Introduction
Breast conserving surgery (BCS) is the preferred treatment method for early-stage breast cancer. BCS is usually followed by radiotherapy, for patients with invasive breast cancer.Reference Onitilo, Engel, Stankowski and Suhail 1 The aim of radiotherapy is to reduce the risk of cancer recurrence through suppressing any tumour cells that might remain after surgery.Reference Veronesi, Marubini and Mariani 2 Several studies showed that the combination of radiotherapy and BCS reduces the risk of cancer recurrence by 50–70%.Reference Owen, Ashton and Bliss 3 , Reference Darby, McGale and Correa 4
The usual method for breast irradiation includes external beam radiotherapy with the total dose of 50–60 Gy in daily fractions of 1·8–2 Gy over 4–6 weeks administered to the whole breast followed by a boost dose directly to the tumour bed.Reference Bitter, Heffron-Cartwright, Wennerstrom, Weatherford, Einstein and Keiler 5 In contrast to the whole breast irradiation (WBI), partial breast irradiation (PBI) is a rational method for early-stage breast cancer, which allows the administration of a lower radiation dose.Reference Njeh, Saunders and Langton 6 By decreasing the treatment volume and increasing the delivered dose in each daily fraction, treatment can be accomplished in a short time (generally 1 week) compared with WBI.
Several approaches are now available for PBI implementation. These methods are multi-catheter interstitial brachytherapy, balloon-based PBI, three-dimensional (3D) conformal radiation therapy and intraoperative radiation therapy (IORT).Reference Njeh, Saunders and Langton 6
Balloon-based PBI methods, which are developed to simplify the brachytherapy procedure, are administered through three different systems, including MammoSite (MS) (Hologic, Inc., Marlborough, MA, USA), Contura system (Hologic, Inc.) and Xoft/Axxent™ (Xoft, Inc., Sunnyvale, CA, USA), known as electronic brachytherapy (eBX).Reference Njeh, Saunders and Langton 6 MS and Contura use after-loading of the radioactive source (192Ir) in the catheter or balloon templates. Xoft/Axxent™ eBX system is a developed form of the balloon-based brachytherapy, which uses a miniature X-ray tube rather than a radioactive isotope.Reference Ivanov, Dickler, Lum, Pellicane and Francescatti 7
IORT allows patients with the wide variety of cancers, such as breast, head and neck, prostate, liver, pancreas, rectum, retroperitoneal sarcoma, peripheral sarcoma, lung, gastric and so on, to receive the entire radiation dose in a single fraction immediately after surgery.Reference Reitsamer, Sedlmayer and Kopp 8
Intraoperative radiation therapy for breast cancer is applicable in low-risk elderly patients (≥60 years old) with early stage and small tumour sizes (G1/G2 stage and tumour sizes <2 cm) that are categorised in Luminal-A biology group and show no distant metastasis. Furthermore, this method is a useful treatment in palliative purposes in which the gross tumour cannot be removed by the surgery.Reference Gunderson, Calvo and Willet 9 , Reference Silverstein, Fastner and Maluta 10
Potential advantages of IORT are delivering the radiation dose before tumour cells have a chance to proliferate, administering the radiation through the direct visualisation at the time of surgery, sparing the surrounding and underlying healthy tissues, reduction of the health care costs and treatment time.Reference Reitsamer, Sedlmayer and Kopp 8 , Reference Gunderson, Calvo and Willet 9 , Reference Esposito, Anninga and Harris 11
The earliest concept of IORT as a treatment modality was introduced by Carl Beck in 1909, when he attempted to treat the patients with gastric and colon cancers.Reference Beck 12 Because of low-beam energies, low-dose rates and limited radiotherapy equipment, the treatments were unsuccessful. As a consequence, these early efforts were abandoned and IORT was stopped until a modern IORT treatment technique using megavoltage radiation, produced by a linear accelerator (LINAC), was proposed by Abe.Reference Abe, Fukuda, Yamano, Matsuda and Handa 13
IORT using conventional radiotherapy accelerators can be performed either in a modified operating room located within the radiotherapy department or in a standard accelerator room. In the first case, the surgical procedure is performed inside the shielded operating suite, which may deprive the patient and surgical team from the facilities which are available in a standard operating room. In the second option, IORT implementation requires to transfer the anesthetised patient from the standard operating room to the accelerator room (this technique is sometimes known as transportation technique), which may increase the risk of infection during transportation of the anesthetised patient with an open incision. Owing to these facts, employing the conventional accelerators for IORT was not highly encouraged.Reference Trifiletti, Jones and Showalter 14 , Reference Kraus-Tiefenbacher, Bauer and Scheda 15 To overcome the mentioned limitations, some mobile dedicated accelerators have been recently introduced for IORT. The development of these machines was the main cause of the IORT popularity.Reference Hanna and Kirby 16 These mobile radiotherapy units can be transported to almost any location within a hospital setting, are assembled in a non-dedicated environment and used to easily perform the IORT in a standard operating room. The most important reason for the popularity of IORT was the impressive results achieved during breast cancer treatment. The results of employing IORT techniques for patients with advanced or recurrent tumours showed a 20–50% improvement compared to the conventional radiotherapy approaches.Reference Beddar, Biggs and Chang 17 – Reference Reitsamer, Sedlmayer and Kopp 19
The aim of this study is to present a narrative review on available IORT modalities during breast cancer treatment, dedicated IORT machines and associated treatment procedures and the results of the main conducted clinical trials in this area.
Materials and Methods
We focussed on studies that were explicitly related to breast cancer radiotherapy. To extract the interested studies, we performed an iterative search in MEDLINE, PubMed, PubMed Central and ISI web of knowledge databases from December 2014 until May 2018. The search was carried out using terms of ‘external beam radiotherapy’, ‘whole breast irradiation (WBI)’, ‘partial breast irradiation (PBI)’, ‘intraoperative radiotherapy (IORT)’, ‘breast conserving surgery (BCS)’, ‘intraoperative electron radiotherapy(IOERT)’, ‘Low-KV IORT’ ‘breast therapy’, ‘breast conserving surgery (BCS) treatment’, ‘IORT equipment’, ‘IORT devices’, ‘IOERT for breast cancer treatment’, ‘IORT versus IOERT’, ‘IORT trials’, ‘TARGIT (targeted intraoperative radiation therapy)’, ‘Electron intraoperative therapy trial (ELIOT)’, ‘Mobetron’, ‘LIAC’, ‘Novac’, ‘INTRABEAM’, ‘Xoft/Axxent’ and ‘balloon-based brachytherapy’. Furthermore, the reference list of each article was reviewed in detail to extend our literature selection. Finally, based on standard search strategies, including title screening, abstract review and scanning the method and conclusion parts of each paper, eligible studies were selected. To better articulate our research, each of selected papers was fully evaluated and the main findings that satisfied the objective of our research were recorded and summarised.
Following the mentioned search method, we found about 150 scientific documents. Finally, 54 documents were selected, based on the screening and scanning strategies, for review.
Results
Nowadays, several dedicated IORT machines are clinically available, including INTRABEAM (Carl Zeiss Meditec, Inc., Oberkochen, Germany) and Xoft/Axxent™ machines, which utilise the low-kilovolt X-rays, and mobile dedicated electron accelerators such as Mobetron (IntraOP Medical, Inc., Santa Clara, CA), Novac [New Radiant Technology (NRT), Rome, Italy] and LIAC (Sordina, Rome, Italy).Reference Esposito, Anninga and Harris 11 , Reference Biggs, Willett, Rutten, Ciocca, Gunderson and Calvo 20
Low-kilovolt IORT
Low-kilovolt IORT refers to implementation of intraoperative radiotherapy using low-kilovolt X-rays. There are two X-ray machines, including INTRABEAM (IB) and Xoft/Axxent™ for IORT implementation.
INTRABEAM
One of the most important machines employed in low-kilovolt IORT technique is IB. The IB is manufactured by Carl Zeiss Surgical (Oberkochen, Germany). This device is a mobile photon radiosurgery system (PRS) which delivers low-energy X-rays (30–50 kV) directly to the tumour bed and after the removal of the tumour.Reference Keshtgar and Wenz 21 This system is composed of a miniature accelerator combined with a balanced floor stand with six degrees of freedom to increase the flexibility of the machine in treating the target sites throughout the body (Figure 1).
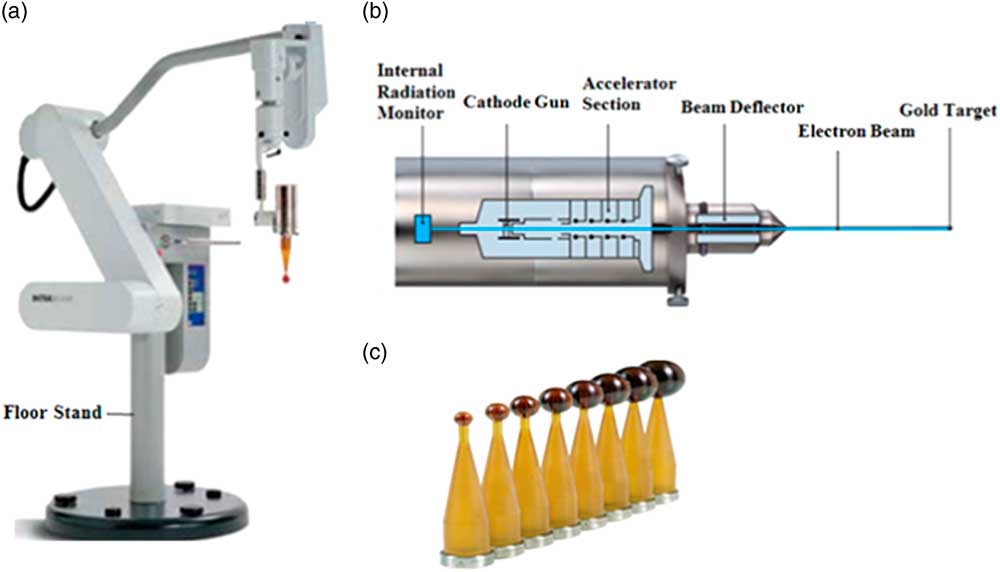
Figure 1 (A) INTRABEAM system, (B) scheme of INTRABEAM miniature accelerator and (C) employed spherical applicators with different diameters.
Miniature accelerator is a lightweight (1·6 kg) X-ray source (PRS) with isotropic radiation emission.
A wide range of different sterilisable applicators including spherical, needle, flat, surface and cylindrical applicators are available for IB.Reference Schneider, Clausen, Thölking, Wenz and Abo-Madyan 22 The spherical ones are employed during breast intraoperative radiotherapy.Reference Schneider, Fuchs and Lorenz 23
Spherical applicators are made of a tissue compatible polyetherimide named Ultem with various diameters ranging from 1·5 to 5 cm with 0·5 cm increments.Reference Schneider, Fuchs and Lorenz 23 They have a glass transition temperature of 216°C and a density of 1·27 g/cm3 and are radiation resistant. Spherical applicators are reusable and can be sterilised by steam.
Treatment procedure
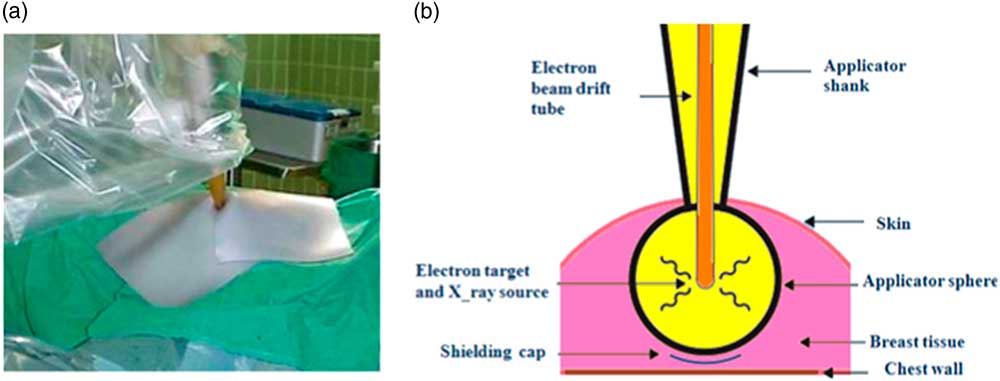
After the tumour resection, the spherical applicator is fixed to the end of the miniature accelerator and placed into the lumpectomy cavity to obtain a homogeneous dose distribution on the surface of the applicator. The planning target volume (PTV) in breast low-kilovolt IORT is considered to be a spherical shell with 1 cm thickness. The diameter of the lumpectomy cavity is measured by a disposable tape to determine the size of applicator. The precise dose administration depends on the diameter of the applicator and beam energy. Both parameters can be changed to optimise the radiation treatment. The prescribed dose at the surface of the applicator is equal to 20 and 10 Gy at 5 mm distance from the applicator surface. Owing to the rapid attenuation of radiation in depth of tissue (~1/r 3), IORT can be performed in a standard operating room with minimal exposure to the staff. Furthermore, the radiation field is shielded by a tungsten sheet to protect the patient and treatment staff as well as localise the irradiation to the treatment site (Figure 2a). If necessary, a radiopaque tungsten shield impregnated polyurethane sheet is used to protect the chest wall (Figure 2b). When the irradiation is completed, the applicator is removed and the wound is closed. This system delivers the prescribed dose over 20–45 min (depending on the size of employed applicator).Reference Vaidya, Tobias and Baum 24 , Reference Vaidya 25 The relative biological effectiveness (RBE) for this system, which is estimated by radiobiological experiments with the cell cultures between 1·2 and 2·5.Reference Herskind, Steil, Kraus-Tiefenbacher and Wenz 26 , Reference Deneve, Hoefer, Harris and Laronga 27
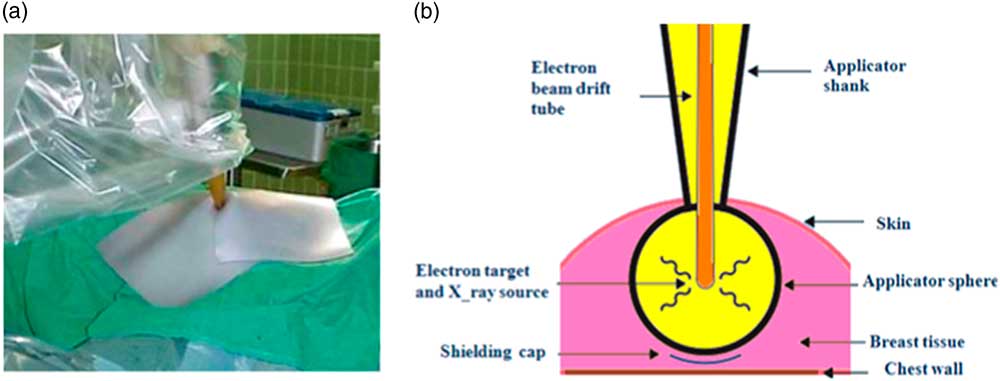
Figure 2 A tungsten sheet is used to localise the irradiated area. (a) Schematic diagram of spherical applicator inserted into the lumpectomy cavity. (b) It shows how the target tissue within the breast is irradiated and how the structures under chest wall can be protected by a shielding cap. Reprint with kind permission from Vaidya JS et al.Reference Vaidya, Tobias and Baum 24
The physics, radiobiology, dosimetry and early clinical applications of IB have been fully evaluated and the device has received the American Food and Drug Administration (FDA) approval for treatment of intracranial tumours in 1999 and breast cancer in 2009.Reference Melhus, Rivard and Narth 29 , Reference Park, Yom and Podgorsak 30
Xoft/Axxent™ eBX system
The novel Xoft/Axxent™ system (Figure 3a) is a modified form of balloon-based brachytherapy. eBX uses a disposable miniaturised X-ray source rather than a radioactive isotope (such as Iridium-192 HDR source). A miniature X-ray tube (Figure 3b), which has a diameter about 2·25 mm, produces X-rays ranging from 20 to 50 kV.Reference Klepczyk, Keene and De Los Santos 31 However, only the 50 kV X-ray is used for breast IORT.Reference Dooley, Wurzer and Megahy 32 The X-ray tube with its high voltage cable is enclosed inside a flexible cooling catheter to provide the possibility of using a water cooling system (Figure 3c). Owing to the presence of water cooling system, dose rate of the eBX is considerably higher than that of INTRABEAM which is cooled by air cooling system.Reference Skowronek, Hojczyk and Ambrochowicz 33 eBX IORT delivers 20 Gy at the surface of the balloon applicator and 9–10 Gy at 1 cm depth. The lifetime of the eBX miniature X-ray tube is very short, approximately 2·5 h compared to the 10 years in the IB X-ray source.

Figure 3 (a) Xoft/Axxent™ controller, (b) miniature X-ray tube and (c) water cooling system (Courtesy: Xoft, Inc.).
The employed applicator for breast radiotherapy is a single-lumen balloon catheter, which contains two additional ports; one for evacuation of air or fluid and the other for balloon insufflations. Furthermore, this applicator provides a channel for loading the soft X-ray source. The essential component of applicator is the balloon catheter. The balloon can be filled with different volumes of saline in order to ameliorate fitting to the lumpectomy cavity counters.Reference Dickler, Patel and Wazer 34 Different components of this applicator are shown in Figure 4.

Figure 4 Various sections of eBX breast applicator (Courtesy: Xoft, Inc.).
Treatment procedure
Breast irradiation procedure using eBX system is as follows. After the tumour resection, a pathway is created to insert the applicator inside the lumpectomy cavity. Then, the miniature X-ray tube is inserted inside the applicator and radiation dose is administered to the patient through stopping the source at some predefined dwell positions for certain dwell times. The PTV for breast irradiation in this technique is also a spherical shell with 1 cm thickness. To reduce the ambient exposure rate, a flexible drape is placed over the wound and a flexible tungsten-loaded silicone sheet (FlexiShield, Xoft, Inc., Sunnyvale, CA) is placed on the top of the drape.Reference Park, Yom and Podgorsak 30 , Reference Mehta, Algan and Griem 35 , Reference Hepel, Hiatt, Cardarelli and Wazer 36 After irradiation, the applicator is removed and the surgeon will close the lumpectomy cavity. Depending on the size of applicator, treatment time ranges between 17 and 26 min.Reference Herskind, Steil, Kraus-Tiefenbacher and Wenz 26
The Axxent eBX system was approved by FDA in 2006. Regarding that this method has been recently introduced, there is almost no long-term clinical experience about this kind of radiotherapy. But, the initial clinical experiences with Axxent technology are generally favourable with good cosmetic results.Reference Mehta, Algan and Griem 35 , Reference Hepel, Hiatt, Cardarelli and Wazer 36
Intraoperative electron radiation therapy (IOERT)
IOERT uses high-energy electron beam during radiotherapy. In the mid-1960s, electron beam was produced by conventional linear accelerators (LINAC) and was employed for IORT implementation. With the appearance of miniaturised mobile-dedicated IOERT accelerators in the mid-1990s, an important phase of IOERT development was completed.Reference Orecchia, Ciocca and Lazzari 37 Three different types of dedicated machines including Mobetron, Novac and LIAC are introduced for IOERT.
Mobetron
Mobetron (IntraOP Medical Inc., Santa Clara, CA, USA) is a mobile-dedicated LINAC for IOERT. The nominal electron energies produced by Mobetron are 4, 6, 9 and 12 MeV.Reference Biggs, Willett, Rutten, Ciocca, Gunderson and Calvo 20 Mobetron is made of three main components, including control console, modulator and therapy module, and is equipped with a beam stopper which follows the gantry movement in all directions (Figure 5a).

Figure 5 (a) The beam stopper (marked with red arrow) follows the gantry movement (left picture). (b) The soft-docking system is used by the Mobetron. As shown in this figure, there is an air gap (4 cm±1 mm) between the end of the gantry and the top surface of the applicator. The green light in the centre of accelerator head shows the proper adjustment of the gantry (right picture). Reproduced with permission from Am. Assoc. Phys. Med and with kind permission from Beddar et al.Reference Beddar, Biggs and Chang 17 , Reference Beddar 39
The configuration of the accelerator’s treatment head is in the form of a C-arm, but with a greater degree of freedom. Employed applicators with the Mobetron are metallic cylindrical tubes with different diameter and base angles (0°, 15° and 30°). The available diameters for the flat applicators are from 3 to 10 cm with 0·5 cm increments, and the bevelled applicators range from 3 to 6 cm with 1 cm steps.Reference Mills, Fajardo, Wilson, Daves and Spanos 38 Flat applicators are predominantly used for flat treatment areas, while bevelled applicators are used to treat sites that appear at an angle. The Mobetron uses the soft-docking (also named air-docking) system for electron beam collimation (Figure 5b).
To evaluate the accuracy of the docking procedure, an optical docking system is employed. As shown in Figure 5, this system consists of a laser-detecting device which is mounted on the accelerator beam collimation system to assist the operator during the soft docking procedure (adjusting the gantry respect to the position of electron applicator).
Regarding the fact that this radiotherapy machine is designed to operate only in the electron mode, electron beam current can be kept at low values, which decreases the inherent radiation leakage. Together with the compact beam stopper located on the opposite side of the gantry, Mobetron can be used in a standard operating room with no additional shielding.
Novac
Novac7 is a robotic mobile intraoperative unit which produces an electron beam. Its radiating head is moved by an articulated arm that can work in a standard operating room. Novac7 produces the electron beam with four different nominal energies of 3, 5, 7 and 9 MeV. The most important feature of Novac7 is the very high dose-per-pulse electron beam ranging from 2·5 to 12 cGy/pulse, values up to 100 times greater than that of a conventional radiotherapy accelerator. This fact is mainly due to the absence of scattering foil in design of this mobile-dedicated IOERT machine.
The electron beam is collimated by means of hard-docking system. The cylindrical polymethyl methacrylate (PMMA) applicators, employed with this machine, are available in different diameters (4, 5, 6, 7, 8 and 10 cm) and base angles (0°, 15° and 45°). The length of applicators varies according to their diameter: 80 cm for diameters up to 8 cm and 100 cm for 10 cm diameter.
The new version of this dedicated accelerator (Novac11) has been developed by NRT Company in 2012. This accelerator can produce four nominal energies of 4, 6, 8 and 10 MeV. Novac11 platform includes mobile LINAC unit, control console with a touch screen monitor and control software for treatment, patient management, disaster recovery system and remote diagnosis.Reference Ronsivalle, Picardi and Iacoboni 40 The Novac11 also uses the hard-docking system for electron beam collimation. This updated system is equipped with biocompatible PMMA applicators, which can be sterilised with a gas plasma steriliser. Each applicator is guaranteed to last for at least 100 clinical applications. The employed applicators have various diameters of 3, 4, 5, 6, 7, 8 and 10 cm and base angles of 0°, 15°, 22·5°, 30° and 45°.
LIAC
LIAC is a mobile electron accelerator for IOERT implementation. The LIAC system consists of a mobile radiant unit and an operator control rack, connected together by a 10-m cable. The output beam has a 3 mm diameter and is collimated by the sterilisable cylindrical PMMA applicator through the hard-docking system. Employed applicators are cylindrical tubes with 5 mm thickness and 60 cm length.Reference Hosseini Aghdam, Baghani, Mahdavi, Aghamiri and Akbari 41 The diameter of these applicators changes between 3 and 10 cm (3, 4, 5, 6, 7, 8 and 10 cm), and their base angle changes from 0° to 45° (0°, 15°, 30° and 45°).Reference Robatjazi, Mahdavi, Takavar and Baghani 42 The length of employed applicators is equal to 60 cm.Reference Baghani, Aghamiri, Mahdavi, Akbari and Mirzaei 43 Recently, a new type of applicator, known as beam shaper, is also introduced for IOERT with this dedicated accelerator.Reference Heidarloo, Baghani, Aghamiri and Mahdavi 44 , Reference Heidarloo, Baghani, Aghamiri, Mahdavi and Akbari 45 This applicator consists of four adjustable blades for producing any arbitrary rectangular electron fields. The beam shaper applicator can easily eliminate the field matching problems in irradiating large tumour areas such as gastric cancers, retroperitoneal and peripheral sarcomas.Reference Heidarloo, Baghani, Aghamiri and Mahdavi 44 , Reference Heidarloo, Baghani, Aghamiri, Mahdavi and Akbari 45
The dose rate of this machine can vary between 2 and 30 Gy/min depending on the beam energy and the size of employed applicator. Innovation in the robotic system permits the LIAC to be extremely mobile, which strongly simplifies the hard-docking procedure. The design of the LIAC head is almost similar to Novac7, except for the presence of a brass scattering foil with 85 µm thickness.
Clinical energies provided by LIAC are 4, 6, 8 and 10 MeV. In order to access the higher nominal energy (12 MeV), the new LIAC machine has been developed. This new machine is equipped with 19 autofocussing cavities that can produce the electron beam with nominal energies of 6, 8, 10 and 12 MeV. To minimise the probability of the neutron production at high energies (12 MeV), the scattering foil of this new LIAC machine consists of an aluminium sheet with 820 µm thickness. The dose rate of this machine varies between 3 and 40 Gy/min.
Treatment procedure
The treatment procedure for breast cancer IOERT is as follows.Reference Rocco, Rispoli and Iannone 46 , Reference Beddar and Krishnan 47 Immediately after the tumour is removed, the remaining parenchyma should be separated in order to place a shielding disk against the pectoral muscle to protect the thoracic wall from irradiation. This disk is composed of two different layers. The first layer is made of a low atomic material such as aluminium, PMMA or Teflon, whereas the second one involves a high atomic material, including copper, lead or stainless steel.Reference Beddar and Krishnan 47 , Reference Robatjazi, Baghani, Mahdavi and Felici 48 The disk is placed in such a way that the low atomic side would be in the beam origin direction. Furthermore, it must be larger than the irradiation area to ensure an optimal chest wall protection.
Then, the shielding disk is placed beneath the tumour bed and its margins are sutured together. The PTV in breast IOERT is considered to be a cylindrical volume. The electron energy is selected based on the target thickness. The electron energy should be selected in such a way that the 90% isodose level fully covers the distal end of tumour bed. The diameter of the applicator is chosen according to cover the entire tumour bed plus a safety margin.
After selection of electron energy and applicator size, the applicator is placed in direct contact with the tumour bed and is connected to the head of the treatment machine through the docking procedure (hard-docking or soft-docking system). Then, the monitor units, needed to deliver the prescribed dose, are calculated, and the prescribed dose is administered to the patient. If IOERT is employed as a sole method for radiotherapy, the prescribed dose would be equal to 21 Gy, otherwise, the prescribed dose would be 10–12 Gy.Reference Forouzannia, Harness and Carpenter 49 , Reference Fastner, Sedlmayer and Merz 50 The time of ‘beam-on’ is <2 min, and the duration of the entire procedure is about 15–20 min. After irradiation, the shielding disk is removed and the surgeon will close the incision.
Clinical outcomes of IORT
Recently, many centres have been applying IORT during BCS, according to the criteria dictated by the working group to monitor the clinical outcome and histological findings. The American Society for Radiation Oncology (ASTRO) or European Society for Radiotherapy and Oncology (ESTRO) are the criteria guidelines followed for accelerated partial breast irradiation (APBI). TARGeted Intraoperative radioTherapy Alone (TARGIT-A) and Electron Intraoperative Therapy Trial (ELIOT) are the two main randomised IORT trials for early-stage breast cancer.
TARGIT-A, a prospective randomised trial, compares the effectiveness of low-kilovolt IORT using IB system with that of conventional WBI (breast EBRT) in early-stage breast cancer. This trial was started in March 2000 and closed in June 2012.Reference Vaidya, Wenz and Bulsara 28 The patients were randomly selected to receive either IORT or EBRT. TARGIT occurred in two stratum including pre-pathology stratum, which was concurrent with lumpectomy and post-pathology stratum, given subsequently by reopening the wound after the lumpectomy.
The ELIOT, a prospective randomised trial, was performed at the European Institute of Oncology (Milan, Italy). The third randomised phase of ELIOT, which compares the IOERT to EBRT in terms of local control, absence of local recurrences and cosmetic outcome, was started in November 2000 and closed in December 2007.Reference Veronesi, Orecchia and Maisonneuve 51 In this trial, the LIAC and the Novac7 dedicated IORT accelerators were employed. The outcomes of the TARGIT-A trial and the ELIOT trial are presented in Table 1.
Table 1 The clinical outcomes of TARGIT-A and ELIOT trials.

Discussion and Conclusion
In this study, a comprehensive review has been conducted about comparing different IORT techniques and associated facilities during breast cancer radiotherapy. This method is introduced as a PBI technique which differs from WBI in many aspects. WBI is a long-term procedure which follows a fractionated strategy, while IORT-based PBI is considered as a short course treatment modality (single session). WBI usually benefits from an image-based treatment planning for quantitative evaluation of radiotherapy, whereas the PBI through IORT is deprived from such a professional image-based treatment planning system. In WBI, the breast is often irradiated by two tangential photon fields (in cases with positive supraclavicular lymph nodes, a supraclavicular photon field is also added), whereas in IORT-based PBI a single field with simplified geometry is often employed. Although employing the two radiation fields can improve the dose uniformity inside the breast, but there are still some technical challenges, including field matching and formation of hotspots and coldspots within the target volume. Therefore, radiation delivery in the case of WBI could be more sophisticated. The development of new modalities for WBI such as 3D conformal radiation therapy and intensity-modulated radiation therapy improves the treatment quality; however, the treatment time and cost are also increased. On the other hand, employing the IORT during PBI can avoid such expensive and time-consuming beam delivery systems. WBI requires for daily attendance in a 4–6 weeks treatment course at the external radiotherapy department. Owing to the limited access to such departments in some countries and high patient loads, many patients cannot attend for postoperative external beam radiotherapy, and therefore will confront mastectomy. In contrast, there is no time delay between the surgery and radiotherapy in IORT-based PBI and thus, patient would benefit from BCS.
The results of our review showed that IORT provides a very precise identification of the tumour bed through direct visualisation of the treatment site. Furthermore, it allows the critical structures or skin to be easily shielded or moved away from the radiation field, leading to excellent clinical and cosmetic results. The advent of mobile-dedicated IORT accelerators has an important role in the popularity of IORT, particularly in the field of breast cancer treatment. They are assembled in a non-dedicated environment and used to easily perform the IORT in a standard operating room. Currently, some types of mobile-dedicated IORT machines are commercially available, including IB and Xoft/Axxent™ machines, which utilise the low-kilovolt X-rays, and mobile-dedicated accelerators such as Mobetron, Novac and LIAC, which produce high-energy electron beams. A comparison between the main characteristics of these IORT facilities has been presented in Table 2.
Table 2 Comparing the currently available dedicated mobile IORT machines

Low-kilovolt IORT uses 50 kV X-ray energy, which limits the treated volume to the maximum depth of 1 cm from applicator surface. But, employing such low-energy photon beam can effectively increase the biologic effectiveness of administered dose to the tumour bed. Furthermore, the weakly penetrating nature of these low-energy photons makes it possible to use the corresponding machines (IB and Xsoft/Axxent™) in a standard and unshielded operating room.
IOERT also refers to employment of high-energy electron beam from either conventional or dedicated electron accelerators for intraoperative irradiation of tumour bed. This method is the most common modality for IORT implementation. At present, the introduced mobile-dedicated IOERT machines have an important role in the progress of this treatment modality. Employment of 12 MeV maximum electron energy in IOERT (in order to reduce the probability of neutron production) allows the irradiation inside an unshielded operating room but in expense of limited treatment depth (up to 4 cm).
Both low-kilovolt IORT and IOERT methods can benefit from direct visualisation of target area, effective shielding of the normal tissue and organs at risks (OARs), which may be located inside the radiation field. Using these methods we can also reduce the treatment time to a single session that can improve both RBE of treatment and patient quality of life. In addition, both modalities are suitable for PBI due to the localised and targeted irradiation of treatment area.
In comparison with the low-kilovolt IORT, IOERT generates essentially a more uniform dose distribution because of employing the electron beam and simplified geometry for tumour bed irradiation Reference Rahimzade Yekta, Mahdavi and Baghani 52 (as described in treatment procedure). Furthermore, due to the limited penetration depth, normal tissues and OARs are more effectively saved in the IOERT approach. The treatment time for IOERT procedure is also considerably lower than that of low-kilovolt X-ray IORT (2 min versus 20–40 min, respectively). Therefore, IOERT is the most effective technical method for IORT implementation.
However, one of the greatest limitations of the IOERT method is the lack of applicator flexibility in treatment of sophisticated anatomical areas. Thus, in cases where the shape and position of the tumour bed is not simply accessible to the intended applicator, IOERT is not suitable. Finally, the IOERT facilities are much more expensive than low-kilovolt IORT devices.Reference Esposito, Anninga and Honey 53
The outcomes of performed clinical trials demonstrate that IORT is almost equivalent to EBRT in specifically selected and well-informed patients. There is no significant difference in overall mortality, breast cancer mortality, non-breast cancer mortality and distant metastases between IORT and EBRT. The breast cancer mortality for IORT and EBRT groups were comparable in TARGIT-A trial (2.6% for TARGIT group and 1.9% for EBRT group). The overall mortality in TARGIT group was equal to 3.9%, whereas for EBRT it was 5.3%.Reference Silverstein, Fastner and Maluta 54 The 5-year overall-survival for IOERT group and EBRT in ELIOT trial was also equal to 96.8 and 96.9%, respectively. The 10-year survival rate estimations for both groups were almost the same (89.8% for ELIOT and 92% for EBRT).Reference Silverstein, Fastner and Maluta 10 Although results of these clinical trials are desirable and encouraging, but due to the lack of long-term follow-up data, using this treatment modality should be restricted to the low-risk patients and under-dedicated protocols.Reference Silverstein, Fastner and Maluta 10 , Reference Silverstein, Fastner and Maluta 54
Currently, about 210 health centres around the world manage the IORT treatments with several dedicated IORT machines. Despite the fact that IORT is faced with some technical challenges involving coordination of tasks with timely and efficient communication among several departments, including operative services, radiation oncology, surgery, anaesthesiology, and engineers, but it is expected that this technique will become more widespread in the immediate future.
Acknowledgements
None.
Financial Support
None.
Conflict of Interest
None.