INTRODUCTION
Late-Quaternary extinctions (LQEs) of megafauna occurred during a period of global, time-transgressive extinctions that selectively affected megafauna (terrestrial vertebrates weighing >44 kg) from 50 to 4 ka (Koch and Barnosky, Reference Koch and Barnosky2006; Stuart, Reference Stuart2015). The causes and consequences of this are still extensively debated (Johnson, Reference Johnson2009; Gill, Reference Gill2014; Sandom et al., Reference Sandom, Ejrnaes, Hansen and Svenning2014; Hempson et al., Reference Hempson, Archibald, Bond, Ellis, Grant, Kruger and Kruger2015; Malhi et al., Reference Malhi, Doughty, Galetti, Smith, Svenning and Terborgh2016; Rabanus-Wallace et al., Reference Rabanus-Wallace, Wooller, Zazula, Shute, Jahren, Kosintsev, Burns, Breen, Llamas and Cooper2017; Galetti et al., Reference Galetti, Moleón, Jordano, Pires, Guimarães, Pape and Nichols2018). In several regions of the world, such as Australia and North America, LQEs appear approximately synchronous with human colonisation events; however, direct causality remains uncertain (e.g., Johnson, Reference Johnson2009; Broughton and Weitzel, Reference Broughton and Weitzel2018). Current research is actively exploring the consequences of LQE for ecosystem functioning and biodiversity. For example, the loss of key megaherbivores has been linked to changes in fire regimes (Gill, Reference Gill2014), differences in nutrient cycling (Doughty et al., Reference Doughty, Wolf and Malhi2013), and dispersal limitation in certain plant species (Peres et al., Reference Peres, Emilio, Schietti, Desmoulière and Levi2016). Understanding both the causes and consequences of past megafaunal extinctions is important because extant megafauna is under threat of extinction today and is a strong focus of global nature conservation efforts.
One critical challenge in the study of LQEs is a better understanding of past megafauna abundance (Bradshaw et al., Reference Bradshaw, Hannon and Lister2003). Past distribution of megaherbivores is typically researched using radiocarbon-dated and identified bone remains recovered from, for example, archaeological sites, sedimentary deposits, and caves. The main weaknesses of this evidence is that, to be compelling in terms of abundance, it needs to be based on extremely comprehensive collections as it focuses on presence of animal remains; absence of evidence cannot be taken as evidence of absence (Stuart, Reference Stuart2015). For example, low abundance may be undetected when using bone remains alone and can be mistaken for extinction events (Haile et al., Reference Haile, Froese, MacPhee, Roberts, Arnold, Reyes and Rasmussen2009).
A useful methodology to address the presence/absence and abundance of megafauna uses the abundance of dung fungal spores along with fossil pollen recovered from sedimentary archives (Davis and Shafer, Reference Davis and Shafer2006; Gill et al., Reference Gill, McLauchlan, Skibbe, Goring, Zirbel and Williams2013; Baker et al., Reference Baker, Cornelissen, Bhagwat, Vera and Willis2016). This method focuses on spores of ascomycete fungi whose life cycle is fully reliant on vertebrate herbivores, and whose presence in sediments are consequently interpreted as compelling evidence for the presence of megaherbivores (van Geel et al., Reference van Geel, Buurman, Brinkkemper, Schelvis, Aptroot, van Reenen and Hakbijl2003; Davis and Shafer, Reference Davis and Shafer2006; Baker et al., Reference Baker, Bhagwat and Willis2013; Johnson et al., Reference Johnson, Rule, Haberle, Turney, Kershaw and Brook2015). The spores produced during sexual reproduction are unintentionally ingested by megaherbivores while grazing. They subsequently germinate after digestion when deposited with dung. Depending on local conditions, such as moisture levels and temperature within the deposited dung, successful mycelium growth and fructification release explosively sticky spores onto surrounding vegetation, ready to be ingested. Some spores do not complete their biological cycle and are lost into the environment. A fraction of these lost spores will be integrated into sedimentary archives after transportation by turbulent air (Gill et al., Reference Gill, McLauchlan, Skibbe, Goring, Zirbel and Williams2013), water flow (Etienne et al., Reference Etienne, Wilhelm, Sabatier, Reyes and Arnaud2013), or slope run-offs (Baker et al., Reference Baker, Cornelissen, Bhagwat, Vera and Willis2016). Despite the promise of this approach, there are some weaknesses, including the reliance on sampling sites such as lakes and bogs that may not be representative of the landscape. In addition, many studies using dung fungal spores rest on the identification of Sporormiella spores and do not include other types of dung fungal spores such as Sordaria and Podospora (Baker et al., Reference Baker, Bhagwat and Willis2013; Perrotti and van Asperen, Reference Perrotti and van Asperen2019).
Radiocarbon-dated bone collections and dung fungal spores are complementary archives but are rarely applied together. However, there is the potential to develop such an approach where bone and spore results are directly compared, when the geographical relevance of both proxies is understood, for example, on islands (Graham et al., Reference Graham, Belmecheri, Choy, Culleton, Davies, Froese and Heintzman2016) and in other isolated regions.
In North America, LQEs were particularly severe, with 69% of megafauna species greater than 45 kg going extinct before the onset of the Holocene (Stuart, Reference Stuart2015). Most species survived until the Pleistocene–Holocene transition (14–10 ka), and dramatic megaherbivore losses, in terms of abundance, have been identified using Sporormiella at sites across North American (excluding east Beringia) at about 14 ka (Robinson et al., Reference Robinson, Burney and Burney2005; Davis and Shafer, Reference Davis and Shafer2006; Gill et al., Reference Gill, Williams, Jackson, Lininger and Robinson2009; Perrotti, Reference Perrotti2018).
In northern Alaska and northeastern Siberia, bone collections have been used to estimate temporal changes in faunal communities. Using a large database of radiocarbon-dated megaherbivore remains from the Alaska North Slope, Mann et al. (Reference Mann, Groves, Kunz, Reanier and Gaglioti2013) suggest that between 45 and 10 ka there were 6 times more individuals and 30 times greater biomass of megaherbivores than at present. Dominant taxa were chiefly Mammuthus (mammoth), Equus (horse), and Bison (bison or buffalo), now extinct in the region (apart from reintroduced bison). Similar values have been calculated by Zimov et al. (Reference Zimov, Zimov, Tikhonov and Chapin2012) for northeastern Siberia, but it is not clear how far these estimates can be extrapolated to other northern regions, such as that of interior Alaska (i.e., the unglaciated region between the Brooks and Alaska Ranges, Fig. 1).
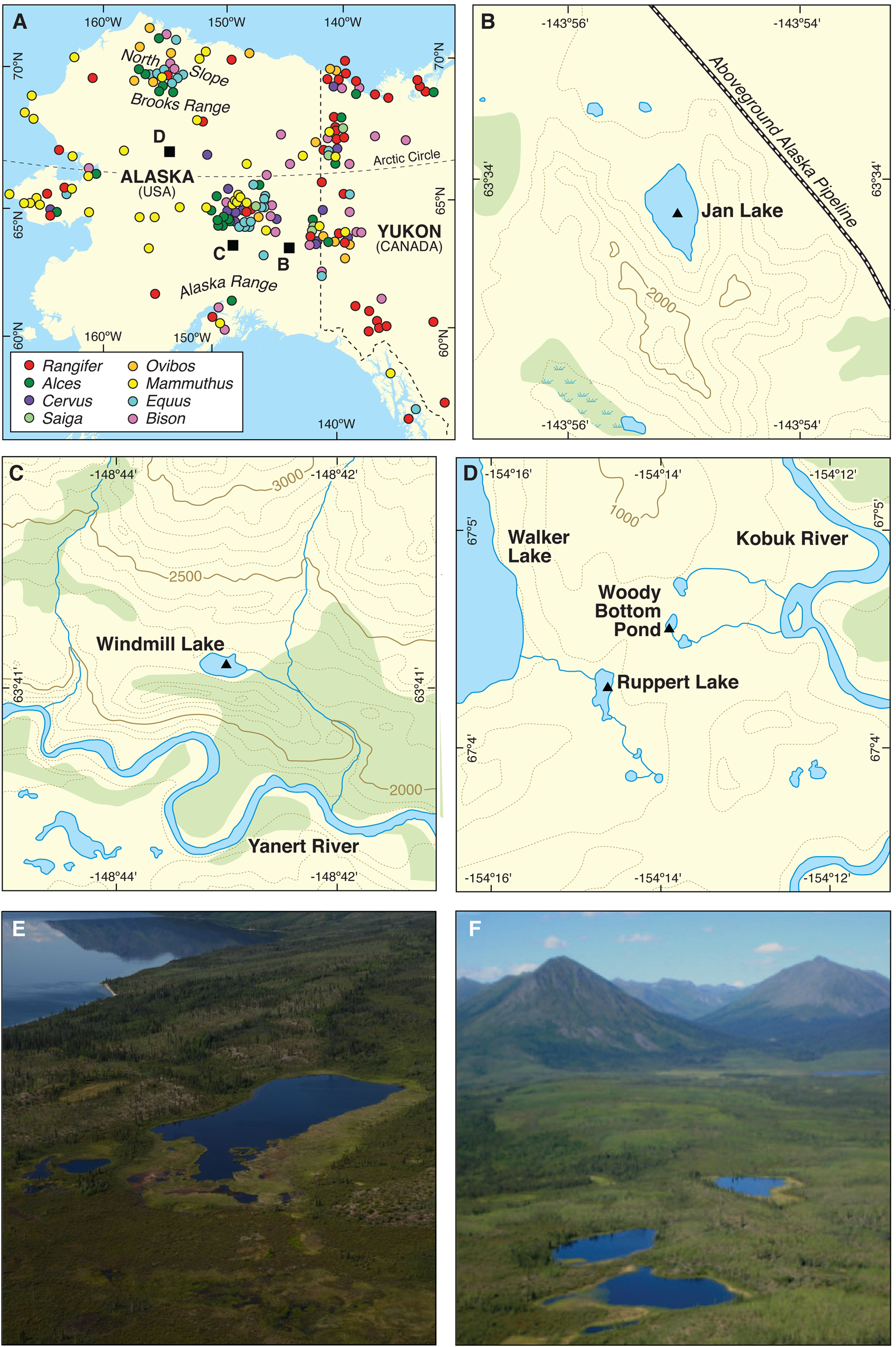
Figure 1. (color online) Location of bone remains and lake sites. (A) Map of Alaska locating megaherbivore bone remains 25 ka or younger (circles; see source publication in methods and Supplementary Table 2) and lake sites (squares). (B) Jan Lake, an alluvium-dammed basin with spore and pollen record spanning 14.2–4.6 ka. (C) Windmill Lake, a moraine-dammed basin with spore and pollen record spanning 14.8–9.5 ka. (D) Ruppert Lake and Woody Bottom Pond, two kettle lakes with spore and pollen records spanning 17–0 ka and 9.5–0 ka, respectively. (E) Oblique aerial photograph of Ruppert Lake with the larger Walker Lake, top left (photo by Tom Roland). (F) Oblique aerial photograph of Woody Bottom Pond (middle lake, photo by Maarten van Hardenbroek). Altitude is reported in feet.
Conditions at high latitudes such as those of interior Alaska were unusual in the last glacial phase because the megafauna inhabited a biome that now has no modern analogue, often termed the “mammoth-steppe” (Guthrie, Reference Guthrie1968, Reference Guthrie and Hopkins1982; Williams and Jackson, Reference Williams and Jackson2007) or “steppe-tundra” (Anderson et al., Reference Anderson, Edwards, Brubaker, Gillespie, Porter and Atwater2004). Paleobotanical records indicate vegetation was dominated by herbs (e.g., Cyperaceae, Poaceae, Artemisia, and other forbs), with only a minor component of trees and shrubs (Larix, Betula, Salix; Anderson et al. Reference Anderson, Edwards, Brubaker, Gillespie, Porter and Atwater2004; Zazula et al., Reference Zazula, Froese, Elias, Kuzmina and Mathewes2007; Gaglioti et al., Reference Gaglioti, Barnes, Zazula, Beaudoin and Wooller2011; Willerslev et al., Reference Willerslev, Davison, Moora, Zobel, Coissac, Edwards and Lorenzen2014). Moreover, the palaeo-geography of Alaska was unusual. During the last glacial maximum, northern and interior Alaska were biologically isolated from the rest of America until the opening of an inland ice-free corridor between the Laurentide and Cordilleran ice sheets along the eastern slopes of the Rocky Mountains (Kitchen et al., Reference Kitchen, Miyamoto and Mulligan2008). The estimated date at which the corridor opened and permitted megaherbivore exchange between interior Alaska and the rest of North America varies between 13.4 ka (Heintzman et al., Reference Heintzman, Froese, Ives, Soares, Zazula, Letts and Andrews2016) and 12.6 ka (Pedersen et al., Reference Pedersen, Ruter, Schweger, Friebe, Staff, Kjeldsen and Mendoza2016).
In contrast with the southern part of the continent, Alaska appears to have had staggered extinctions similar to those observed in Eurasia (Stuart, Reference Stuart2015), including an Equus species (“hemione-like” ass) prior to the last glacial maximum (Guthrie, Reference Guthrie2003). The last stage of LQEs in Alaska happened during the Pleistocene–Holocene transition and saw the loss of other species of Equus (caballine horses), Mammuthus, and Saiga (Saiga antelope). Evidence from the North Slope and Fairbanks regions has provided an estimate of temporal change in megafaunal abundance in the region around the last stages of the LQE, but this is based exclusively on an extensive collection of radiocarbon-dated bones (Mann et al., Reference Mann, Groves, Kunz, Reanier and Gaglioti2013).
The combination of Sporormiella records and bone data that we use here promises a more robust estimate of change in animal numbers. Our main aim is to study changes in population size and diversity of megaherbivores in interior Alaska across the final stage of LQEs (i.e., the last extinctions believed to have occurred by about 13 ka, [Stuart, Reference Stuart2015]). We hypothesise that the faunal loss during the final stage of the LQE in interior Alaska was associated with a sharp reduction in total population size across all megaherbivores, as indicated by Sporormiella and changes in vegetation cover. Our objectives are as follows:
(1) Identify the timing of the last extinctions by compiling a database including all existing, spatially explicit, radiocarbon-dated megaherbivore bone remains in the region.
(2) Assess whether three new Sporormiella spore records from interior Alaska have different Sporormiella spore accumulation rates before and after the last extinctions (as defined in 1).
(3) Examine the temporal sequences of 1 and 2 in relation to increasing dominance of woody vegetation across the Pleistocene–Holocene transition (based on existing dated pollen records) to assess any systematic signs of correlation between megaherbivore abundance and tree cover.
The secondary aim is to compare records of Sporormiella and other obligate dung fungal spores to improve the interpretation of dung fungal spore records in this study and in future studies. Sporormiella records have been shown to be informative in their own right, but they could be improved by extending identification to a wider range of spore types. Here, our objectives are as follows:
(1) Use a wider range of dung fungal spores, such as Sordaria and Podospora, to assess the added interpretative values of these spore types.
(2) Compare the spore record at two nearby sites to assess the local variation of dung fungal spore records.
MATERIALS AND METHODS
Faunal remains database
Geo-located and radiocarbon-dated megaherbivore bone remains from Alaska and the Yukon dated 25,000 calibrated 14C years before present or younger were compiled from relevant published work (Guthrie, Reference Guthrie2006; Campos et al., Reference Campos, Willerslev, Sher, Orlando, Axelsson, Tikhonov and Aaris-Sørensen2010; Lorenzen et al., Reference Lorenzen, Nogués-Bravo, Orlando, Weinstock, Binladen, Marske and Ugan2011; Mann et al., Reference Mann, Groves, Kunz, Reanier and Gaglioti2013; Meiri et al., Reference Meiri, Lister, Collins, Tuross, Goebel, Blockley and Zazula2014; Martindale et al., Reference Martindale, Morlan, Betts, Blake, Gajewski, Chaput, Mason and Vermeersch2016). This time range was chosen to cover a long period before and after the last stage of LQEs. This database approach provides the most comprehensive knowledge of the past distribution of megaherbivores in the region to date and includes the following taxa: Mammuthus, Equus, Saiga, Bison, Alces (moose or elk in British English), Ovibos (muskox), Rangifer (caribou, or reindeer in British English), and Cervus (elk or red deer in British English). Records of exceptional Holocene survival of Mammuthus on St. Paul Island (Graham et al., Reference Graham, Belmecheri, Choy, Culleton, Davies, Froese and Heintzman2016) were excluded.
The dated megaherbivore bone remains are drawn from very large collections of bones made over more than a century, and both the original collecting and the selection of bones for dating were made largely without regard to stratigraphic context, as they were surface finds made opportunistically on river bars and point bluffs (Guthrie, Reference Guthrie2006; Mann et al., Reference Mann, Groves, Kunz, Reanier and Gaglioti2013). In this respect, the dates are a near-random reflection of faunal remain presence or absence in space and time. Importantly, although species composition changes dramatically, the overall record of bone dates is almost continuous through the study period. In addition, the geographical distribution of bone remains is widespread across Alaska and the western Yukon (Fig. 1A). This supports the assumption that the rise and decline of remains for individual taxa in our database is likely to be a reflection of past population dynamics.
All dates were made on bone or tooth collagen and 95% (519 out of 546) were obtained by accelerator mass spectrometry (AMS). Further confidence in the dating is provided by the contexts in which they were found—permafrost and/or anaerobic contexts—which maximises endogenous collagen preservation and limits diagenesis (Guthrie, Reference Guthrie2006; Mann et al., Reference Mann, Groves, Kunz, Reanier and Gaglioti2013). Although ultrafiltration and hydroxyproline dating can increase accuracy (Kosintsev et al., Reference Kosintsev, Mitchell, Devièse, van der Plicht, Kuitems, Petrova, Tikhonov, Higham, Comeskey, Turney, Cooper, van Kolfschoten, Stuart and Lister2019), even in permafrost-preserved bones (Zazula et al., Reference Zazula, MacPhee, Southon, Nalawade-Chavan, Reyes, Hewitson and Hall2017), this mostly affects specimens dated to >25 ka, beyond the range of the present study. Raw radiocarbon age determinations were calibrated using IntCal13 in OxCal (Reimer et al., Reference Reimer, Bard, Bayliss, Beck, Blackwell, Ramsey and Buck2013), and all ages are presented as thousands of calibrated 14C years before present (cal ka BP).
Coring sites, pollen, and fungal spores
Four lakes in interior Alaska, Jan Lake, Windmill Lake, Ruppert Lake, and Woody Bottom Pond, were cored. Table 1 summarises site characteristics, coring years, and original publications for core chronologies, while the location of each lake is mapped in Figure 1. Jan Lake's basin (Fig. 1B) is formed by an alluvium dam from the Tanana River, and its watershed is characterised by metamorphic bedrock hills, with no major inlets. The surrounding vegetation is spruce, birch, and aspen forest. Windmill Lake (Fig. 1C) lies in a closed moraine-dammed basin with no major inlets. It is surrounded today by open sedge tussock vegetation with willow and nearby birch and spruce. Ruppert Lake (Figs. 1D, 1E) is a kettle lake on a terminal moraine. It has one inlet and one outlet, and the surrounding forest is dominated by spruce, aspen, birch, and willow. Woody Bottom Pond (Figs. 1E, 1F) is another kettle lake situated in close proximity (about 650 m) to Ruppert Lake. It has no inlet and is located in a bowl-shaped depression, surrounded by a well-drained moraine ridge. The lake is fringed by sedges, peat mosses, and spruce trees, while the surrounding slopes support shrub birch and aspen. All four lakes are located at similar elevations and have similar surface areas of open water (Fig. 1, Table 1).
Table 1. Characteristics of the lake study sites

To study changes in population size and diversity of megaherbivores (our first aim), we counted Sporormiella spores and pollen from the same samples. Samples were prepared for pollen analysis from 1 cm3 of wet sediment using a standard method outlined in Moore et al. (Reference Moore, Webb and Collinson1991). Pollen and spores were identified at 400× and 1000× magnification. In order to maximise reliability, we counted a minimum of 300 pollen grains (Bennett and Willis, Reference Bennett, Willis, Smol, Birks and Last2001), and Sporormiella spores observed during pollen counting were identified and counted. In order to calculate pollen and spore concentrations and accumulation rates, an exotic marker (i.e., a known number of Lycopodium annotinum spores) was added to the samples during the laboratory procedure (Wood and Wilmshurst, Reference Wood and Wilmshurst2013). Tablets of Lycopodium annotinum spores were provided by Lund University, and the tablet batch number for each sample is included in Supplementary Table 1. Identification of dung fungal spores was based on resources listed in Baker et al. (Reference Baker, Bhagwat and Willis2013). Figure 2 was created using the programme C2 v 1.3 (Juggins, Reference Juggins2007) and the R package Beeswarm (Eklund, Reference Eklund2016). At two sites, Ruppert Lake and Woody Bottom Pond, we also extended spore identification to other dung fungal spores (including Sordaria and Podospora) commonly reported in the literature (e.g., van Geel et al., Reference van Geel, Buurman, Brinkkemper, Schelvis, Aptroot, van Reenen and Hakbijl2003) (our second aim).
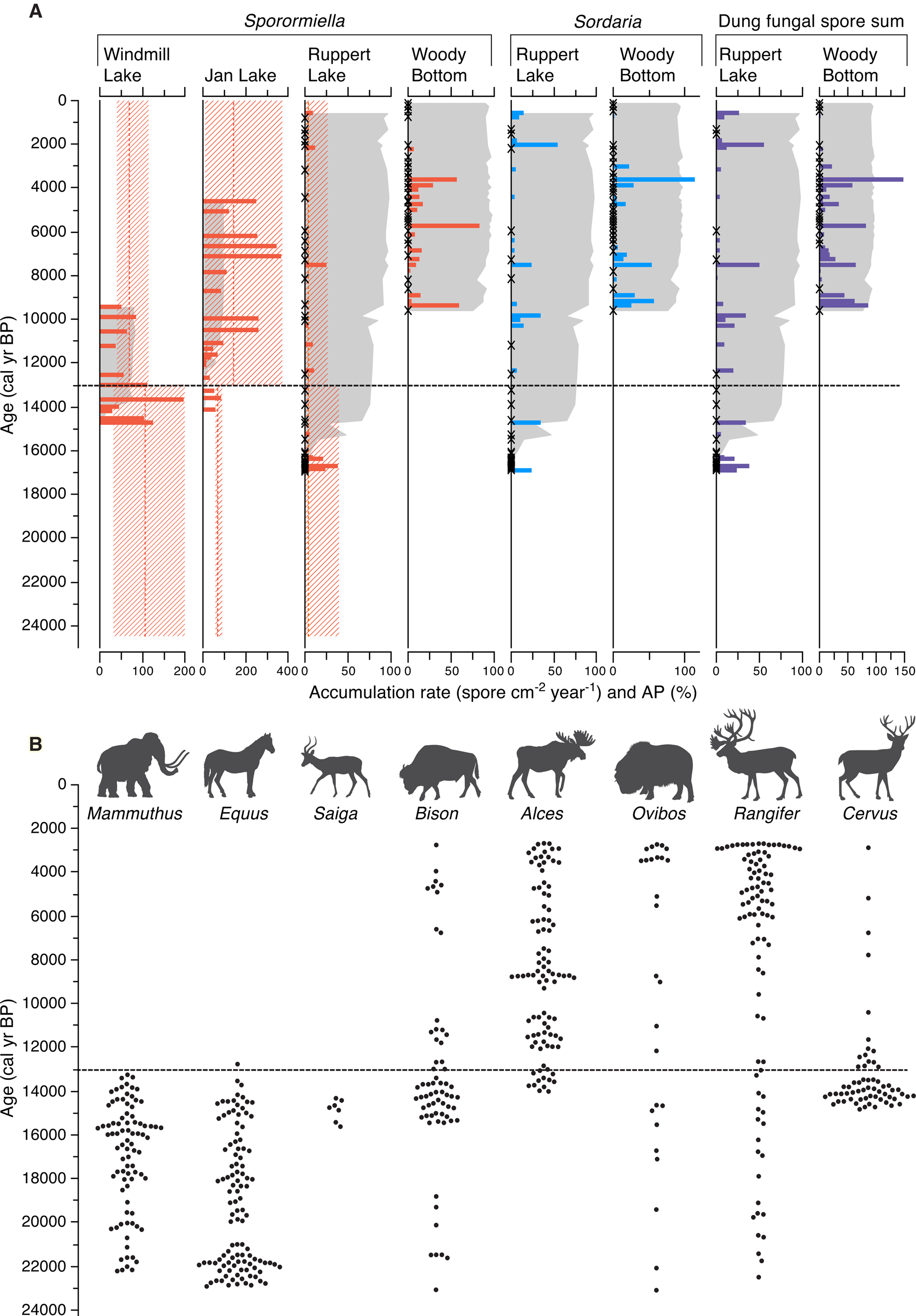
Figure 2. (color online) Spore and bone diagram. (A) Dung fungal spore accumulation rates (spore per cm2 per year) are shown as coloured bars, and percent arboreal pollen (%AP) is shown as grey shading. The mean and range of spore accumulation rates before and after the last extinctions is indicated with a dotted line and hashed zones, respectively. A small cross indicates samples where no dung fungal spores were encountered. (B) Megaherbivore bone remains 25 ka or younger from Alaska, plotted against time. Each bone is plotted as a dot using the median calibrated radiocarbon date.
RESULTS
Faunal remains database
Records of 546 georeferenced and radiocarbon-dated megaherbivore fossils from Alaska and the Yukon were collated (Fig. 1, Supplementary Table 2). The most numerous megaherbivore genera for the study period (25 ka to present) were Equus (n = 108), followed by Rangifer (n = 95), Alces (n = 93), Mammuthus (n = 85), Cervus (n = 69), Bison (n = 63), Ovibos (n = 26), and finally Saiga (n = 7). Taken at face value, temporal distribution of radiocarbon-dated megaherbivore remains indicates that Mammuthus and Equus were dominant until about 16–15 ka, when Bison and Cervus numbers increased (Fig. 2). Following the extinction of Mammuthus and Equus at about 13 ka, and subsequently during the Holocene, Alces was dominant, with Rangifer increasing in number from the mid-Holocene. We consider the marked regional turnover of megaherbivore dominant taxa around 13 ka to indicate the last stage of the LQE in interior Alaska.
The small proportion of bones dated by gas counting (i.e., pre-AMS) do not affect this result: they comprise pre-15 ka Equus, Bison, and Mammuthus; Holocene Rangifer, Alces, and Ovibos; and three Bison in the range 14–13 ka (Supplementary Table 2). All of these are corroborated by AMS dates on other specimens, and none determines a first or last appearance datum. When plotted chronologically (Fig. 2), the data show that the number of megaherbivore bones recovered in Alaska remained high and relatively constant throughout the period under consideration, despite an important turnover of dominant taxa.
Coring sites, pollen, and fungal spores
The stratigraphies and chronologies for the four cores have been published previously (Table 1), and the age-depth models are reproduced in Supplementary Figure 1. The core sediments are described in Supplementary Table 3. The median age and sediment accumulation rates (for each sample analysed for pollen or spores) are presented in Supplementary Table 1.
Ages were calibrated and age-depth models were created using the IntCal13 calibration curve (Reimer et al., Reference Reimer, Bard, Bayliss, Beck, Blackwell, Ramsey and Buck2013) and the R package “rbacon” version 2.3 (Blaauw and Christen, Reference Blaauw and Christen2011). The models used 21, 9, 11, and 9 AMS radiocarbon dates based as far as possible on macro- and micro-fossils for Jan Lake, Windmill Lake, Ruppert Lake, and Wood Bottom Pond, respectively. In addition, a tephra layer related to the Aniakchak Caldera Forming Event II, dated to 3.595 ± 0.004 ka in Greenland ice cores (Denton and Pearce Reference Denton and Pearce2008; Pearce et al., Reference Pearce, Varhelyi, Wastegård, Muschitiello, Barrientos, O'Regan and Cronin2016), was identified and used to further constrain the models in Ruppert Lake and Woody Bottom Pond (Monteath et al., Reference Monteath, van Hardenbroek, Davies, Froese, Langdon, Xu and Edwards2017). Finally, the start of a distinct rise in Alnus pollen in a high-resolution pollen record from Ruppert Lake (Higuera et al., Reference Higuera, Brubaker, Anderson, Hu and Brown2009) was dated to 7.56 ± 0.050 ka. Because of the very close proximity of the two sites, this pollen-inferred date was transposed to the Woody Bottom Pond and included in the age model.
At the three sites straddling the last stage of LQEs (i.e., Jan Lake, Windmill Lake, and Ruppert Lake), the overall abundance of Sporormiella does not diminish after the last extinctions at the transition between the late Glacial and the Holocene (Fig. 2, Table 2). Spore values are highly variable, and spore accumulation is plotted together with spore abundance relative to the pollen sum in Supplementary Figures 2–5. At these three sites, Sporormiella spores occurred in fewer than half the counted samples at Ruppert Lake, but in all samples at Jan Lake and Windmill Lake (Table 2). In all, 208 individual Sporormiella spores were identified at these sites (20, 131, and 57, respectively). Accumulation rates varied between 0 and 371.6 spores cm-2 yr-1 (Table 2). Ruppert Lake spore average accumulation values (Table 2) are similar before and after the last extinctions (4.1 vs 3.4 spores cm-2 yr-1). At Windmill, after the last extinctions, values are lower than before (70.2 vs 105.5; Table 2); here, pre-LQEs higher values pre-dating the last extinctions are mostly driven by a peak Sporormiella accumulation rate occurring near 13 ka (Fig. 2). Jan Lake has high average accumulation rates after the last extinctions (146.0), compared with an average of 70.9 before. At this site, the earliest record may have been derived from terrestrial or very shallow sediments, and thus may reflect differential recruitment of spores to the site (Carlson and Finney, Reference Carlson and Finney2004; ME and MvH personal observations). Our fourth site, Woody Bottom Pond, does not span the last stage of the LQE, and 30 individual Sporormiella spores were counted with an average accumulation rate of 8.4 spores cm-2 yr-1 over the Holocene.
Table 2. Summary of spore counts. Note that total dung fungal spore counts in Ruppert Lake includes one Podospora spore, in addition to Sordaria and Sporormiella spores.

For the two sites with a more detailed spore analysis, Ruppert Lake and Woody Bottom Pond, the most common fungal spore was Sordaria (n = 36, n = 47, respectively), followed by Sporormiella (n = 20, n = 39, respectively) with only one occurrence of Podospora. At Ruppert Lake, there was a near-significant correlation (p = 0.052) between Sporormiella and Sordaria using a Spearman's rank test, while at Woody Bottom Pond this correlation was highly significant (p < 0.001).
At Ruppert Lake, Sordaria shows higher accumulation rates after the last extinctions (average 6.7 spores cm-2 yr-1) compared with an average of 2.6 spores cm-2 yr-1 before the last extinctions. The Holocene record from Woody Bottom Pond has greater average Sporormiella accumulation rates than the entire Ruppert Lake record, with an average accumulation rate of 8.4 spores cm-2 yr-1 (range 0–84.3 spores cm-2 yr-1). Sordaria has an average accumulation rate of 9.2 spores (Table 2). Few spores are recorded in the last ~3000 years of the record and, as at Ruppert Lake, values fluctuate from sample to sample.
At the three sites straddling the last stage of LQEs, pollen of woody taxa rises around 14 ka, signalling the shift from herbaceous vegetation to shrub tundra and then to woodland and boreal forest as a response to deglacial climate change (Anderson et al., Reference Anderson, Edwards, Brubaker, Gillespie, Porter and Atwater2004). There is no correlation between Sporormiella abundance and arboreal pollen percentages (Fig. 2).
DISCUSSION
Our main aim was to study changes in the population size and diversity of megaherbivores in interior Alaska at the final stage of LQEs (i.e., the last extinctions believed to have occurred by about 13 ka). We hypothesise that faunal loss at the final stage of LQEs in interior Alaska is associated with a sharp reduction in megaherbivore population size and changes in vegetation cover. Our secondary aim was to investigate the significance of using a wider range of dung fungal spores at two nearby sites to improve the interpretation of dung fungal spore records in this study and in future studies.
At four sites covering different time intervals (Ruppert Lake 17–0 ka, Jan Lake 14.2–4.6 ka, Windmill Lake 14.8–9.8 ka, and Woody Bottom Pond, 9.5–0 ka), significant numbers of Sporormiella spores occur from 17 ka to the present. Spore numbers and estimated accumulation rates show variability within each core and among sites. This is typical of Sporormiella records (e.g., Davis and Shafer, Reference Davis and Shafer2006), which are often described informally as a ‘noisy’ signal. However, the presence of Sporormiella in all samples at two of our sites (Windmill Lake and Jan Lake) suggests the continuous presence of megaherbivores into the Holocene. At Ruppert Lake, Sporormiella spores were not observed in all samples, which could be interpreted as low numbers (or absence) of megaherbivores visiting the lake. Here, overall Sporormiella counts are low when compared with the other sites (Table 2), and it is therefore difficult to assess whether these zero-counts represent true absence or whether the small quantity falls below the detection limit (e.g., Walanus and Nalepka, Reference Walanus and Nalepka2013). For future investigations, uncertainty regarding Sporormiella zero-counts could be avoided by more strictly adhering to the guidelines set by Etienne and Jouffroy-Bapicot (Reference Etienne and Jouffroy-Bapicot2014) recommending high exotic marker counts, independently from pollen sum. We also found that, when Sordaria, another reliable indicator of megaherbivores (Baker et al., Reference Baker, Bhagwat and Willis2013; Perrotti and van Asperen, Reference Perrotti and van Asperen2019) is counted, the number of samples with indicative spores (i.e., Sporormiella and/or Sordaria) is doubled (12 vs 25 samples of 48 total). Therefore, including other dung fungal spores such as Sordaria appears to be an effective additional measure to minimise the uncertainty associated with Sporormiella zero counts.
Besides megaherbivore abundance, other factors affect spore abundance (van Asperen et al., Reference Van Asperen, Kirby and Shaw2019). These factors include (1) moisture availability for fungal growth and hydrology (Wood and Wilmshurst, Reference Wood and Wilmshurst2011), (2) shoreline morphology, (3) seasonality of climate and wind impacting fungal growth and dispersal (van Asperen, Reference van Asperen2017), (4) taphonomic effects including the sedimentary environment and spore preservation, and (5) laboratory procedures (van Asperen et al., Reference van Asperen, Kirby and Hunt2016). Thus, with each site having unique environmental and sedimentary conditions, there is unlikely to be a direct relationship between the number of dung fungal spores and the abundance of megaherbivores that applies across sites. Despite these caveats, when megaherbivore abundance is driven by a region-wide process, synchronous and local changes in dung fungal spore abundances can be expected at each site.
Comparison of values of spores before and after the last extinctions (Fig. 2) shows that at Ruppert Lake the range of values remained similar, at Jan Lake there was an increase, and at Windmill Lake the values remained similar but for a peak at 14–13 ka. From these data, we can conclude that there was no dramatic change in megaherbivore biomass associated with the LQE and the onset of the Holocene at any of the sites, and by extension at the regional level.
At Ruppert Lake, we extended the counting and identification to a wider range of dung fungi, including Sporormiella, Sordaria, and Podospora. We also analysed an additional well-dated core from nearby Woody Bottom Pond that spanned most of the Holocene. We found substantial differences between these two sites, but these differences were reduced when accounting for a wider range of dung fungal spores (Fig. 2). Different species of dung fungi have preferences for certain types of dung (e.g., Richardson, Reference Richardson1972, Reference Richardson2001) and demonstrate species-specific responses to different environmental conditions (Dix and Webster, Reference Dix and Webster1995; Krug et al., Reference Krug, Benny, Keller, Mueller, Foster and Bills2004). Consequently, some taxa may grow better in certain conditions, resulting in spore assemblages dominated by one species or a group of species with similar ecology. Dix and Webster (Reference Dix and Webster1995) also highlight the importance of competition among species as a driver for the composition of the dung fungal community. This suggests that dung fungal biomass, and thus ultimately spore production, is strongly limited by factors such as space, nutrients, and moisture availability. Therefore, the sum of individual dung spores, and not the types taken individually, may provide the most appropriate proxy for megaherbivore biomass. In fact, we find that some of the apparent randomness in our record can be reduced when considering both Sporormiella and Sordaria together (Fig. 2).
Even when considering Sporormiella and Sordaria together, however, Ruppert Lake and Woody Bottom Pond did not show the same temporal pattern of abundance. Although appearing counter-intuitive because these two sites are located close together, this result reinforces earlier findings that the dung fungal spore signal can relate to extremely small spatial scales (e.g., Kamerling et al., Reference Kamerling, Schofield, Edwards and Aronsson2017; Davies, Reference Davies2019). This may be explained by the observation of Baker et al. (Reference Baker, Cornelissen, Bhagwat, Vera and Willis2016), who found that shore run-off (within <10 m distance from the water) was the most significant process transporting spores into a series of ponds in the Oostvaardersplassen nature reserve in the Netherlands. In the only quantitative dispersal study we are aware of, Gill et al. (Reference Gill, McLauchlan, Skibbe, Goring, Zirbel and Williams2013) demonstrated the importance of short-distance wind dispersal (<100 m) to explain the significant relationship between local bison distribution and spore abundance in the Konza Prairie, Kansas, USA. While the latter was conducted in grasslands, away from wetland depositional environments, both studies indicate key dispersal distances considerably less than the 650 m separating these two lakes. The emerging pattern within these studies and others (e.g., Raper and Bush, Reference Raper and Bush2009; Etienne et al., Reference Etienne, Wilhelm, Sabatier, Reyes and Arnaud2013; Davies, Reference Davies2019) is that dung fungal spores produce a local, short-distance signal of megaherbivore activity. In a vast region such as interior Alaska there is a need for using as many sites as possible to gain a landscape-wide understanding of megaherbivore distribution and abundance.
Uniquely, we are able to compare spore counts with fossil bone data. During the period leading to the final stage of LQEs, there was a significant taxonomic turnover of dominant megaherbivores in the bone fossil record (Fig. 2). An initial assemblage dominated by Mammuthus and Equus (and in the late-glacial, Cervus) was followed, after a period of transition ending around 13 ka, by an assemblage dominated by Bison, Alces, and, later in the Holocene, by Rangifer. The period of transition is characterised by the extinction in North America of three genera (Mammuthus, Equus, and Saiga; Stuart, Reference Stuart2015), although Mammuthus survived on the Island of St. Paul until 5600 years ago (Graham et al., Reference Graham, Belmecheri, Choy, Culleton, Davies, Froese and Heintzman2016). It appears that most of the extinctions and the period of dominance turnover occurred before the opening of the ice-free corridor, estimated at 13.4 ka (Heintzman et al., Reference Heintzman, Froese, Ives, Soares, Zazula, Letts and Andrews2016) or 12.6 ka (Pedersen et al., Reference Pedersen, Ruter, Schweger, Friebe, Staff, Kjeldsen and Mendoza2016). It is unclear how isolation from the rest of North America would have impacted megaherbivore abundance. The lack of north–south exchange in North America may have limited long-distance dispersal, believed to be key to megaherbivore species survival in the region (Mann et al., Reference Mann, Groves, Reanier, Gaglioti, Kunz and Shapiro2015, Reference Mann, Groves, Gaglioti and Shapiro2019). The extinctions associated with the transition around 13 ka seen here represent the final wave of megafauna extinction in the area.
Several records from North America (not Alaska) indicate a causal relationship linking megaherbivore extinctions to subsequent changes in forest composition and wildfire regimes (Robinson et al., Reference Robinson, Burney and Burney2005; Gill et al., Reference Gill, Williams, Jackson, Lininger and Robinson2009; Perrotti, Reference Perrotti2018). In interior Alaska our three sites spanning the final stage of LQEs show that the period 15–12 ka features an increase in woody taxa (largely Salix and Betula; Bigelow and Edwards, Reference Bigelow and Edwards2001; Carlson and Finney, Reference Carlson and Finney2004; Higuera et al., Reference Higuera, Brubaker, Anderson, Hu and Brown2009). In two out of three instances the increase in arboreal pollen precedes faunal turnover, and in none of the three instances is major synchronous decline in Sporormiella abundance evident. Spore accumulation rates either increase or remain stationary following the last extinctions. The last extinctions in the bone dataset at about 13 ka actually represent a major taxonomic turnover, but there is no apparent change in total herbivore numerical abundance. Thus, the stability, or even increase, of spore accumulation rates into the Holocene, which might seem unexpected given results from other regions, is in fact consistent with the maintenance of herbivore population size, albeit via a new suite of taxa. The dominant grazers of the Pleistocene, Mammuthus and Equus, vanished from the record shortly after the vegetation shift, and their places are taken primarily by Alces, which is a browser, and later by Rangifer.
In interior Alaska, a major ecosystem change developed through the Pleistocene–Holocene transition, and as available feeding niches changed, taxa better adapted to woodland conditions became dominant. The Holocene interior climate likely favoured higher plant biomass than that of the Pleistocene, providing the nutrition (largely but not entirely) for browsers (see Guthrie Reference Guthrie2003). Our finding of a shift from a predominantly grazing to browsing herbivore guild, following an increase in woody taxa, suggests that megafaunal extinction may be at least partly related to climate-induced vegetation change, at least in this region. Another significant factor to account for when studying megaherbivore abundance in Alaska is the antiquity of human settlement, potentially dating back earlier than 15 ka, and the impact these populations had on their environment with activities such as fire and hunting (Vachula et al., Reference Vachula, Huang, Longo, Dee, Daniels and Russell2019). Moreover, megaherbivore abundance may have remained relatively constant due to a complex history of isolation from the rest of North America until about 13 ka, allowing already resident taxa to expand.
CONCLUSION
In North America, the LQEs were particularly severe, with 69% of megafauna species becoming extinct, mostly around the time of the Pleistocene–Holocene transition (14–10 ka; Stuart, Reference Stuart2015). Dramatic megaherbivore abundance losses associated with the final stage of LQEs have been identified using spores of dung fungus Sporormiella at sites from North America and across the globe. However, such studies are lacking from interior Alaska, a region biologically isolated by ice from the rest of North America until about 13 ka. To study changes in population size and diversity of megaherbivores in interior Alaska, we used a combination of radiocarbon-dated bone data and Sporormiella records spanning before and after the final stage of LQEs (i.e., the last extinctions believed to have occurred by about 13 ka).
Based on the bone data, we found that there was a major turnover of dominant megaherbivore species around 13 ka attributable to the final stage of LQEs, but, taken at face value, the abundance of bone did not markedly change over time. At the three coring sites spanning the final stage of LQEs, the overall abundance of Sporormiella spores did not diminish after the last extinctions. Because there were no synchronous decreases in spore abundance across the three sites, there is no indication that megaherbivore abundance was driven by a region-wide process during this period.
There did not appear to be any direct correlation between Sporormiella abundance and major vegetation change in the region, which saw pollen of woody taxa rise around 14 ka as a response to deglacial climate change. The rise in woody taxa is associated with an increase in bones of browser species such as Alces at two out of three of our sites, suggesting important changes in ecosystem function. By the same token, the loss of the grazing taxa Mammuthus and Equus, and great reduction in Bison, may suggest extinction causality, at least in part, due to climate-driven vegetation change. However, our work did not focus on assessing causes of LQEs, and other potential drivers of extinction such as ancient human populations active in interior Alaska during this period would require consideration in future research.
Spore values show high variability, which is dampened when including Sordaria, the only other dung fungal spore type found in significant abundance in our study. From a methodological point of view, our results indicate that high counts and the inclusion of Sordaria (and any additional spore type reliably indicating megaherbivore) can improve reliability of results when applying the dung fungal spore method. To confirm the turnover and spatial distribution in megaherbivores across the region, future work should also include more sites with spore studies and additional lines of evidence such as ancient sedimentary DNA or other biomarkers preserved in lake sediments.
The significant strength of our approach is the use of two complementary and independent indicators regarding the presence and abundance of megaherbivores in the past, namely radiocarbon-dated bone remains and accumulation rates of dung fungal spores recovered from radiocarbon-dated lake cores. These two congruent lines of evidence based on state-of-the-art methodology (including three Sporormiella records and one bone database) make us relatively confident that there was no long-lasting dramatic decrease in megaherbivore abundance in interior Alaska around 13 ka associated with LQEs. This new and robust evidence should act as a cautionary tale against the assumption that regional LQEs are systematically associated with megaherbivore biomass crashes, in addition to taxonomic turnovers. The implication is that studying the consequences of LQEs requires thoroughly testing changes in megaherbivore abundance in time, a critical first step we achieved for interior Alaska. Studies of megaherbivore abundance during periods of substantial climate change such as the last stage of LQEs and the onset of the Holocene can provide valuable insight as we attempt to predict changes in biodiversity and ecosystem function that may occur under future climate scenarios.
ACKNOWLEDGMENTS
This work was funded by NERC; Lakes and the arctic carbon cycle NE/K000306/1. Radiocarbon dating was supported by the NERC Radiocarbon Facility NRCF010001 (allocation numbers 1726.0813 and 1847.1014), and we kindly acknowledge the efforts of Dr. Charlotte Bryant. Tony Stuart kindly shared some dates from the Lister/Stuart megafaunal extinctions project (NERC grants GR3/12599 and NE/D003105/1). We thank Dr. Xiaomei Xu at the Keck Carbon Cycle AMS Facility, University of California, Irvine, USA, for her expertise in low current AMS. We also thank Hannah Laurens, Tom Roland, and Kim Davies for their invaluable help in the field, and Nancy Bigelow and the Alaska Quaternary Center for the loan of equipment. The manuscript was greatly improved by the comments of two anonymous referees, Associate Editor Jeff Pigati, and Senior Editor Derek Booth.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/qua.2020.19.







