioedema). There are other causes which are unknown. Mast cell-mediated angioedema is often accompanied by other signs of mast cell-mediator release such as urticaria, flushing, generalised pruritis, bronchospasm and/or hypotension. Bradykinin-mediated angioedema is not associated with other allergic symptoms (Bingham & Zuraw Reference Bingham, Saini and Feldweg2010).
Delayed-type hypersensitivity reactions can be classified according to Coombs & Gell (Reference Coombs and Gell1968) as type IV hypersensitivity reactions which are mediated by T cells . This classification was prior to the availability of a detailed analysis of T cell subsets and functions. It now seems that T cells are involved in all types of hypersensitivity reactions (types I–IV). T cells differ in the cytokines they produce causing different types of immune responses. T-helper type 2 T cells secrete the cytokines IL-4 and IL-5 (Romagnani, Reference Romagnani1997) which can promote mast cell response (Posadas & Pichler, Reference Posadas and Pichler2007).
On balance, it is probable that AB developed mast cell-mediated angioedema given the presence of a generalised rash with widespread pruritis. Activated mast cells release inflammatory mediators, including histamine, heparin, leukotriene C4 and prostaglandin D2 which result in dilation of venules in the dermis and enhance vascular permeability causing tissue oedema (Bingham et al. Reference Bingham, Saini and Feldweg2010). In severe cases, mucosal oedema may involve the larynx, throat and tongue leading to dyspnoea, dysphagia, respiratory distress and even death due to laryngeal oedema (Kaplan & Greaves, Reference Kaplan and Greaves2005). Early diagnosis and management (including withdrawal of the causative agent) is thus required. As with AB, investigations are often unremarkable, and a high index of suspicion regarding the aetiological factor including medications, whether commenced recently or not should be considered. The causative agent should be withdrawn with appropriate supportive action enacted (Freiman et al. Reference Freiman, Borsuk and Sasseville2005).
Allergic reactions with associated angioedema have been noted as a potentially serious adverse effect subsequent to the use of a variety of antipsychotic agents including clozapine. Based on this case report, we believe that such late-onset allergic reactions are possible secondary to clozapine treatment. Failure to recognise the association between clozapine and this late-onset reaction may result in an initial failure to treat symptoms and withdraw clozapine, causing more severe symptomotology and a treatment delay.
Conclusion
This case serves as a clinical reminder that clozapine can be associated with the development of angioedema. It highlights the possibility that the development of angioedema may be delayed for several months. An earlier identification of a drug-induced angioedema can significantly reduce patient suffering.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for profit sectors.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committee on human experimentation with the Helsinki Declaration of 1975, as revised in 2008. The study protocol was approved by the institutional review board of the participating institution. Written informed consent was obtained from the patient.
Conflicts of Interest
None.