Introduction
Extravascular bleeding due to perforation of a blood vessel is one of the major complications associated with cardiac catheterisation. Severe extravascular bleeding generally induces tamponade and a decrease in circulating blood volume. However, phrenic nerve palsy is a rare complication associated with cardiac catheterisation.
For patients with a CHD, paediatric cardiologists sometimes approach the branch of the aorta, namely the subclavian artery, or the internal mammary artery. Both arteries have several branches that originate and run differently for different patients. Consequently, it is sometimes difficult to approach these branches. We describe a case of transient phrenic nerve palsy that was related to extravascular bleeding from a branch of the internal mammary artery during a diagnostic cardiac catheterisation procedure.
Case
We report on a 7-month-old male who was diagnosed with tetralogy of Fallot. At 1 month of age, the patient underwent a left-modified Blalock−Taussig shunt procedure for palliative surgery because of progressive cyanosis. After the procedure, the patient exhibited an improvement in cyanosis. A diagnostic cardiac catheterisation was performed to evaluate intracardiac repair when the patient was 7 months old. Though we tried to approach the left-modified Blalock−Taussig shunt from the left subclavian artery, it is difficult to approach the left-modified Blalock–Taussig shunt. Angiography to detect the best way to approach the left-modified Blalock–Taussig shunt showed that the 0.025 inch guidewire (SURF [PIOLAX, Yokohama, Japan]) had entered the left internal mammary artery and accidentally perforated a branch of this artery (Fig 1a and b). A careful observation was made due to minimal extravascular bleeding. The bleeding stopped after some time. After completing the catheterisation procedure, we used echocardiogram and X-ray fluoroscopy to confirm that there was no pericardial effusion or pleural effusion.
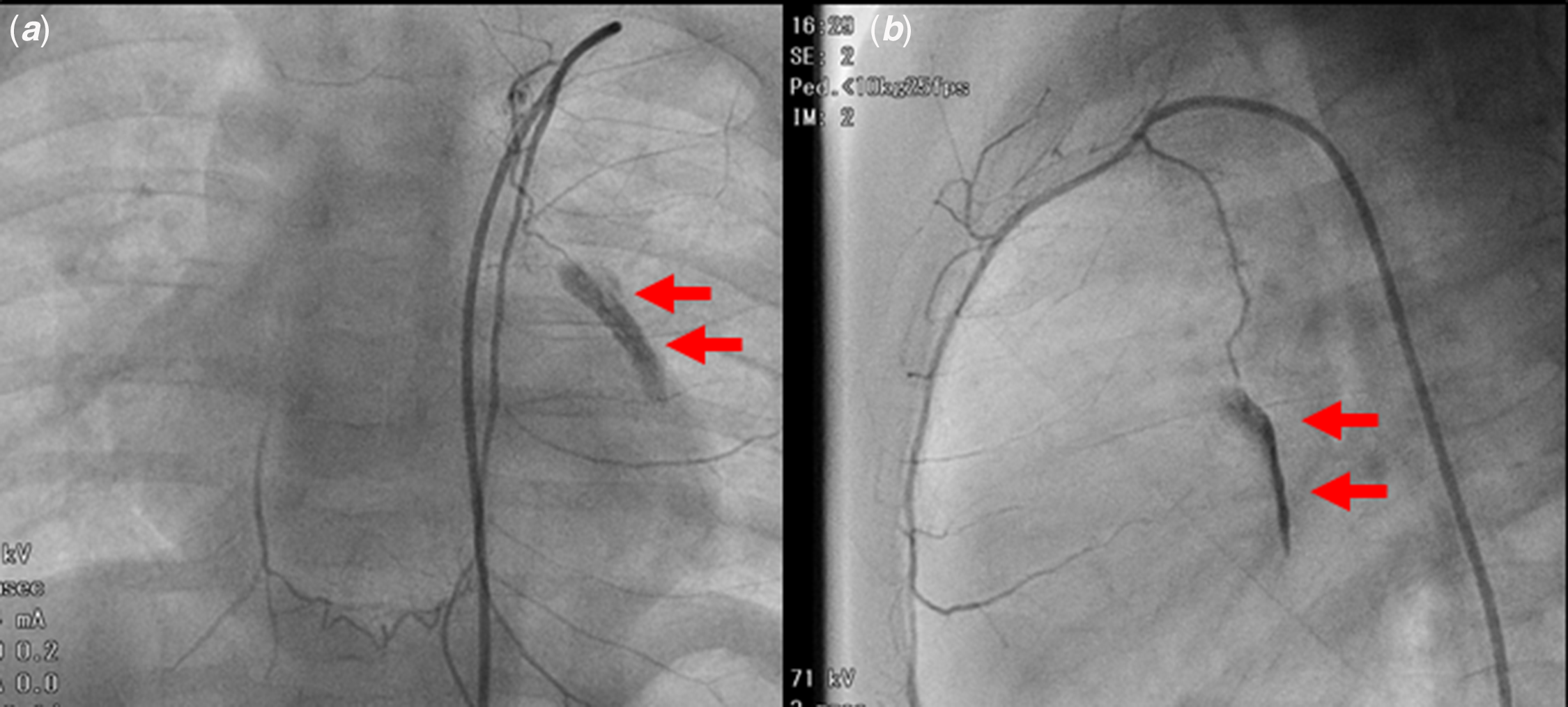
Figure 1. Extravascular bleeding from the branch of the left internal mammary artery. Anterior–posterior view ( a ) and lateral view ( b ). The arrows indicate the ruptured artery.
It was subsequently noticed that the left thoracic diaphragm was elevated, thus indicating phrenic nerve palsy (Fig 2a and b). The patient did not have any respiratory symptoms, such as retraction of the chest wall, cough, tachypnoea, or hypoxia, after the cardiac catheterisation procedure. A chest X-ray taken 1 day after the cardiac catheterisation procedure confirmed elevation of the left thoracic diaphragm (Fig 2c and d). Four days later, a chest X-ray showed a normalised left thoracic diaphragm and indicated improved phrenic nerve palsy (Fig 2e).

Figure 2. Transient phrenic nerve palsy after perforation of the artery. Anterior–posterior view of the X-ray during cardiac catheterisation before perforation ( a ) and after perforation ( b ). Chest X-rays taken one day before ( c ), one day after ( d ), and 4 days after cardiac catheterisation ( e ). The arrows indicate the elevated thoracic diaphragm.
Discussion
The phrenic nerve is a bilateral nerve that originates in the neck and descends through the thorax to the diaphragm. Reference El-Boghdadly, Chin and Chan1 The pericardiophrenic artery is a branch of the internal mammary artery that runs adjacent to the phrenic nerve, near the pericardium. In the current case, arterial bleeding was identified to be from the pericardiophrenic artery. Reference Henriquez-Pino and Prates2
A nerve injury can result from either compression to the haematoma or direct trauma from the needle or catheter. Compression injuries secondary to bleeding usually improve with resolution of the associated haematoma, while a direct injury may take much longer to improve. Reference Depierraz, Essinger, Morin, Goy and Buchser3 In our patient, haemorrhage caused by the rupture of the pericardiophrenic artery compressed the phrenic nerve, which resulted in transient phrenic nerve palsy.
Transient phrenic nerve palsy induced by cardiac catheterisation is rare. However, there are some reports about catheterisation-induced phrenic nerve palsy. Catheterisation-related phrenic nerve palsy was well-known complications for pulmonary vein isolation of radiofrequency catheter ablation. Reference Abbadessa, Lavorgna and Cirillo4 Phrenic nerve paralysis is also a rare complication associated with central venous catheterisation or cardiac electronic device implantation due to the anatomical position between the central vein and the phrenic nerve. Reference Depierraz, Essinger, Morin, Goy and Buchser3,Reference Godcharles, Safarzadeh, Oliver, Roman and Al-Kouatly5,Reference López-Gil, Fontenla, Juliá, Parra and Arribas6
It may be difficult to detect phrenic nerve palsy under general anaesthesia because the symptoms will be masked by mechanical respiration. A chest X-ray or fluoroscope could also be useful for detecting phrenic nerve palsy. In unilateral phrenic nerve palsy as in our case, patients are usually asymptomatic, have a good prognosis, and do not always need treatment. Reference Abbadessa, Lavorgna and Cirillo4 However, some patients experience severe heart failure or severe respiratory failure, are sometimes symptomatic, and need oxygen therapy or respiratory support.
Generally, coil embolisation is a better choice to stop bleeding from a ruptured artery. In our case, bleeding from the perforated site was minimal, and the patient’s vital signs were stable during careful observation. Thus, we continued the diagnostic catheterisation without performing coil embolisation.
In this case, it was difficult to access the left-modified Blalock–Taussig shunt because the entrance of the shunt was very close to that of the left internal mammary artery. We were not able to immediately recognise the wrong pathway, and the guidewire was inserted into a branch of the left internal mammary artery, although we had checked the lateral view. To avoid these complications, we suggest that operators perform angiography without hesitation if they lose their way.
In conclusion, phrenic nerve palsy is a rare complication associated with cardiac catheterisation. Cardiologists should keep in mind that the phrenic nerve runs adjacent to the pericardiophrenic artery when they access this artery.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
Informed consent was obtained from the patient for publication of this case report.





