Protein-losing enteropathy is an infrequent but severe end-organ dysfunction occurring after Fontan operation and limiting survival of Fontan patients. Onset of protein-losing enteropathy varies from weeks to years after Fontan palliation and is reported to affect 3.7–11.3% of the Fontan population.Reference Mertens, Hagler, Sauer, Somerville and Gewillig1–Reference John, Johnson, Khan, Driscoll, Warnes and Cetta5 The precise pathophysiological mechanisms remain unknown but reduced cardiac output, venous congestion, increased mesenteric vascular resistance, and intestinal inflammation might contribute to disease development.Reference Rychik6,Reference Rychik and Spray7 Although development of protein-losing enteropathy was found to be associated with right ventricular morphology of the single ventricle in some studies, other reports could not confirm this finding.Reference Mertens, Hagler, Sauer, Somerville and Gewillig1-Reference Powell, Gauvreau, Jenkins, Blume, Mayer and Lock4 Beyond that, only a limited number of further risk factors associated with manifestation of protein-losing enteropathy such as phrenic palsy have been identified.Reference Schumacher, Stringer and Donohue3
Protein-losing enteropathy is characterised by an excessive enteric loss of proteins resulting in low serum albumin and total protein levels, hypercoagulability, and immunodeficiency due to intestinal loss of immunoglobulins and lymphocytes.Reference Rychik6-Reference Udink Ten Cate, Hannes and Germund8 Clinical manifestations are variable and include chronic diarrhoea, abdominal cramps, peripheral oedema, persistent pleural effusions, ascites, and malnutrition. Approaches to management of protein-losing enteropathy generally focus on optimising haemodynamics of the Fontan circulation, reducing symptoms, and suppressing intestinal inflammation. Medical treatment options that have been reported include reducing volume overload and increasing intravasal oncotic pressure with diuretics, albumin infusions, and dietary modifications, using the intestinal membrane stabilisation effect of subcutaneous heparin, anti-inflammatory therapy with oral steroids, and pulmonary vasodilator therapy.Reference Rychik and Spray7–Reference Thacker, Patel, Dodds, Goldberg, Semeao and Rychik11 Surgical treatment strategies aim at addressing haemodynamic issues affecting the Fontan circulation and include plication of paralysed diaphragm, Fontan fenestration, and implementation of atrial pacing.Reference Rychik and Spray7,Reference Cohen, Rhodes, Wernovsky, Gaynor, Spray and Rychik12,Reference Vyas, Driscoll, Cabalka, Cetta and Hagler13 Catheter-based interventional therapy strategies such as elimination of Fontan pathway obstructions by angioplasty and stent implantation, creation of a fenestration, and reducing volume load by embolisation of aorto-pulmonary collaterals have been successfully utilised.Reference Mertens, Hagler, Sauer, Somerville and Gewillig1,Reference John, Johnson, Khan, Driscoll, Warnes and Cetta5,Reference Rychik6,Reference Menon, Hagler, Cetta, Gloviczki and Driscoll14
However, given the multifactorial and largely unknown pathogenesis, none of the numerous proposed treatment strategies has proven universally successful and survival of Fontan patients with protein-losing enteropathy remains poor with reported 5-year survival rates after onset ranging from 46 to 88%.Reference Feldt, Driscoll and Offord2,Reference John, Johnson, Khan, Driscoll, Warnes and Cetta5 Treatment of refractory protein-losing enteropathy remains challenging and cardiac transplantation is considered the only successful treatment strategy leading to definitive disease resolution.Reference Bernstein, Naftel and Chin15,Reference Schumacher, Gossett and Guleserian16
Since protein-losing enteropathy is an infrequent condition, most reported cohorts include a limited number of patients. Consequently, more studies are necessary to identify successful treatment strategies and prognosis of this unique end-organ dysfunction. Therefore, we performed a retrospective analysis of our institutional cohort of Fontan patients with protein-losing enteropathy to comprehensively investigate long-term clinical outcome and to describe the effects of various treatments on disease remission rate and mortality.
Materials and methods
Study design and patient cohort
We reviewed our institutional database for Fontan patients, who had at least one follow-up visit after Fontan completion in our institution during the study period from January, 1986 to October, 2019. Of 439 identified Fontan patients, 327 (74.5%) were originally operated in our institution. Median follow-up time for the entire cohort was 8.6 years [interquartile range 13.9]. Medical charts were reviewed for established diagnosis or symptoms of protein-losing enteropathy. The study was approved by the institutional review board and the institutional ethics committee (decision number EA2/126/15). Individual informed consent was not considered mandatory.
Definition of protein-losing enteropathy
Diagnostic criteria for protein-losing enteropathy were retrospectively redefined referring to recently proposed diagnostic criteria as a combination of persistent diarrhoea and/or recurring oedema and/or pleural effusions and/or ascites, decreased serum albumin (<3.5 g/dL) and total serum protein levels (<6.0 g/dL), and confirmation of intestinal protein loss with increased faecal alpha-1antitrypsin levels.Reference Udink Ten Cate, Hannes and Germund8 Other causes of hypoproteinaemia such as nephrotic syndrome or inflammatory bowel disease were required to be excluded by clinical and laboratory examination. A total of 33 patients (7.5%) of the entire cohort were diagnosed with protein-losing enteropathy by these criteria, while the suspected diagnosis was retrospectively ruled out in 3 patients. The prevalence in patients operated in our institution was 15 of 327 patients (4.6%). Three patients were only included in the analysis of factors associated with disease manifestation as well as survival analysis but excluded from further analyses due to missing follow-up: one patient refused recommended treatments and discontinued follow-up after two visits, one patient had only one single follow-up visit in our institution to obtain a second opinion, and another patient was diagnosed only shortly before conclusion of data acquisition.
Sustained protein-losing enteropathy was defined as clinical symptoms or abnormally low albumin and total protein levels over a period of ≥6 months. Stable remission was defined as regression of symptoms and elevation of serum albumin levels >3.5 g/dL over a period of ≥12 months. Relapse was defined as clinical deterioration or decrease in serum albumin levels <3.5 g/dL after a minimum symptom-free period of 6 months.
Data acquisition
Details on Fontan surgery and post-operative course, anthropometric, clinical, echocardiographic, laboratory, and invasive haemodynamic findings at onset of protein-losing enteropathy as well as at last follow-up and details of initiated treatments for protein-losing enteropathy during follow-up were retrieved from medical charts. Estimated glomerular filtration rate was calculated based on the Schwartz formula in children <18 years and based on Chronic Kidney Disease – Epidemiology Collaboration formula in adult patients.Reference Schwartz, Munoz and Schneider17,Reference Levey, Stevens and Schmid18 Z-scores for weight, height, and body mass index were calculated from recent national reference charts.Reference Neuhauser, Schienkiewitz, Schaffrath Rosario, Dortschy and Kurth19,20 Echocardiographic parameters were extracted from reports in the electronic medical charts. Systolic function of the single ventricle was graded as normal or mildly, moderately, or severely impaired either according to ejection fraction (>55, 45–54, 30–44, and <30%, respectively) or reported corresponding to visual assessment. The degree of atrioventricular valve regurgitation was classified as absent/trace, mild, moderate, or severe as reported. Invasive haemodynamic measurements retrieved include mean pulmonary artery pressure, systemic ventricular end-diastolic pressure, and transpulmonary pressure gradient, calculated as difference between mean pulmonary artery pressure and pulmonary capillary wedge pressure. Surgical era was defined by equally dividing the time period between the first and last operation in the entire cohort of Fontan patients (cut-off 20 December, 2001). For duration of post-operative thoracic and/or peritoneal drainage, days from operation until extraction of last drain were counted. Bradyarrhythmia was determined by requirement of a pacemaker implantation secondary to Fontan operation. Fontan pathway stenosis was defined by any catheter intervention performed after Fontan operation that included balloon angioplasty and/or stent implantation of/within the pulmonary vasculature and Fontan pathway. Patency of fenestration was evaluated at last follow-up for Fontan patients without protein-losing enteropathy and at the time of disease manifestation in patients with protein-losing enteropathy. Diagnosis of phrenic palsy was determined if it had been documented in medical charts and/or if a diaphragm plication had been performed after Fontan operation.
Statistical analysis
Demographic and surgical data were obtained from institutional medical records. Follow-up data were collected from clinical and laboratory examinations during subsequent visits to our institution or from available correspondence from referring physicians. Data are expressed as figures (percentages) and median [interquartile range, calculated as 75.-25. percentile]. Continuous variables were compared using Mann–Whitney test for unpaired or Wilcoxon matched-pairs signed rank test for paired data; categorical data were compared using chi-square test. Survival and freedom from protein-losing enteropathy were assessed using Kaplan–Meier survival analysis; differences between groups were analysed using the log-rank test. Statistic analysis was performed using SPSS statistical software (Version 23, IBM Corp., NY, United States of America). A p-value < 0.05 was considered statistically significant.
Results
Patients
Patient details are listed in Table 1. Median age at diagnosis of protein-losing enteropathy was 7.3 years [interquartile range 7.8] and disease manifestation occurred early, at a median of 2.9 years [interquartile range 5.8] after Fontan operation. Overall freedom from protein-losing enteropathy was 93.2% at 10 and 20 years (Fig 1a). The most common underlying cardiac morphologies were double inlet left ventricle (n = 9) and tricuspid atresia (n = 6) with left ventricular morphology of the systemic ventricle predominating (Table 1). The most frequent Fontan modification was the total cavopulmonary connection with extracardiac conduit (n = 20, Table 1). Fifteen patients had received a primary fenestration; however, fenestration was patent in only four patients at onset of protein-losing enteropathy. Fenestrations had been closed percutaneously (n = 6) or surgically (n = 1) in seven patients and spontaneous closure was documented in four patients prior to disease manifestation. The most frequent clinical manifestations of protein-losing enteropathy included oedema and ascites, followed by diarrhoea and pleural effusions (Table 1). Echocardiographic assessment at onset of protein-losing enteropathy revealed good ventricular function and absent or only mild atrioventricular valve regurgitation in the majority of the patients (Table 2). Also, invasive assessment at disease manifestation showed haemodynamic parameters well within tolerable ranges for a Fontan circulation with median pulmonary artery pressure of 13.0 mmHg [interquartile range 6.0], systemic ventricular end-diastolic pressure of 8.0 mmHg [interquartile range 4.3], and transpulmonary pressure gradient of 5.0 mmHg [interquartile range 3.0].
Table 1. Patient characteristics

Data are presented as median [interquartile range] or frequencies (percentages).
PLE = protein-losing enteropathy; TCPC = total cavopulmonary connection.
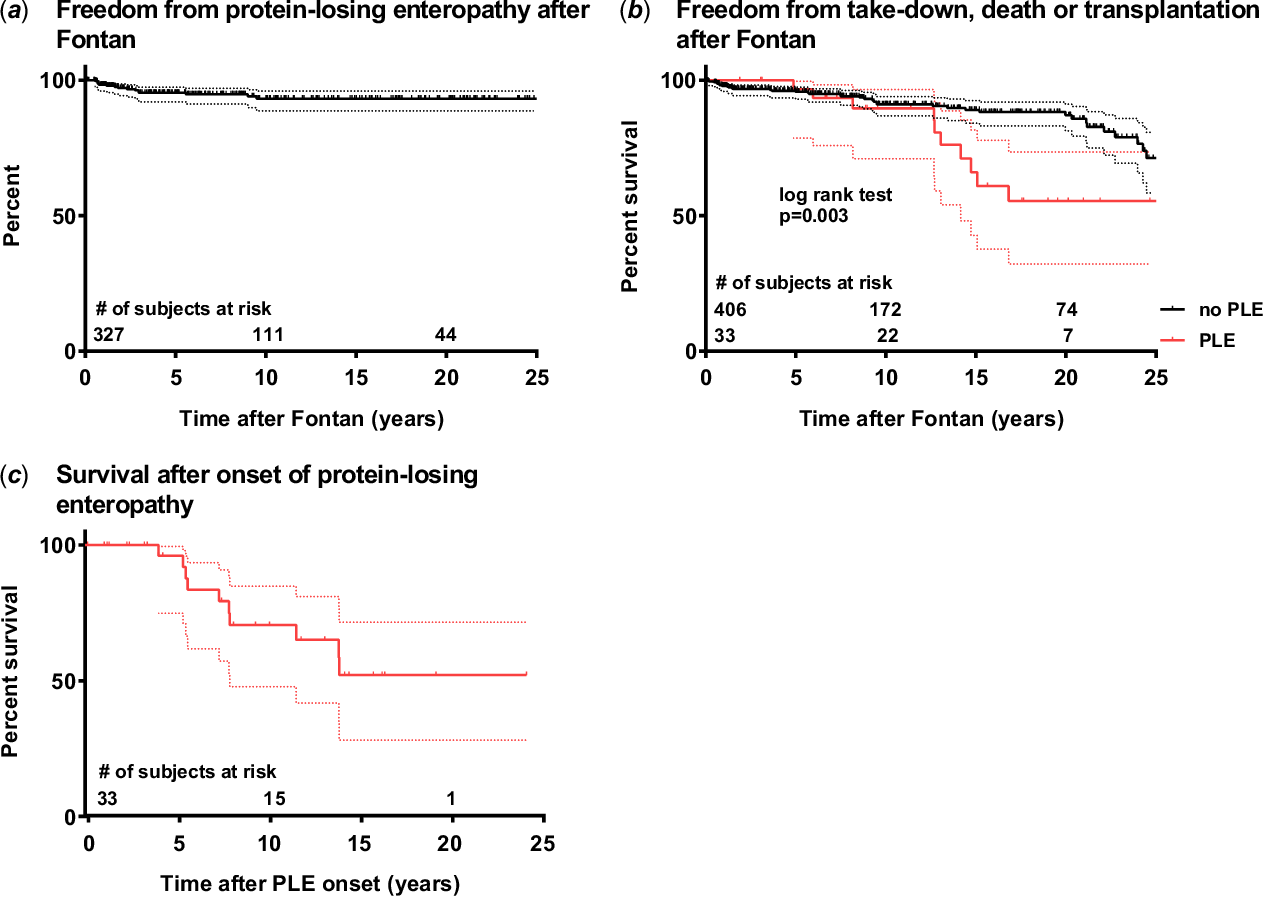
Figure 1. (a) Kaplan–Meier curves depicting freedom from protein-losing enteropathy, (b) freedom from Fontan take-down, death, or cardiac transplantation according to the presence of protein-losing enteropathy, and (c) survival after manifestation of protein-losing enteropathy. Dotted lines represent 95% confidence intervals. 1a: Figure includes only patients operated in our institution to avoid selection bias. 1b: Curves were compared using the log-rank test. Three patients of the Fontan group without protein-losing enteropathy had a Fontan take-down and were censored at time point of take-down.
Table 2. Anthropometric, clinical, haemodynamic, and laboratory parameters during follow-up

Data are presented as median [interquartile range] or frequencies (percentages). p-Values < 0.5 considered statistically significant are highlighted in bold. Ventricular dysfunction and AV valve regurgitation were graded as described in Materials and Methods section.
AV valve = atrioventricular valve; BMI = body mass index; EDP = systemic-ventricular end-diastolic pressure; eGFR = estimated glomerular filtration rate; PAP = mean pulmonary artery pressure; TPG = transpulmonary pressure gradient.
Addressable haemodynamic restrictions of the Fontan circulation were identified in 25 of 30 (83.3%) patients at manifestation or during course of disease including unilateral diaphragmatic paralysis in 11, stenosis of central pulmonary arteries in 14, obstruction of Fontan conduit, superior or inferior vena cava in 8, and bradyarrhythmia in 5 patients, respectively (Fig 2). Further compromising haemodynamic issues identified were severe outflow tract obstruction (n = 2) as well as severe atrioventricular valve regurgitation (n = 2) and complete obstruction of the left pulmonary arterial vasculature (n = 1). In two (6.7%) patients, only collateral vessels were identified as possible factors negatively impacting haemodynamics while in three (10.0%) patients, comprehensive evaluation did not reveal addressable haemodynamic restrictions.

Figure 2. Graphic depiction of individual disease course and treatment strategies in patients with protein-losing enteropathy. The abscissae represent approximated time intervals after Fontan operation in (a) patients with stable remission and (b) patients without remission or with frequent relapses; 0 on the abscissae marks the time point of Fontan operation. In the ordinates, individual patients are represented. Three patients without follow-up were excluded (see Materials and Methods section). Grey bars represent time intervals after Fontan operation before onset of protein-losing enteropathy, red bars represent time intervals of active protein-losing enteropathy, green bars represent time intervals with remission of protein-losing enteropathy, and black bars represent death. Vertical lines represent an initiated treatment, either medical (M), surgical (S), or catheter-based interventional (I), followed by a number representing a specified procedure or medication: M: Medical treatments: 1 = budesonide; 2 = sildenafil; 3 = heparin; 4 = bosentan; 5 = prostacyclin. S: Surgical procedures: 1 = plication of diaphragm; 2 = AV valve reconstruction/ replacement; 3 = pacemaker implantation; 5 = creation of fenestration; 6 = Fontan revision; 7 = other surgical procedure. I: Catheter-based interventional procedures: 1 = balloon angioplasty/stent placement pulmonary arteries; 2 = embolisation of collaterals; 3 = balloon angioplasty/stent placement IVC/SVC; 4 = EPS; 5 = creation/dilatation of a fenestration; 6 = other interventional procedure (The information on numbers of therapies initiated is also provided in a tabulated fashion as Supplemental Table 1). AV valve = atrioventricular valve; EPS = electrophysiological study; IVC = inferior vena cava; LVOTO = left ventricular outflow tract obstruction; SVC = superior vena cava.
Factors associated with development of protein-losing enteropathy
Of the possible risk factors investigated, we found bradyarrhythmia, phrenic palsy, and stenosis within the Fontan pathway to be significantly associated with manifestation of protein-losing enteropathy (Table 3). In addition, earlier surgical era but not type of Fontan modification was associated with protein-losing enteropathy. Patency of a fenestration on the other hand was associated with a decreased odd of protein-losing enteropathy. Anatomical factors such as ventricular morphology and heterotaxy were unrelated to disease manifestation. Moreover, length of ICU and hospital stay were significantly longer in patients eventually developing protein-losing enteropathy, while further perioperative factors such as cardiopulmonary bypass time or duration of drainage were not significantly different.
Table 3. Risk factors associated with protein-losing enteropathy

Data are presented as median [interquartile range] or frequencies (percentages). Odds ratio (OR) (95% confidence interval (CI)) is given for categorical variables. p-Values < 0.5 considered statistically significant are highlighted in bold.
APC = atriopulmonary connection; AVC = atrioventricular connection; PLE = protein-losing enteropathy; RV = right ventricle; TCPC = total cavopulmonary connection.
Treatment strategies
After diagnosis of protein-losing enteropathy, surgical treatment was initiated in 19 patients (Fig 2) and included diaphragmatic plication (n = 11), pacemaker implantation (n = 5), resection of ventricular outflow tract obstruction (n = 2), Fontan revision (n = 2), atrioventricular valve reconstruction or replacement (n = 2) as well as creation of a fenestration and implantation of an aorto-pulmonary shunt for obstruction of left pulmonary artery perfusion (each n = 1). A total of 64 percutaneous interventional procedures were performed in 25 patients and predominantly focused on relief of Fontan pathway obstructions including balloon angioplasty and/or stenting of pulmonary arteries (n = 14 patients) as well as of Fontan conduit, inferior vena cava, or superior cavopulmonary anastomosis (n = 8 patients). A total of 18 (60.0%) patients had at least one intervention for Fontan pathway obstruction at any level. Median pressure gradient across obstructions was 2 mmHg [interquartile range 2] which was significantly reduced by intervention to a median of 0 mmHg [interquartile range 0] (p < 0.001), indicating successful relief of obstruction.
Medical treatment was initiated in 28 patients. In the recent decade, the principal therapy concept was the combination of pulmonary vasodilator and anti-inflammatory therapy (n = 14): eight patients received a combination of sildenafil and budesonide (of whom budesonide was added to existing therapy with sildenafil in two patients), five patients a combination of sildenafil and budesonide with bosentan, and one patient a combination of budesonide and sildenafil with oral prostacyclin. Pulmonary vasodilator therapy only was initiated in seven patients (sildenafil n = 5, bosentan n = 1, and prostacyclin n = 1) and two patients received only anti-inflammatory therapy with budesonide. In the early study period, subcutaneous heparin therapy was initiated in seven patients but eventually discontinued due to ineffectiveness and/or severe side effects such as heparin-induced osteopenia. In addition, somatostatin was used temporarily in one patient but did not result in clinical improvement. Adjuvant symptomatic medical therapy included diuretics, anti-congestive heart failure therapy, and substitution of electrolytes as well as vitamin D. During acute disease exacerbation, patients usually received intravenous diuretic treatment, albumin infusions, and intravenous immunoglobulin substitution as considered necessary according to clinical status and laboratory values; however, these medications were not analysed in detail.
Treatment results
Individual courses of protein-losing enteropathy including episodes of relapse and remission, individual treatments, and their effects on disease resolution are depicted in Figure 2. Overall, 15 patients are currently in remission from protein-losing enteropathy. Surgical therapy alone or after unsuccessful medical therapy was able to establish a stable remission in three patients. Medical treatment alone also achieved stable remission in three patients, while percutaneous interventional procedures alone did not result in stable remission in any patient. With multimodal combination of surgical, interventional, and medical therapies, sustained clinical remission was attained in an additional eight patients while remission was ultimately achieved by cardiac transplantation in one patient. In 4 patients, initiated treatment leads only to temporary remission from protein-losing enteropathy while disease persisted in 11 patients despite initiated therapy (Fig 2b).
Morbidity
Haemodynamic and laboratory parameters at disease onset and at last follow-up are shown in Table 2. Overall, albumin and total protein levels significantly increased from onset of protein-losing enteropathy to last follow-up in the entire cohort. In general, there was a considerable progressive decline in ventricular function with the number of patients having moderate and severe ventricular dysfunction increasing from two to nine patients (p = 0.003). The degree of atrioventricular valve regurgitation did not change significantly during follow-up. Invasive haemodynamic data revealed no increase in pulmonary artery or systemic ventricular end-diastolic pressure in the entire cohort during follow-up, whereas transpulmonary pressure gradient slightly but statistically significantly decreased (Table 2).
Renal dysfunction
Median serum creatinine significantly increased during follow-up (Table 2). Correspondingly, a significant decrease of estimated glomerular filtration rate from 120.0 ml/min/1.73 m² [interquartile range 42.3] to 98.0 ml/min/1.73 m² [interquartile range 41.5] (p = 0.009) was observed, indicating an overall decline of renal function during follow-up. Overall, estimated glomerular filtration rate however fell still in the normal range for healthy individuals.Reference van den Brand, van Boekel, Willems, Kiemeney, Den Heijer and Wetzels21 Pronounced impairment of renal function was uncommon and frequency did not significantly increase during follow-up. At least moderate renal dysfunction, defined as estimated glomerular filtration rate <60.0 ml/min/1.73 m², was present in one patient at onset of protein-losing enteropathy and in three patients at last follow-up (p = 0.50).
Infections
Seven (23.3%) patients were prone to recurrent infections including dermal and soft tissue infections (n = 5), sepsis (n = 4), severe pneumonia (n = 2), and endocarditis (n = 1). Persisting or temporary lymphocytopenia was present in 26 (86.7%) patients with a minimal median lymphocyte count of 0.7 K/µL [interquartile range 0.5].
Somatic growth and physical development
Median height z-score significantly decreased during course of the disease, reflecting impaired somatic growth of Fontan patients with protein-losing enteropathy (Table 2). Z-scores of weight and body mass index did not significantly change during follow-up. However, abnormally low weight was frequent with 11 (36.7%) patients having a weight z-score < −2. Growth impairment was proportional though in most cases and consequently, body mass index z-scores < −2 were infrequent (n = 5 patients, 16.7%). Compared to our institutional Fontan cohort without protein-losing enteropathy, z-scores for height (−1.9 [interquartile range −1.9] versus −0.8 [interquartile range −1.5]; p < 0.001) and body weight (−1.3 [interquartile range −1.8] versus −0.8 [interquartile range −1.4]; p < 0.001) at last follow-up were significantly lower in patients with protein-losing enteropathy, while z-score for body mass index did not differ significantly (−0.9 [interquartile range −1.4] versus −0.4 [interquartile range −1.0]; p = 0.087).
Of note, in 12 of 23 (52.2%) of the adolescent and adult patients (>13 years of age), a profoundly delayed puberty was observed. In three male patients, onset of puberty was delayed until the ages of 16 and 17 years in two patients with both not yet having achieved complete pubertal development (Tanner stages <5) at the ages of 19 and 21 years, respectively, while the third male patient died at the age of 16 years and had no onset of puberty. Of the nine female patients, seven had no menarche as well as Tanner developmental stages ≤P2 and ≤B2 by the ages of 16–24 years at last follow-up. Only two female patients had menarche at 17 and 19 years of age but have still not completed physical development with Tanner stages <5 at ages of 20 and 22 years, respectively. This finding was more prevalent in patients without stable remission from protein-losing enteropathy though frequency did not differ statistically significantly (8/13 versus 4/10, p = 0.301).
Vitamin D deficiency/osteopenia
Vitamin D deficiency was observed in 19 of 30 patients (56.7%). However, prevalence might be underestimated in our cohort since vitamin D status was only routinely assessed in the more recent study period, while six (20.0%) patients from the early study period had no measurements. In seven patients (23.3%), osteopenia was diagnosed. One of them suffered from several repetitive pathologic fractures and one had a compressive thoracic vertebra fracture. Five of the patients with osteopenia had received subcutaneous heparin therapy.
Cardiac transplantation
Indications for cardiac transplantation were severe clinical heart failure (NYHA functional class III/IV) and persistent protein-losing enteropathy refractory to treatment. Overall, cardiac transplantation was considered in 12 (40%) patients during follow-up and 7 patients were evaluated for transplantation in our institution. Of these, two patients declined transplantation and one patient was not approved by our institutional transplantation board due to precarious clinical condition. Four patients were eventually listed after a median interval of 7.5 years [interquartile range 16.6] after onset of protein-losing enteropathy. Two of these patients were transplanted but only one survived, resulting in complete remission of protein-losing enteropathy. The second patient died 1 day after transplantation due to unmanageable bleeding after early graft failure requiring mechanical circulatory support. Two patients died on the waiting list. Evaluation for transplantation had been recommended in three additional patients of whom one discontinued follow-up and later died from severe pneumonia and one patient from abroad deceased from sepsis before scheduled evaluation. The third patient chose to continue evaluation in another institution and died shortly afterwards on the waiting list from circulatory arrest. Two patients are currently being evaluated for transplantation in our institution.
Mortality
During follow-up, 10 patients (33.3%) died after a median interval of 7.2 years [interquartile range 6.8] after onset of protein-losing enteropathy. Freedom from death or cardiac transplantation was significantly lower in patients with protein-losing enteropathy compared to Fontan patients without (Fig 1b). Survival estimates were 96.1, 70.5, and 50.1% at 5, 10, and 20 years after disease manifestation, respectively (Fig 1c). Patients who died were exclusively those with protein-losing enteropathy refractory to treatment and consequently, mortality rate was significantly higher in patients without sustained remission (p < 0.001). Total time of active episodes of protein-losing enteropathy was significantly longer in non-survivors compared to survivors (7.0 years [interquartile range 6.7] versus 2.0 years [interquartile range 6.1]; p = 0.010). Major causes of death were terminal circulatory failure (n = 5) and sepsis (n = 3), while two patients died after reoperation. Haemodynamic assessment revealed no significant differences between survivors and non-survivors at protein-losing enteropathy onset (Table 4). Serum albumin and total protein levels were lower non-survivor group at last follow-up, reflecting poor treatment response. Impairment of ventricular function progressed significantly in the non-survivor group (p = 0.026), while differences were statistically not significant in the survivor group (p = 0.053). In addition, pulmonary artery pressure was significantly higher at last follow-up in non-survivors. In an unadjusted subgroup analysis, mortality was significantly lower in patients treated with a combination of budesonide and pulmonary vasodilator therapy (2/14 versus 8/16, p = 0.038) during a comparable follow-up duration after protein-losing enteropathy manifestation (median 9.9 years [interquartile range 12.1] versus 7.8 years [interquartile range 8.1]; p = 0.580).
Table 4. Comparison of protein-losing enteropathy survivors and non-survivors

Data are presented as median [interquartile range] or frequencies (percentages). p-Values < 0.5 considered statistically significant are highlighted in bold. Ventricular dysfunction and AV valve regurgitation were graded as described in Materials and Methods section.
AV valve = atrioventricular valve; BMI = body mass index; EDP = systemic-ventricular end-diastolic pressure; eGFR = estimated glomerular filtration rate; PAP = mean pulmonary artery pressure; TPG = transpulmonary pressure gradient.
Discussion
Protein-losing enteropathy is an infrequent but severe end-organ dysfunction causing substantial morbidity and mortality in patients after Fontan palliation. In our current study, we present a large institutional cohort of patients with a comparably long median follow-up period of 13.1 years [interquartile range 10.6]. The aim of the study was to describe clinical, haemodynamic, and laboratory findings and evaluate the effects of medical, surgical, and interventional treatment strategies on clinical outcome and mortality. Importantly, we also found several factors to be associated with manifestation of protein-losing enteropathy.
Protein-losing enteropathy manifestation and risk factors
To date, there is no universal diagnostic definition of protein-losing enteropathy, but a recently published meta-analysis proposed detailed diagnostic criteria to establish the diagnosis.Reference Udink Ten Cate, Hannes and Germund8 We adopted these criteria to identify patients in our Fontan cohort leading to the exclusion of three patients in whom protein-losing enteropathy was suspected but diagnostic criteria were not fulfilled. This underlines the importance of the use of a standardised diagnostic definition for protein-losing enteropathy not only to initiate early comprehensive assessment and treatment but also to allow a more feasible inter-institutional comparison of clinical practice and research results.
The prevalence of protein-losing enteropathy in Fontan patients operated in our institution was 4.6% which is within published ranges of 3.7–11.3% of the Fontan population.Reference Mertens, Hagler, Sauer, Somerville and Gewillig1-Reference John, Johnson, Khan, Driscoll, Warnes and Cetta5 Onset occurred early after Fontan operation at a median of 2.9 years [interquartile range 5.8] in our cohort, a finding also described by other authors.Reference Mertens, Hagler, Sauer, Somerville and Gewillig1,Reference Schumacher, Stringer and Donohue3 This early disease onset is suggestive for patient-specific characteristics, and post-operative haemodynamic compromises to play a significant role in disease manifestation. As an important finding, we identified several factors, namely bradyarrhythmia, phrenic palsy, and Fontan pathway stenosis to be associated with disease manifestation, while patency of a fenestration was associated with decreased odds of protein-losing enteropathy. The association of phrenic palsy with development of protein-losing enteropathy was recently also observed by Schumacher et al; however, the association of bradyarrhythmia and Fontan pathway stenosis, though not entirely surprising, was not previously described.Reference Schumacher, Stringer and Donohue3 In addition, we found an association of surgical era but not of type of Fontan modification with development of protein-losing enteropathy. Although not specifically investigated by our study, this may suggest that evolutions and improvements in Fontan patient selection, surgical management, and post-operative care could reduce the incidence of protein-losing enteropathy in more contemporary Fontan cohorts, while the type of modification may be not related or a less important contributor to disease manifestation. Appreciation of this association is however limited by the shorter follow-up duration in patients from the recent surgical era (median 5.0 years [interquartile range 7.5]). This follow-up period is still considerably longer than the median interval to onset of protein-losing enteropathy in our cohort but may nonetheless result in underestimation of its frequency.
As additional interesting finding, we observed a patent fenestration to be associated with decreased frequency of protein-losing enteropathy manifestation. Although there is considerable controversy about the general necessity of a fenestration in Fontan patients, it is not unreasonable to hypothesise that in Fontan patients who might have suboptimal haemodynamic and anatomic prerequisites, a fenestration might convey haemodynamic benefits such as reduced venous congestion and increased ventricular preload which could result in decreased risk for protein-losing enteropathy. Conversely, early creation of a fenestration in Fontan patients who have developed protein-losing enteropathy and do not have identifiable haemodynamic issues to address might represent a strategy worth exploring. Ventricular morphology was not associated with protein-losing enteropathy in our study. Also, previously published results concerning right ventricular morphology as a risk factor for protein-losing enteropathy are inconsistent.Reference Mertens, Hagler, Sauer, Somerville and Gewillig1-Reference Powell, Gauvreau, Jenkins, Blume, Mayer and Lock4 In general, evidence regarding risk factors for protein-losing enteropathy is clearly limited, essentially due to the rarity of the disease and consequently limited numbers of patients studied in published reports. This lack of evidence together with the limited understanding of disease pathophysiology highlights the necessity of further multi-institutional studies.
Treatment strategies
Various medical, interventional, and surgical strategies have been used to treat protein-losing enteropathy, but no single strategy proved universally successful.Reference Mertens, Hagler, Sauer, Somerville and Gewillig1,Reference Schumacher, Stringer and Donohue3,Reference Rychik and Spray7 Clinical manifestation, onset, and course of protein-losing enteropathy are distinct in individual patients and no standardised therapeutic approach exists. Of note, it has to be recognised that there is a considerable disparity between haemodynamic findings and manifestation of protein-losing enteropathy in Fontan patients which might explain the unpredictability of treatment results and, above all, emphasises the tremendous knowledge gap concerning disease pathomechanisms. From our own experience, though not specifically analysed in our study, there is a considerable number of patients with upsettingly compromised or even failing Fontan haemodynamics without any sign of protein-losing enteropathy, while a majority of our patients with protein-losing enteropathy had haemodynamic parameters well within acceptable ranges for a Fontan circulation. This perception is also shared by other authors.Reference Powell, Gauvreau, Jenkins, Blume, Mayer and Lock4,Reference Meadows and Jenkins22,Reference Rychik, Goldberg and Rand23
Interventional and surgical therapy
Addressable haemodynamic compromises of the Fontan circulation can be identified in a substantial number of patients as may be concluded from the reported figures of procedures performed in previous studies. Frequencies of 45–72% of patients with protein-losing enteropathy have been reported to have received some form of surgical procedure and/or catheter-based intervention to treat protein-losing enteropathy.Reference Mertens, Hagler, Sauer, Somerville and Gewillig1,Reference John, Johnson, Khan, Driscoll, Warnes and Cetta5,Reference Meadows and Jenkins22 In line with these studies, an addressable haemodynamic restriction was identified in 83.3% of our patients. The most frequent findings were obstructions at various levels of the Fontan pathway, phrenic palsy, and bradyarrhythmia. Plication of the diaphragm as a successful surgical treatment option resulted in resolution of protein-losing enteropathy in three patients, indicating that impaired ventilation represents a severe obstacle to the passive lung perfusion of the Fontan circulation which should be eliminated immediately when clinical deterioration occurs. This is also emphasised by our finding of phrenic palsy to be associated with protein-losing enteropathy. Pacemaker implantation after disease onset was indicated in five patients due to bradyarrhythmia. In two of these patients, atrial pacing resulted in a temporary or complete remission of protein-losing enteropathy, emphasising the importance of an atrial rhythm to maximise cardiac output in Fontan haemodynamics.Reference Cohen, Rhodes, Wernovsky, Gaynor, Spray and Rychik12 Stenoses of the Fontan pathway are also significantly associated with protein-losing enteropathy and 60.0% of the patients required balloon angioplasty and/or stent implantation. The fact that interventional procedures alone, especially relief of obstructions within the Fontan pathway, did not generally result in remission from protein-losing enteropathy in our study must however be interpreted with caution. Clinical improvement remained unappreciated if not fulfilling our definition of remission which potentially results in underestimation of treatment effectiveness, and in addition, interventional therapies were often accompanied with other therapy modalities which may impede unambiguous identification of respective treatment effects.
More recently, not only surgical but also interventional thoracic duct decompression by rerouting the innominate vein blood flow to the atrial compartment has been suggested.Reference Hraska24-Reference Smith, Hoffman, Dori and Rome26 This might represent a promising additional treatment approach in Fontan patients with protein-losing enteropathy; however, this was not yet attempted in our institution. Also, mid-term or long-term outcome data after this procedure are still missing.
Medical therapy
In our cohort, the combination of oral anti-inflammatory therapy with budesonide in addition to pulmonary vasodilation with sildenafil was the most effective medical therapy resolving protein-losing enteropathy in three patients without identifiable haemodynamic restrictions. As an oral corticosteroid, budesonide has few systemic adverse effects and seems efficient in treatment of protein-losing enteropathy by diminishing intestinal inflammation concomitantly found in these patients.Reference Rychik6,Reference Thacker, Patel, Dodds, Goldberg, Semeao and Rychik11,Reference Schumacher, Cools and Goldstein27 The pulmonary vasodilator sildenafil may increase cardiac output in Fontan patients and result in lower cavopulmonary pressure by reducing pulmonary vascular resistance which consequently may improve protein-losing enteropathy.Reference Rychik and Spray7,Reference Reinhardt, Uzun and Bhole10,Reference Uzun, Wong, Bhole and Stumper28 Being a vasodilatative agent, sildenafil is also thought to decrease mesenteric arterial resistance and increase mesenteric arterial flow possibly contributing to resolution of the disease.Reference Reinhardt, Uzun and Bhole10,Reference Uzun, Wong, Bhole and Stumper28 Since intestinal inflammation and diminished mesenteric flow are considered being major pathogenetic factors of protein-losing enteropathy, the combination of budesonide and sildenafil appears to represent the most rational medical treatment option. Importantly, our data suggest that the use of a combination of both drugs might be associated with reduced mortality. However, since our analysis is unadjusted for additional therapies and other possible confounders, this finding requires verification by further studies.
Data concerning the efficiency of other pulmonary vasodilators such as bosentan or prostacyclin are lacking. In our cohort, two patients received prostacyclin in the earlier study period, which did not resolve protein-losing enteropathy. Bosentan was used in combination with sildenafil in nine patients leading to sustained remission in six patients.
More than two decades ago, subcutaneous heparin has been described to eliminate enteric protein loss and resolve protein-losing enteropathy, possibly due to membrane stabilisation effects on intestinal mucosa.Reference Ryerson, Goldberg, Rosenthal and Armstrong9,Reference Donnelly, Rosenthal, Castle and Holmes29 In our cohort, seven patients received subcutaneous heparin as treatment in the early study period. In all patients, heparin was ineffective and did not lead to clinical remission or regression of symptoms.
Treatment results and current treatment approach
Despite the efficiency of single surgical or medical treatment strategies in several patients, a considerable number did not comparably respond to initiated treatments and mortality was substantial if remission from protein-losing enteropathy could not be achieved (Fig 2). In fact, mortality is significantly associated with duration of active periods of protein-losing enteropathy which emphasises the importance of an aggressive, multimodal approach to treat protein-losing enteropathy.
Based on these results and considerations as well as previously published recommendations from Rychik et al, our current institutional treatment approach is outlined in Figure 3.Reference Rychik, Goldberg and Rand23 After confirmation of disease and initial detailed haemodynamic assessment, an aggressive, multimodal, and individual treatment is commenced. Whenever deemed possible, identified restrictions of the Fontan circulation will be addressed by surgery or catheter-based interventions. We however initiate medical therapy with budesonide and sildenafil in all patients, regardless of identifiable haemodynamic issues or planned procedures, to increase the probability of stable remission. Adjuvant treatments include diuretics, dietary modifications, as well as replacement of albumin and/or immunoglobulins as indicated by clinical course and laboratory findings. In patients with increased pulmonary vascular resistance, we commence a dual pulmonary vasodilator therapy with addition of an endothelin receptor antagonist. Depending on haemodynamic findings, we also consider initiation of heart failure medication such as angiotensin converting enzyme inhibitors. In one patient with progressive decline of systolic cardiac function who is currently being evaluated for cardiac transplantation, we recently achieved a significant clinical improvement, though not remission, after start of sacubitril/valsartan. This might represent an additional therapeutic option worth further exploration in patients with protein-losing enteropathy and impaired ventricular function; however, recommendations cannot be made at present since this is only an anecdotal observation. Importantly, it has to be stated that evidence of efficiency of most of these medical heart failure therapies in failing Fontan patients, including those with protein-losing enteropathy, is virtually non-existent.Reference Rychik, Atz and Celermajer30

Figure 3. Proposed algorithm for diagnostic and therapy of protein-losing enteropathy in Fontan patients. α1-AT = α1-antitrypsin; ACEi = angiotensin-converting-enzyme inhibitor; BAP = balloon angioplasty; CPET = cardiopulmonary exercise testing; ECG = electrocardiography; EPS = electrophysiological study; GIT = gastrointestinal tract; HTX = cardiac transplantation; IVIG = intravenous immunoglobulin; PLE =protein-losing enteropathy; SVOTO = systemic ventricular outflow tract obstruction.
In patients with remission of symptoms and normalisation of serum albumin and protein, we consider tapering off budesonide, while sildenafil is generally continued. If initiated treatments fail to achieve significant improvement or remission within 6–12 months and surgical and interventional options are considered exhaust, patients will be recommended evaluation for cardiac transplantation (Fig 3).
Growth impairment and delayed physical development
We observed a significant growth impairment in our cohort with 46.7% of the patients having a height z-score below −2 at last follow-up. Although impaired somatic growth in Fontan patients in general has been noted previously, height z-scores in these studies vary from −1.15 to −0.7, which is considerably higher compared to a height z-score of −1.9 in our patients.Reference Cohen, Bush and Ferry31-Reference Francois, Bove and Panzer33 In particular, our cohort of Fontan patients with protein-losing enteropathy had significantly lower z-scores for height and body weight compared to our Fontan patients without protein-losing enteropathy. It may thus be concluded that protein-losing enteropathy increases the risk of additional growth impairment in Fontan patients. Impaired somatic growth in Fontan patients with protein-losing enteropathy was also described by a small number of previous studies.Reference Schumacher, Stringer and Donohue3,Reference Goldberg, Dodds and Avitabile34 The reasons for growth impairment remain speculative since systematic investigations are lacking but are likely multifactorial. Chronic low cardiac output state and malabsorption, caloric loss, negative nitrogen balance, and disturbance of the delicate hormonal balance due to chronic protein loss have been discussed.Reference Rychik6,Reference Johnson, Driscoll and O’Leary35 As a remarkable finding, 12 of 23 (52.2%) of our patients in adolescent or adult age at last follow-up had profoundly delayed puberty which supports the hypothesis that protein-losing enteropathy may cause significant hormonal imbalances. Delayed puberty in Fontan patients in general was also reported in a recent study; however, mean overall delay compared to a normative cohort was modest with 0.89 years for females and 1.19 years for males.Reference Menon, Al-Dulaimi and McCrindle36 Unfortunately, physical development was not assessed in all our patients and could therefore not be analysed systematically. However, in these documented cases, onset or progression of puberty was markedly delayed within the range of several years.
Reduced bone mineral density has also been reported in patients with protein-losing enteropathy.Reference Goldberg, Dodds and Avitabile34 However, it remains unclear what the major causes for this observation are, since not only various factors such as intestinal malabsorption, vitamin D deficiency, hypocalcaemia, reduced physical activity but also therapies such as corticoid therapy or subcutaneous heparin might contribute to osteopenia. Importantly, vitamin D deficiency was frequent in our cohort and regular laboratory evaluation of vitamin D status seems recommendable in these patients.
Mortality and cardiac transplantation
Death occurred in 10 patients (30.3%) following manifestation of protein-losing enteropathy. Serum albumin and total protein levels were significantly lower in the non-survivor group reflecting treatment failure. In addition, non-survivors had significantly higher pulmonary artery pressure and a more pronounced progression of ventricular dysfunction. This implicates that these factors might have a significant impact on clinical deterioration during the course of protein-losing enteropathy. Consequently, it might be reasonable to initiate evaluation for cardiac transplantation early, if pulmonary artery pressure increases and progressive ventricular systolic dysfunction becomes evident. Since severe hypoalbuminaemia limits the interpretation of invasive haemodynamic assessment due to low oncotic pressure, measurements of pulmonary artery and ventricular end-diastolic pressures might underestimate pulmonary vascular resistance or presence of ventricular dysfunction.Reference Simpson37 Therefore, invasive haemodynamic parameters within normal ranges for Fontan patients should not delay treatment.
Our comparably long follow-up period illustrates protein-losing enteropathy being a chronic disease with frequent and ultimately most likely inevitable relapses (Fig 2). Even after long periods of stable remission, relapses are common and can lead to quick deterioration of patients’ conditions. Nevertheless, 5-year survival estimate after disease onset markedly improved during the past decades from 50.0% in earlier reports to 88.0% in more recent studies and up to as high as 96% in our study cohort (Fig 1).Reference Mertens, Hagler, Sauer, Somerville and Gewillig1,Reference Feldt, Driscoll and Offord2,Reference John, Johnson, Khan, Driscoll, Warnes and Cetta5 Overall improved survival might be based on a more aggressive and multimodal therapeutic approach, improvements in surgical, interventional, and medical treatment strategies, and cardiac transplantation as ultimate treatment option over recent decades. Since our study showed a significant correlation between total time of active protein-losing enteropathy episodes and mortality, cardiac transplantation should be considered in time when treatment fails to achieve stable remission. Various studies have accumulated evidence that protein-losing enteropathy resolves in most cases after cardiac transplantation and is not associated with increased transplantation mortality.Reference Schumacher, Gossett and Guleserian16,Reference Schumacher, Yu and Butts38
Conclusions
Protein-losing enteropathy is a serious end-organ dysfunction limiting survival of Fontan patients. Since no universally successful treatment exists, individualised multimodal treatment strategies combining medical, interventional, and surgical procedures are mandatory. Survival after diagnosis of protein-losing enteropathy in Fontan patients has improved; however, relapse is frequent. In patients with refractory disease, cardiac transplantation should be considered timely before clinical deterioration and progressive multi-organ dysfunction jeopardise success of transplantation. To further improve our understanding of disease pathomechanisms, risk factors, and most importantly treatment and outcome, larger multi-institutional studies will be indispensable.
Limitations
There are several important limitations to this study that should be noted. Since our study is a retrospective analysis covering a long study period, some data (e.g. laboratory and haemodynamic) were not available for every patient. Also, protein-losing enteropathy is an infrequent disease with a high clinical variability. Therefore, patients with mild presentation might not have been detected and the number of unreported cases might be higher. Likewise, consideration of patients with comparably short follow-up duration by our defined inclusion criteria might have led to underestimation of the prevalence of protein-losing enteropathy. Due to the limited number of patients and the variations of treatment concepts during the long study period, a meaningful statistic comparison of treatment strategies cannot be performed. Some therapeutic interventions might have led to clinical improvement but remained unappreciated if not fulfilling our definition of remission which might to some extent have resulted in misjudgement of therapy effectiveness. In addition, combinations of therapy modalities as applied in this study may impede unambiguous identification of respective treatment effects. Moreover, Fontan pathway obstructions were not angiographically quantified, limiting interpretation of success of interventional procedures. Furthermore, half of our patients with protein-losing enteropathy were referred to us from other institutions with variable prior intervals and initiated therapies after disease onset, further limiting the appreciation of therapy effects. Also, the analysis of risk factors might be affected by the long study period including contemporary and more historic Fontan patients which may differ considerably. Moreover, due to the retrospective nature of the study, risk factors such as Fontan stenosis or bradyarrhythmia were only imprecisely defined according to the procedures and some patients with comparable risk factors might have been missed. Therefore, results have to be interpreted with caution.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951120000864
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the institutional review board and the institutional ethics committee (decision number EA2/126/15). The requirement of individual informed consent was waived.









