Introduction
Feed intake and digestibility are the main factors impacting animal productive performance (Mertens, Reference Mertens and Fahey1994). However, particularly in experiments with grazing ruminants, both dry matter (DM) intake and digestibility cannot be directly measured and indirect methods are used to estimate these variables. Diet digestibility can be estimated through in vitro or in situ techniques which, however, are under criticism because they do not simulate all animal digestion processes and, additionally, the in vitro assays are under high variability between measurement series (Peyraud, Reference Peyraud1997). Alternatively, diet digestibility can also be estimated by using internal markers, which are unitary chemical entities of feedstuffs which are expected to be completely excreted in faeces (Lippke, Reference Lippke2002).
Either the indigestible dry matter (iDM) or the indigestible neutral detergent fibre (iNDF) is commonly used as an internal marker and, in general, their concentration is determined by incubating feed and faecal samples in vitro (Cochran et al., Reference Cochran, Adams, Wallace and Galyan1986; Lippke et al., Reference Lippke, Ellis and Jacobs1986) or in situ (Tamminga et al., Reference Tamminga, Robinson, Meijs and Boer1989) for a long period of time and by assuming that the residual DM or neutral detergent fibre (NDF) are chemically identical in feed and faeces. However, although it is assumed that both markers have zero digestibility, the faecal recovery rate (RR) of iDM and iNDF reported in previous studies have been variable and different from 100% (Lippke et al., Reference Lippke, Ellis and Jacobs1986; Huhtanen et al., Reference Huhtanen, Kaustell and Jaakkola1994). Moreover, there is not a standard technique to determine these markers and previous studies have evaluated the impact of bag porosity and/or incubation time on iDM or iNDF concentration in feedstuffs or even their reliability on estimating the in vivo digestibility (Tamminga et al., Reference Tamminga, Robinson, Meijs and Boer1989; Huhtannen et al., Reference Huhtanen, Kaustell and Jaakkola1994; Berchielli et al., Reference Berchielli, Andrade and Furlan2000; Casali et al., Reference Casali, Detmann, Valadares Filho, Pereira, Cunha, Detmann and Paulino2009; Krizsan and Huhtannen, Reference Krizsan and Huhtanen2013; Lee and Hristov, Reference Lee and Hristov2013; Krizsan et al., Reference Krizsan, Rinne, Nyholm and Huhtanen2015; Norris et al., Reference Norris, Tedeschi and Muir2019). Results of these studies have been broadly variable and, in fact, the reliability of using a marker for estimating diet digestibility is greatly dependent on its RR in faeces. However, most published studies which have used either internal marker to estimate diet digestibility assumed that its faecal RR was 100% or that the average RR measured within the trial was the same in all experimental animals. However, it is relevant to consider that the individual variability on marker RR may impact the accuracy of individual digestibility estimates and, as a consequence, may produce a bias on diet treatment means in field trials.
The aim of this study was to evaluate the impact of the in situ technique, NDF content in diet and trial on the relationship between intake and faecal excretion and, consequently, on faecal RR of iDM and iNDF in trials with sheep.
Materials and methods
Data set and in situ techniques
A data set of individual observations was compiled from digestibility trials conducted at the Federal University of Santa Maria, Santa Maria, RS, Brazil (S 29°29′, W 54°13′, a.l. 102 m) with sheep (17 trials) housed in metabolic cages and fed only forage or forage plus supplements. All trials were conducted using a Latin Square design with experimental periods varying from 15 to 21 days, with 10 to 14 days for adaptation and 5 to 7 days for measurements, when the feed offered, refusals and faeces were weighed, recorded, and sampled. General description of the experiments and relevant variables are presented in Table 1. All samples were dried at 55°C for at least 72 h, ground through a 1-mm screen and pooled by the animal within each experimental period for analysis. Total DM content was determined by oven drying at 105°C for 24 h. Samples of diet and faeces from 8 trials (n = 257) were weighed (2 g) in triplicate in polyester filter bags (5 × 5 cm, 40 μm of porosity) and incubated for 144 h in the rumen (method A) of a cannulated steer grazing tropical or temperate grass pastures and receiving supplementation with concentrate feedstuffs. This incubation time was defined based on results reported by Lippke et al. (Reference Lippke, Ellis and Jacobs1986) who observed that RR of indigestible fibre in faeces of sheep did not change appreciably after 144 h of in vitro incubation of feed and faeces samples. After rumen incubation, the bags were rinsed with tap water until the water remained clear, dried at 105°C for 24 h and weighed to obtain the iDM content. Thereafter, the bags containing the dried residues were treated with a neutral detergent solution in an autoclave at 110°C for 40 min (Senger et al., Reference Senger, Kozloski, Sanchez, Mesquita, Alves and Castagnino2008), washed in tap water, dried at 105°C for 24 h and weighed to obtain the iNDF content. Alternatively, samples of diet and faeces of 13 trials (n = 321) were also incubated and treated as described above, except that they were weighed in bags (5 × 5 cm) with 16 μm of porosity and incubated in situ for 288 h (method B), as proposed by Krizsan and Huhtannen (Reference Krizsan and Huhtanen2013). The daily intake and faecal excretion (g/kg body weight (BW)) of both iDM and iNDF were calculated as: DM intake or faecal output (g/kg BW) × marker concentration (g/g DM). Marker RR was calculated as: marker excreted in faeces (g/kg BW)/marker intake (g/kg BW).
Table 1. Descriptive variables of digestibility trials carried out with sheep between 2003 and 2018 at the Universidade Federal de Santa Maria, Santa Maria, RS, Brazil (29°4′S, 53°5′W)
a References: Trials 1, 2, 9,12, 14 and 15, unpublished; Trial 3, Amaral et al. (Reference Amaral, Kozloski, Santos, Castagnino, Fluck, Farenzena, Alves and Mesquita2011); Trial 4, Ruggia Chiesa et al. (Reference Ruggia Chiesa, Kozloski, Bonnecarrère Sanchez, Lima, Oliveira, Härter, Fiorentini and Cadorin Júnior2008); Trial 5, Kozloski et al. (Reference Kozloski, Cadorin Júnior, Härter, Oliveira, Alves, Mesquita and Castagnino2009b); Trial 6, Kozloski et al. (Reference Kozloski, Reffatti, Bonnecarrère Sanchez, Lima, Cadorin Júnior, Härter and Fiorentini2007); Trial 7, Lima et al. (Reference Lima, Kozloski, Bonnecarrère Sanchez, Ruggia Chiesa, Härter, Fiorentini, Oliveira and Cadorin Júnior2008); Trial 8, Kozloski et al. (Reference Kozloski, Perez Netto, Bonnecarrère Sanchez, Lima, Cadorin Júnior, Fiorentini and Härter2006); Trial 10, Stefanello et al. (Reference Stefanello, Mezzomo, Zeni, Ebling, Soares and Kozloski2018); Trial 11, Mibach et al. (Reference Mibach, Demarco, Barbosa, Oliveira, Corrêa, Del Pino, Rabassa, Schmitt, Kozloski and Brauner2021); Trial 13, Hentz et al. (Reference Hentz, Kozloski, Orlandi, Ávila, Castagnino, Stefanello and Pacheco2012); Trial 16, Orlandi et al. (Reference Orlandi, Pozo, Mezzomo and Kozloski2020a); Trial 17, Orlandi et al. (Reference Orlandi, Stefanello, Mezzomo, Pozo and Kozloski2020b).
b Method A: 144 h of in situ incubation of samples in bags of 40 μm porosity; Method B: 288 h of in situ incubation of samples in bags of 16 μm porosity.
Statistical analysis
In two trials some experimental treatments included tannins and, once tannins complex with carbohydrates and proteins in the digestive tract producing neutral detergent insoluble compounds which are excreted in faeces (Van Soest, Reference Van Soest1994), data of animals receiving tannins were excluded from the analysis. Samples of only four trials were used to test both methods and thus, results from both methods were not directly compared. The linear relationship between ingestion and faecal excretion of either marker within each method was performed using the PROC MIXED of SAS (SAS Institute Inc., Cary, NC, USA), including the random effect of trial in the model. The confidence interval (95%) of the equation parameters were calculated on the basis of standard error (SE) values (i.e. ± 2 s.e.) and used to evaluate the deviation of either the slope from 1 or intercept from 0. Significance was declared at P ≤ 0.05. The effect of trials on the average RR of markers within each method was analysed using the PROC GLM of SAS. The standard deviation of means within trials was assumed to indicate the individual variability on RR values within trials. The potential impact of the NDF content in diet on individual RR of markers within each method was determined through linear relationship using the PROC MIXED of SAS, including the random effect of trial in the model.
Results
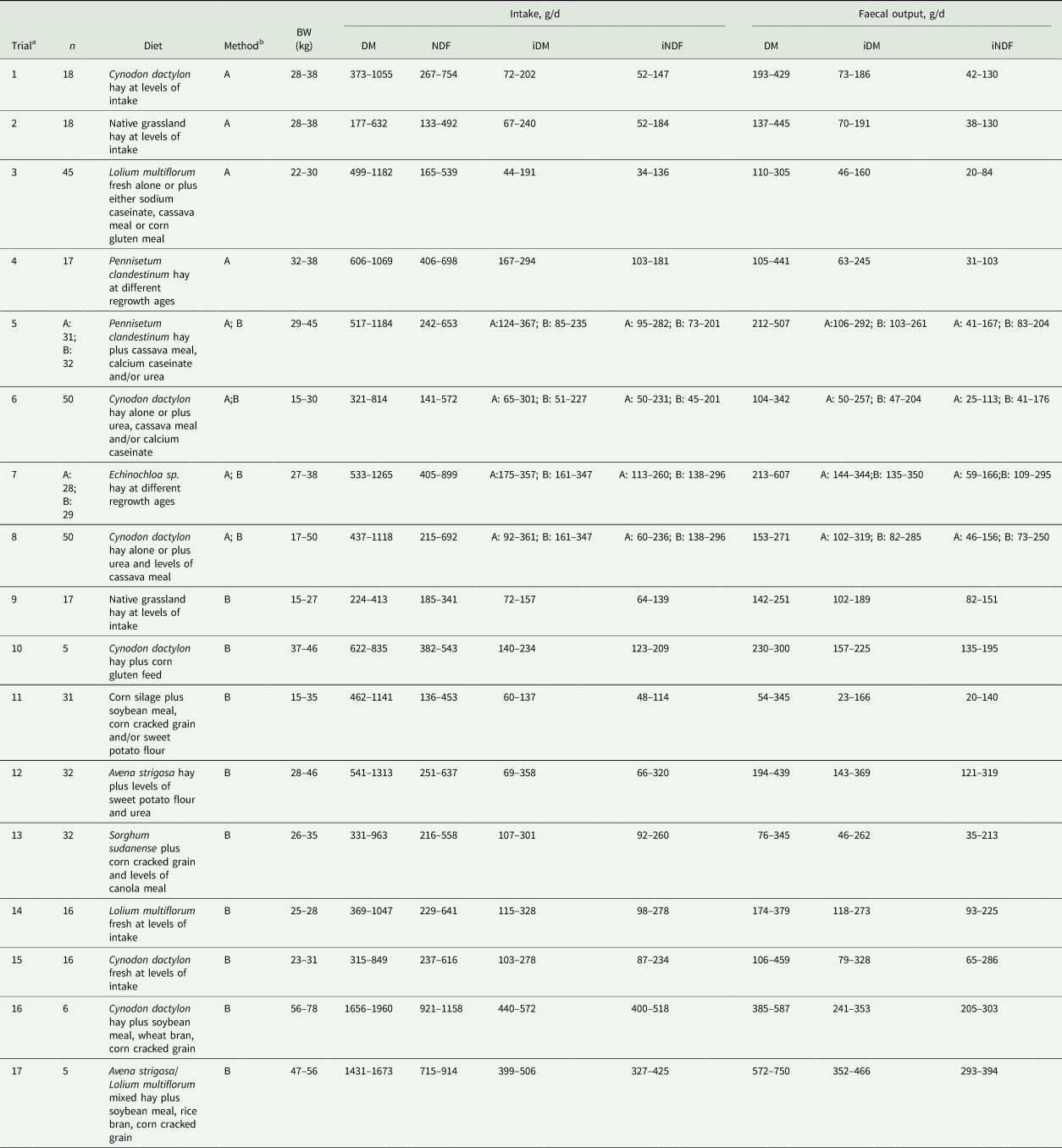
The intake of either iDM or iNDF determined by method A was linearly (P < 0.05) related to the amount excreted in faeces (Fig. 1). The intercept of both regressions was not different from 0 whereas the slopes were lower than 1 (i.e. 0.71 and 0.53 for iDM and iNDF, respectively, P < 0.05). The RMSE of the linear relationship was 0.745 for iDM and 0.225 for iNDF. The average RR was different between trials (P < 0.05) with values varying from 0.64 to 1.08 for iDM and from 0.49 to 0.92 for iNDF (Fig. 2). The standard deviation within trials varied from 9% to 30% of their respective average RR values for iDM, and from 10% to 38% for iNDF.
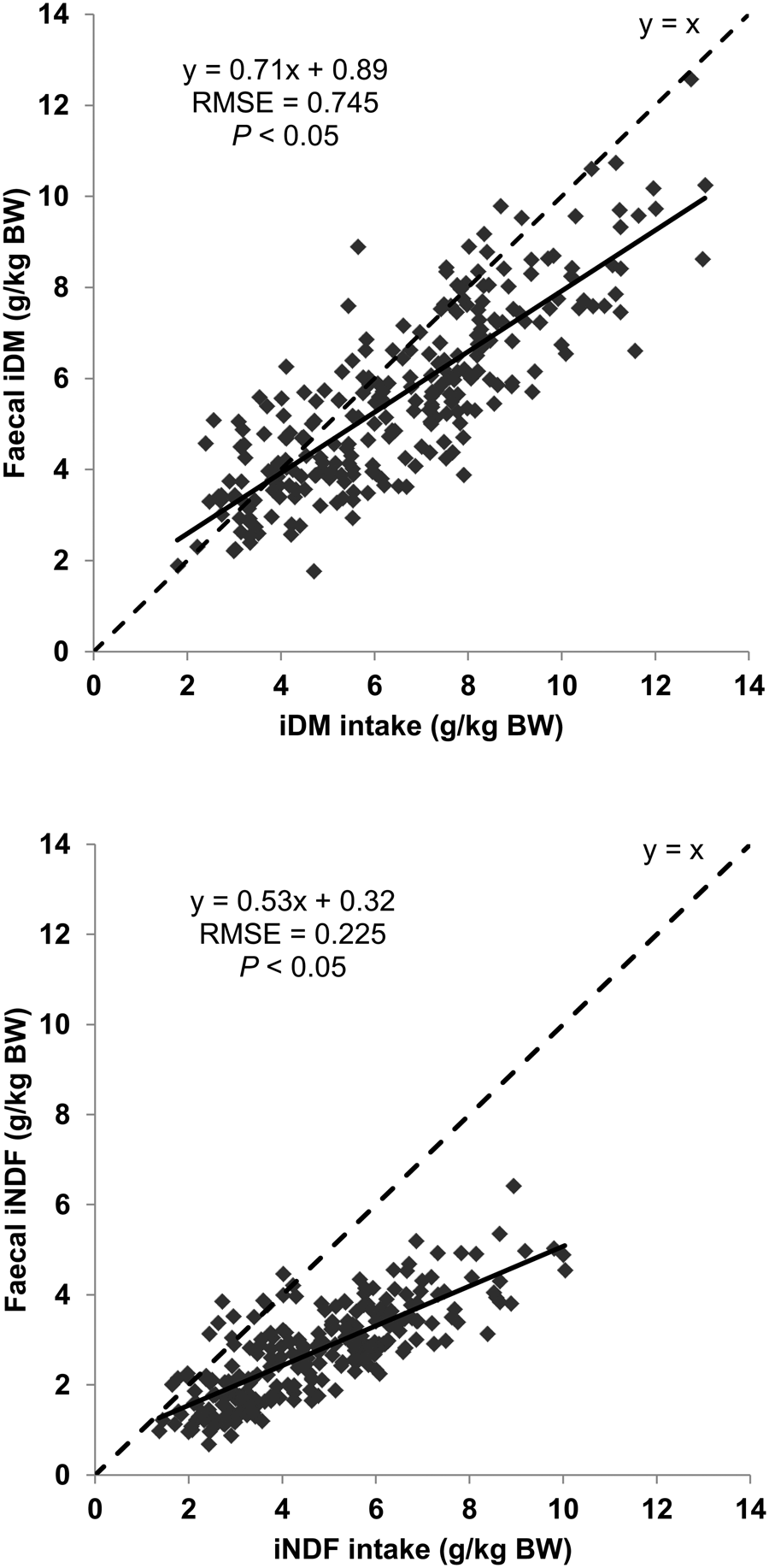
Fig. 1. Relationship between intake and faecal output of indigestible dry matter (iDM) or indigestible neutral detergent fibre (iNDF) in trials with sheep (8 trials, n = 257). Description of trials is shown in Table 1. Total faeces were collected during the last 5 days of experimental periods varying from 14 to 17 days. Indigestible fractions were obtained after 144 h of in situ incubation of feed and faecal samples in bags of 40 μm porosity. For both linear relationships, the intercept was not different from 0 and the slope was different from 1 (P < 0.05). BW, body weight; RMSE, root mean square error.

Fig. 2. Mean recovery rates of indigestible dry matter (iDM) and indigestible neutral detergent fibre (iNDF) in trials with sheep. Description of trials is shown in Table 1. Total faeces were collected during the last 5 days of experimental periods varying from 14 to 17 days. Indigestible fractions were obtained after 144 h of in situ incubation of feed and faecal samples in bags of 40 μm porosity. Bars on columns are standard deviation of means. Effect of trial: P < 0.05.
As by method A, the intake of either marker determined by method B was also linearly (P < 0.05) related to the amount excreted in faeces (Fig. 3). In both linear regressions the intercept was higher than 0 (P < 0.05) and the slope was lower than 1 (i.e. 0.70 and 0.69 for iDM and iNDF, respectively, P < 0.05). The RMSE of these linear relationships were 1.095 for iDM and 0.880 for iNDF, respectively. The average RR was also different between trials (P < 0.05) with values varying from 0.61 to 1.32 for iDM and from 0.57 to 1.23 for iNDF (Fig. 4). The standard deviation within trials varied from 5% to 36% of their respective average RR values for iDM, and from 5% to 38% for iNDF.
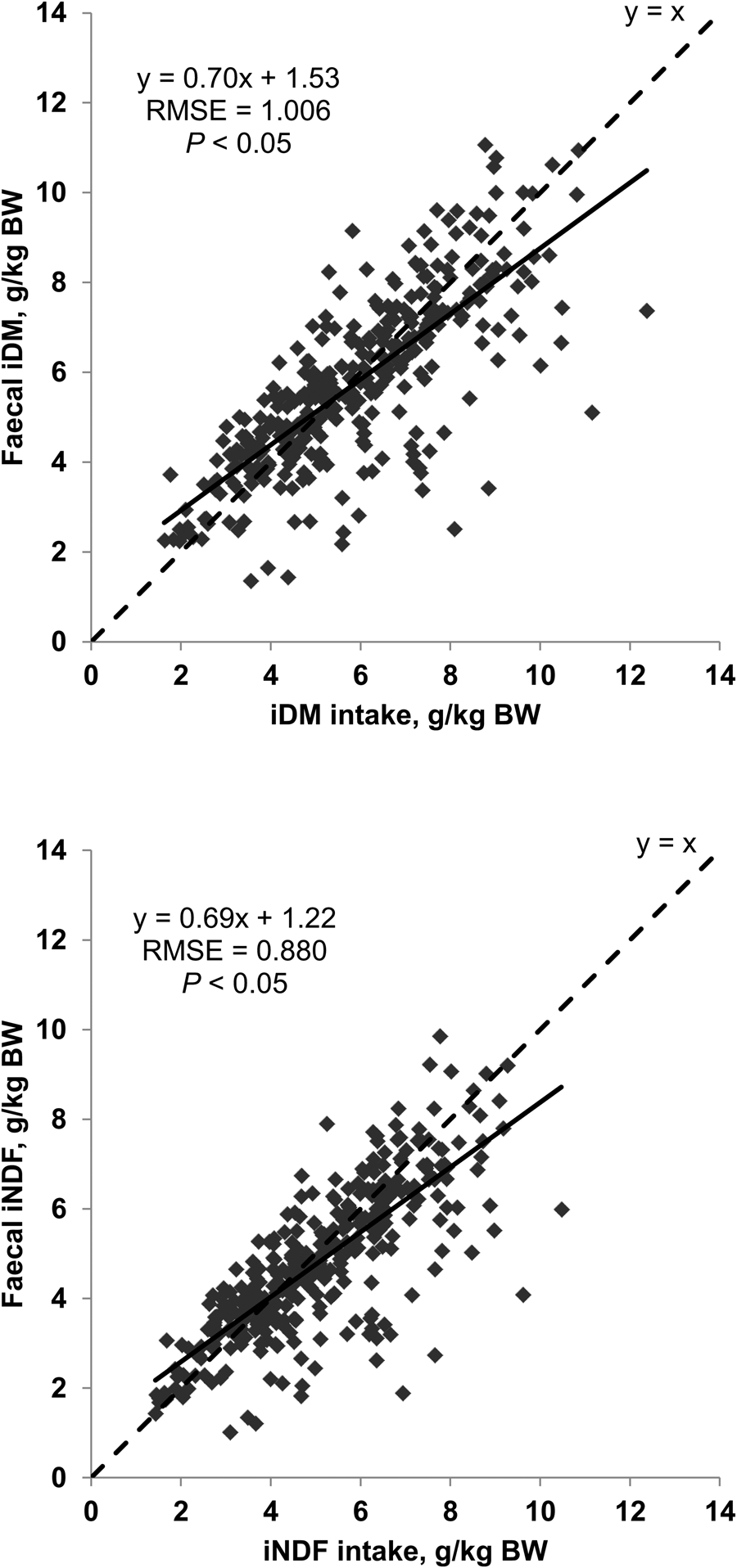
Fig. 3. Relationship between intake and faecal output of indigestible dry matter (iDM) or indigestible neutral detergent fibre (iNDF) in trials with sheep (13 trials, n = 321). Description of trials is shown in Table 1. Total faeces were collected during the last 5 days of experimental periods varying from 14 to 17 days. Indigestible fractions were obtained after 288 h of in situ incubation of feed and faecal samples in bags of 16 μm porosity. For both relationships the intercept and the slope of the linear regressions were different from 0 and 1, respectively (P < 0.05). BW, body weight; RMSE, root mean square error.

Fig. 4. Mean recovery rates of indigestible dry matter (iDM) and indigestible neutral detergent fibre (iNDF) in trials with sheep. Description of trials is shown in Table 1. Total faeces were collected during the last 5 days of experimental periods varying from 14 to 17 days. Indigestible fractions were obtained after 288 h of in situ incubation of feed and faecal samples in bags of 16 μm porosity. Bars on columns are standard deviation of means. Effect of trial: P < 0.05.
Within both methods, the RR of both markers linearly decreased at increased NDF content in diet (P < 0.05, Fig. 5). The RMSE of the linear relationships varied from 0.147 to 0.189 which represented from 19% to 24% of the respective average RR of markers. The general RR of iDM and iNDF were on average 0.90 and 0.61 by Method A and 1.00 and 0.97 by Method B, respectively.
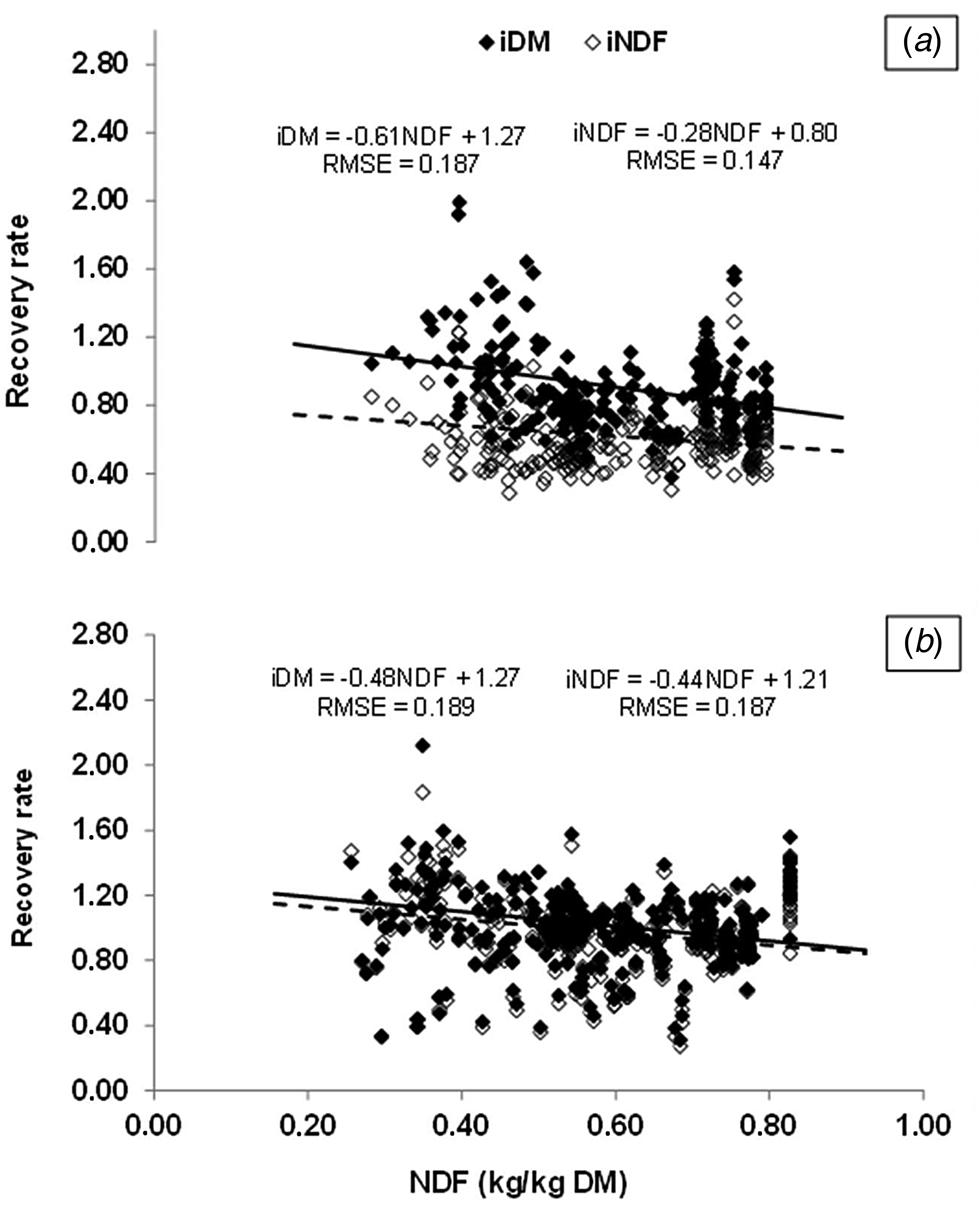
Fig. 5. Relationship between neutral detergent fibre (NDF) content in diet and faecal recovery rate of indigestible dry matter (iDM) and indigestible neutral detergent fibre (iNDF) in trials with sheep. Description of trials is shown in Table 1. Total faeces were collected during the last 5 days of experimental periods varying from 14 to 17 days. Indigestible fractions were obtained after 144 h of in situ incubation of feed and faecal samples in bags of 40 μm porosity (A, n = 257) or after 288 h of in situ incubation of feed and faecal samples in bags of 16 μm porosity (B, n = 321). RMSE, root mean square error. Effect of NDF content in diet for each marker within method: P < 0.05.
Discussion
Several published digestibility studies, as Berchielli et al. (Reference Berchielli, Andrade and Furlan2000), Berchielli et al. (Reference Berchielli, Oliveira, Carrilho, Feitosa and Lopes2005) and Casali et al. (Reference Casali, Detmann, Valadares Filho, Pereira, Cunha, Detmann and Paulino2009), have determined the indigestible fractions of DM or NDF by weighing the samples in bags with 40 to 50 μm of porosity and incubating them in situ or in vitro for 144 h. However, this methodology was under criticism due to a potential loss of undegraded sample through the bag pores and/or incomplete and variable degradation of the indigestible fraction at this relatively short incubation time (Huhtanen et al., Reference Huhtanen, Kaustell and Jaakkola1994; Krizsan and Huhtanen, Reference Krizsan and Huhtanen2013; Krizsan et al., Reference Krizsan, Rinne, Nyholm and Huhtanen2015). As to obtain more biologically accurate estimates of the NDF fraction that would be unavailable for microbial digestion in ruminants, an alternative technique was initially proposed by Huhtanen et al. (Reference Huhtanen, Kaustell and Jaakkola1994) and further evaluated by Krizsan and Huhtanen (Reference Krizsan and Huhtanen2013), by which feed and faecal samples are incubated in situ during 288 h in bags with very low porosity (i.e. 6 or 12 μm). In fact, in the present study, when calculated as the simple mean value of the individual RR values, the RR of both markers was on average higher and closer to 100% by Method B than by Method A. However, by assuming the slope of the linear regressions between intake and faecal excretion as an index of markers RR (i.e. a slope of 1.0 meaning 100% of RR), both methods were equally inaccurate (i.e. how much the slope was different from 1) on determining iDM whereas the performance on determining iNDF was lower by Method A than by Method B (i.e. slope of 0.53 v. 0.69, respectively).
Regardless of the method of marker determination, there was a high variability on RR between trials with some of them showing average values below and others above 100%, which is in accordance with results previously reported by Kozloski et al. (Reference Kozloski, Mesquita, Alves, Castagnino, Stefanello and Sanchez2009a) and other groups. For example, the RR of iNDF determined in vitro and reported by Lippke et al. (Reference Lippke, Ellis and Jacobs1986) in trials with sheep and cattle varied from an average 83% to 130% whereas Huhtanen et al. (Reference Huhtanen, Kaustell and Jaakkola1994) obtained average RR for iDM and iNDF determined in situ varying from 83% to 116% in trials with cattle. In the present study, the differences on RR between trials could be due, at least partially, to differences on diet types. Velazquez et al. (Reference Velásquez, Oliveira, Martins, Balieiro, Silva, Fukushima and Sousa2021), for example, reported increased RR of iNDF in trials with Nelore bulls fed grass hay compared to those fed corn silage based diets and, in the study of Krizsan and Huhtanen (Reference Krizsan and Huhtanen2013), the iNDF content in feed samples incubated in situ increased at an increased proportion of concentrate in diet of rumen fistulated cows. Moreover, in dairy cows fed diet with low but not in those fed diet with adequate crude protein (CP) content, the faecal output was overestimated and digestibility underestimated when iNDF was used as a marker (Lee and Hristov, Reference Lee and Hristov2013). In the present study, the CP content of diets was not broadly variable across the trials (data not shown) and, thus, only the potential impact of the NDF content in diet on markers RR was analysed. Regardless of the method of determination, the RR of both markers linearly decreased at increased NDF content of sheep diets. However, these linear relationships were not consistent showing a high individual variability (i.e. high RMSE values). A clear explanation for the NDF effect, and its variability, on RR is not available. However, it could be linked to the grinding process of feeds and faeces samples. Even though all feed and faecal samples were grounded to pass a 1 mm screen in the present study, faecal samples consist of a more fragmented and uniform material, containing fibre particles which are probably not more susceptible to further mechanical breakdown during the grinding process. In turn, the grinding process of forage samples, mainly of those of low quality, usually results in a more heterogeneous material containing since very small powder particles to particles longer than the 1 mm sieve screen. As a consequence, the susceptibility of this heterogeneous substrate to microbial degradation during the in situ incubation could be also variable yielding variable contents of iNDF (Ovani et al., Reference Ovani, Abdalla, Márquez, da Costa, Bizzuti, Lima, Moreira, Gerdes and Louvandini2022) even with at incubation time as long as 288 h. Recently, Norris et al. (Reference Norris, Tedeschi and Muir2019) and Adams et al. (Reference Adams, Norris, Dias Batista, Rivera and Tedeschi2020) reported that the iDM and iNDF fractions in feeds and faeces decreased by increasing the time of in situ incubation from 288 to 576 h. However, the impact of the longer incubation time was more pronounced in feed than in faeces samples. On average of both studies, whereas the iDM and iNDF contents in feed samples decreased by approximately 21% this reduction was of only 3% in faeces samples.
As relevant as the variability observed between trials, there was also a high variability of markers RR between animals within trials, which is indicated by both the relatively high RMSE values of the linear relationships between intake and faecal excretion of markers across all trials and the high standard deviation of the mean values within trials. A high individual variability on RR values of iNDF was also reported by Berchielli et al. (Reference Berchielli, Oliveira, Carrilho, Feitosa and Lopes2005) in trials with beef cattle. As a consequence, it would be expected that individual digestibility values estimated with either marker in a field trial would be under bias, regardless they are corrected or not for a mean RR obtained in a controlled digestibility trial. Thus, even though the accuracy of markers RR was apparently improved, not relevant precision (i.e. how much the RMSE or standard deviation value is close to 0) advantage was observed in the present study by using bags with lower porosity and longer in situ incubation time for determining iDM or iNDF.
Conclusion
Regardless of the incubation method, the faecal RR of both iDM and iNDF obtained in digestibility trials with sheep showed high variability not only between trials but also within trials. As a consequence, using average RR values obtained in controlled digestibility trials as an index to correct the individual digestibility values obtained with either marker in field trials have the potential to improve the accuracy but not the precision of the digestibility estimates.
Acknowledgements
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) for scholarship support.
Author contributions
G. V. Kozloski and C. A. Pozo conceived and designed the study; C. A. Pozo, M. P. Mezzomo, J. Frasson, L. E. Leonardi, C. M. Christmann and D. C. Rösler conducted data gathering; G. V. Kozloski and C. A. Pozo performed statistical analyses; G. V. Kozloski and C. A. Pozo wrote the article.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
All experimental procedures in trials contributing to the present study complied with the regulations governing the use of animals in the experimentation of the Federal University of Santa Maria, Santa Maria, RS, Brazil.









