Introduction
Tropical lowland rainforests are one of the ecosystems that habour the highest plant biodiversity on earth, and they provide many valuable ecosystem services. However, these forests are under severe threat due to human impact throughout the world (Morris, Reference Morris2010). Sri Lankan rainforests are similarly under anthropogenic pressure and are facing the threat of destruction due to increased consumer demands for timber and cropping (Sarathchandra et al., Reference Sarathchandra, Abebe, Wijerathne, Aluthwattha, Wickramasinghe and Ouyang2021). There is a greater need to conserve and restore these pristine ecosystems to ensure future ecosystem services (Corlett and Primack, Reference Corlett and Primack2008). Direct seeding and seedling planting are two of the key methods used in the restoration of degraded areas in tropical regions. Knowledge of seed biology of native species used in such seed-based restoration is, therefore, essential for restoration success (Hamilton et al., Reference Hamilton, Offord, Cuneo and Deseo2013; Miller et al., Reference Miller, Brose and Gottschalk2017). Most direct seeding and seedling planting activities throughout the world have insufficient seed biological information of the species used for these activities leading to failure of many restoration attempts (Baskin and Baskin, Reference Baskin and Baskin2005). When dormancy is not considered in seeding programmes, there is a likelihood of restoration failures (Merritt and Dixon, Reference Merritt and Dixon2011).
Baskin and Baskin (Reference Baskin and Baskin2014) have compiled information on seed dormancy of 1430 tropical rainforest species and found that 48% of the species have non-dormant (ND) seeds. Furthermore, they recorded that 25, 3, 14, 9 and 1% of the species have seeds with physiological dormancy (PD), morphological dormancy (MD), morphophysiological dormancy (MPD), physical dormancy (PY) and PY + PD, respectively. However, this key study does not relate dormancy to biomes such as lowland rainforests.
Although there is information available on the seed biology of tropical rainforest species in many other parts of the world [Malaysia (Ng, Reference Ng1973, Reference Ng1980), Mexico (Vázquez-Yanes and Orozco-Segovia, Reference Vázquez-Yanes and Orozco-Segovia1993), India (Murali, Reference Murali1997) and Brazil (Valio and Scarpa, Reference Valio and Scarpa2001; de Souza et al., Reference de Souza, Torres, Steiner and Paullo2015; Daibes et al., Reference Daibes, Amoedo, Moraes, Fenelon, da Silva, Lopes, Vargas, Monteiro and Frigeri2019)], published information on seed dormancy of South Asian tropical rainforest species is scant. The available information from other regions of the tropics suggests that seeds of many rainforest species are ND. However, a considerable percentage of these species can also produce dormant seeds (Marques and Oliveira, Reference Marques and Oliveira2008). Furthermore, many studies have shown that proportions of ND to dormant seed-producing species vary within a tropical rainforest biome depending on local habitat conditions (Lan et al., Reference Lan, Yin, He, Tan, Liu, Xia and Baskin2018).
Moreover, seed dormancy and other seed biological traits at the plant community level are important in understanding the dynamics of these plant communities (Tweddle et al., Reference Tweddle, Dickie, Baskin and Baskin2003). Community-level information on seed traits enables us to answer key ecological questions that underpin restoration planning decisions when seed is involved such as the relative proportion of different dormancy classes and how these relate to vegetation strata. Although there are many studies of plant community structure for tropical ecosystems (Sautu et al., Reference Sautu, Baskin, Baskin, Deago and Condit2007), there are no studies available on community-level seed dormancy in lowland tropical rainforest plant communities.
Thus, we studied seed dormancy and other related seed biology traits of 42 tropical lowland rainforest tree species from Sri Lanka. This covers about 20% of the dominant tree species in this ecosystem. The study mainly aimed to provide seed dormancy information to assist the restoration of rainforest on degraded lands in southwestern Sri Lanka, which is, together with the Western Ghats of India, a biodiversity hotspot (Gunawardene et al., Reference Gunawardene, Daniels, Gunatilleke, Gunatilleke, Karunakaran, Nayak and Vasanthy2007). The specific aims are to determine: (1) what seed dormancy class(es) is/are important for tropical lowland wet zone rainforest community in Sri Lanka and (2) what dormancy classes are present in species representing the different forest strata and seed dispersal modes under different climatic conditions. Moreover, the dormancy profile constructed for the tree species in Sri Lankan lowland tropical rainforest species was compared with the dormancy profile constructed by Baskin and Baskin (Reference Baskin and Baskin2014) for tree species of the tropical rainforest biome to test the hypothesis that seed dormancy profiles for different plant communities of the same biome can be different from the generalized seed dormancy profile constructed for the biome due to variations in climate.
Materials and methods
Study species and collection of seeds
The studied tree species (Supplementary Table S1) were selected from the tropical lowland rainforests in Sri Lanka based on their ecological significance, threatened status, the availability of seeds and endemicity. Fruits were collected at least from six individuals from each species located in five rainforests in Sri Lanka, such as Sinharaja, Kanneliya, Yagirala, Kottawa, Pituwala and Dombagaskanda forest reserves, from October 2017 to September 2019 during the peak fruiting season of each species. Mature ripened fruits were collected from trees whenever possible. When fruits were inaccessible and less number of fruits were available on trees, fruits fallen on the ground were collected. Additional care was taken to collect healthy non-predated freshly fallen fruits when they were collected from the ground. Collected fruits were stored in labelled paper or polythene bags and transported to the seed biology laboratory, Department of Botany, University of Peradeniya, where laboratory experiments were conducted. Seeds were extracted from fruits and air-dried to remove surface water if seeds were extracted by soaking in water. Extracted seeds were stored in closed plastic bottles until experiments were initiated within 2–3 d from seed collection, as literature indicates that species may have recalcitrant or very short-lived seeds (Umarani et al., Reference Umarani, Aadhavan and Faisal2015). The stratum (canopy, subcanopy, understory trees and understory shrubs) was identified by using the height information of plants based on Ashton et al. (Reference Ashton, Gunatilleke, de Zoysa, Dassanayake and Gunatilleke1997) and field observations. Month of seed collection was used to determine the time of seed dispersal, which was further divided into three sessions according to rainfall data: wet (100–200 mm), super wet (200–400 mm) and peak (400–500 mm) rainy season.
Seed germination
To determine whether seeds are dormant or not, four replicates, each of at least ten seeds were incubated on moistened tissue papers in Petri dishes at 25°C in light/dark (12 h/12 h) conditions. Seeds were observed for germination at 3-d intervals for 100 d or until all the viable seeds germinated. Radicle emergence (>1 mm) was the criterion for germination. At the end of the experiments, non-germinated seeds were dissected to determine the viability (white firm viable embryos vs grey soft non-viable embryos). Seed germination progression curves were prepared, and T 50 was determined using the curves. Species with T 50 < 30 d were categorized as fast-germinating seeds, while remaining species with T 50 > 30 were categorized as slow-germinating seeds (as suggested by Athugala et al., Reference Athugala, Jayasuriya, Gunaratne and Baskin2021).
Imbibition of seeds
To determine whether the seeds of the studied species have water permeable seed coat or not, seeds incubated at 25°C under conditions mentioned under ‘seed germination’ were observed for changes due to water imbibition (seed coat rupture and seed size increase) in 1-d intervals for consequent 5 d. If the seeds show seed coat rupture and the increment of seed size, they were considered to have a permeable seed coat. Other species with no sign of imbibition were subjected to an imbibition test. Initial mass of 15 fresh intact and manually scarified seeds was measured to the nearest 0.001 g using a digital analytical balance (GR 200, A&D Company Pvt. Ltd.). These seeds were placed on moistened tissue papers in Petri dishes separately and retrieved 2, 5, 8, 24 and 48 h after incubation, blotted dry, reweighed and returned to the Petri dish. Imbibition curves of intact and scarified seeds were prepared and compared.
Embryo length:seed length ratio
Studies were conducted to determine the presence or absence of MD or MPD of seeds (developed vs underdeveloped embryo). If slow-germinating seeds have underdeveloped embryos they can be categorized as seeds with MPD, while fast-germinating seeds with underdeveloped embryos can be categorized as seeds with MD. Eight samples of ten seeds were placed on filter papers moistened with distilled water in 9-cm diameter Petri dishes and incubated at 25°C. Seven samples were retrieved after 12 h, and then after 2-week intervals until germination of seeds was complete. The eighth sample was retrieved soon after the seed coat rupture for radicle protrusion. Seeds were dissected, and seed length and embryo length were measured using a Vernier caliper (AVM Scientific, India). Embryo length:seed length ratio (E:S ratio) was calculated for each seed. Embryo development at the time of radicle emergence was calculated using the equation given below:
 $$\eqalign{&{\rm Percentage}\,{\rm embryo}\,{\rm development} = \cr & \displaystyle{{{\rm E\colon S}\,{\rm ratio}\,{\rm at}\,{\rm the}\,{\rm time\;}{\rm of}\,{\rm radicle}\,{\rm protrusion}-{\rm E}\,{\rm \colon S}\;{\rm ratio\;}{\rm of}\,{\rm fresh\;}\,{\rm seed}} \over {{\rm E\colon S\;}\,{\rm ratio}\,{\rm of}\,{\rm fresh}\,{\rm seed}}}\; \cr & \times 100\% } $$
$$\eqalign{&{\rm Percentage}\,{\rm embryo}\,{\rm development} = \cr & \displaystyle{{{\rm E\colon S}\,{\rm ratio}\,{\rm at}\,{\rm the}\,{\rm time\;}{\rm of}\,{\rm radicle}\,{\rm protrusion}-{\rm E}\,{\rm \colon S}\;{\rm ratio\;}{\rm of}\,{\rm fresh\;}\,{\rm seed}} \over {{\rm E\colon S\;}\,{\rm ratio}\,{\rm of}\,{\rm fresh}\,{\rm seed}}}\; \cr & \times 100\% } $$Seeds with an E:S ratio of >0.5 or if the E:S ratio did not increase prior to germination were considered to have fully developed embryos, while seeds having an E:S ratio of <0.5 and the E:S ratio increased significantly prior to germination were considered to have underdeveloped embryos. Embryo growth studies were not conducted on species with seeds <1 mm in diameter.
Embryo morphology
Five seeds of each species (except the minute seeds) were cut into half using a razor blade. The embryo shape was observed and categorized according to the modified Martins classification system (Baskin and Baskin, Reference Baskin and Baskin2007). Embryos were observed under a light microscope if seeds were <5 mm in length.
Effect of GA3 on germination
This experiment was conducted for the species that had T 50 > 30 d to confirm the physiological dormancy of seeds. Four replicates each of at least ten seeds were placed on Whatman No. 1 filter paper moistened with 500 ppm GA3 solution and incubated at 25°C under light/dark (12 h/12 h) condition. Seeds were observed for radicle and shoot emergence at 3-d intervals for at least 30 consecutive days. Radical emergence (>1 mm) was recorded as the criterion for germination. Non-germinated seeds were dissected to check the viability of the embryo as explained above.
Analysis of data
Four-parameter logistic sigmoidal curves were fitted to the germination data of all species to identify the germination pattern (as suggested by Brown and Mayer, Reference Brown and Mayer1988). T 50 values were determined from the graphs. The relationship between strata and the time of dispersal was analysed with a nominal regression analysis using the dormancy class as the dependant variable and other variables as independent variables. Dormancy profiles created during the present study were compared with that constructed for the tropical rainforest biome (Baskin and Baskin, Reference Baskin and Baskin2014) using the χ 2 test.
Results
Seed germination
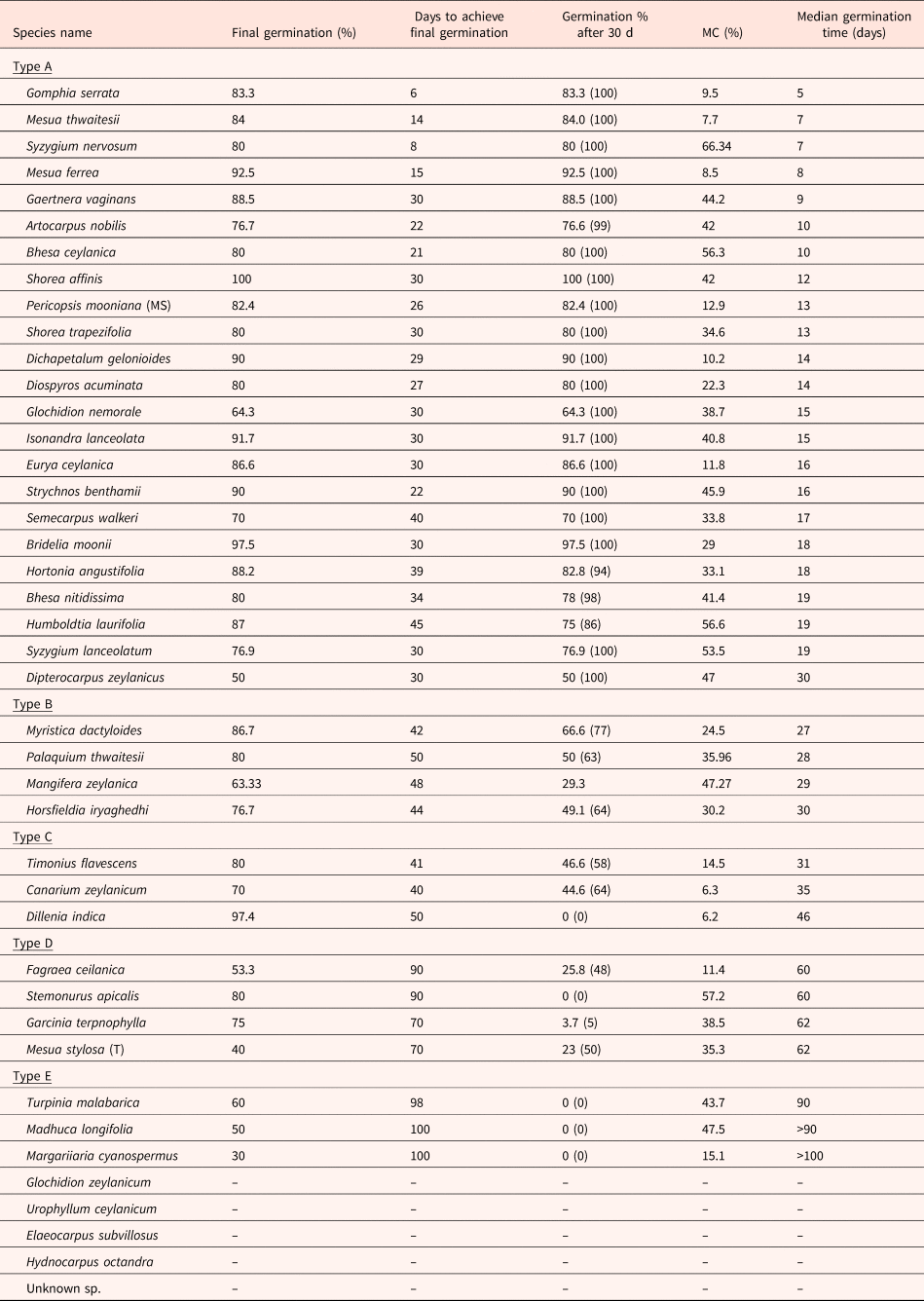
Five types of germination behaviour were identified among the 42 studied species based on the cumulative germination curves (Fig. 1) and other germination parameters (Table 1). Seeds showing A and B types of germination behaviour could be described as ‘fast-germinating seeds’ and seeds with C, D and E types of germination behaviour could be grouped as ‘slow-germinating seeds’. Species showing germination behaviour type A had seeds that reached >90% germination (of the seeds which were viable till the end of the experiment) within 30 d (Table 1). Type A was represented by 46% of the study species. Type B germination behaviour was shown by 16% of the study species with T 50 < 30 d and >90% germination after 30 d (Table 1). However, seeds of type B species reached 100% germination within at least ~55 d. Species with type C germination behaviour consisted of seeds having T 50 between 30 and 50 d represented by 9% of the study species. Species with seeds that had T 50 between 50 and 65 d were grouped into germination behaviour type D. This type contained 7% of the study species. Finally, type E germination behaviour was seen in species having seeds with T 50 >80 d. This type was observed in only 21% of the studied species.

Fig. 1. Cumulative germination of Isonandra lanceolata (type A), Horsfieldia iryaghedhi (type B), Canarium zeylanicum (type C), Fagraea ceilanica (type D) and Margaritaria cyanospermus (type E). Four-parameter logistic sigmodial curves were fitted to identify the germination behaviour.
Table 1. Characteristics of the study species having different germination behaviour types A, B, C, D and E
Imbibition of seeds
Visual signs of imbibition (rupturing of seed coat and increasing seed size, Supplementary Fig. S2) were observed in all the studied species, except in seeds of Pericopsis mooniana when incubated on water alone. When non-scarified P. mooniana seeds were incubated on distilled water at 25°C, a negligible amount of mass increment was observed within 4 d (Supplementary Fig. S3). In contrast, scarified seeds showed a >100% mass increase followed by the enlargement of the seed and the rupturing of the seed coat. The mass increment of manually scarified seeds was significantly higher than that of the intact seeds (F = 54.73, P < 0.001).
Embryo length:seed length ratio
Among the 42 study species, 31 species (Table 2) such as Elaeocarpus subvillosus, Hydnocarpus octandra, Palaquium thwaitesii and Bhesa ceylanica had an E:S ratio of >0.5 (Fig. 2). They were categorized as seeds with fully developed embryos. The remaining 11 species had an E:S ratio of <0.5. However, although the initial E:S ratio of Myristica dactyloides and Horsfieldia iryaghedhi was <0.5, it did not increased >0.5 prior to radicle emergence (15% embryo development). Their cotyledons also did not develop into a functioning leaf, instead leaves developed from the shoot meristem separately and hence, cotyledons act as haustoria to supply nutrients to the developing seedling from the shoot meristem after germination. They were also categorized as seeds with developed embryos. The E:S ratio of the seeds of remaining species (10 species) developed prior to radicle emergence, while at the time of radicle emergence the E:S ratio was >0.5 (Tables 2 and 3), and this group was represented by Stemonurus apicalis and Hortonia angustifolia (Fig. 3). They were categorized as seeds with underdeveloped embryos.

Fig. 2. Developed embryos of fresh seeds of Elaeocarpus subvillosus (A), Hydnocarpus octandra (B), Palaquium thwaitesii (C) and Bhesa ceylanica (D). EM, embryo; EN, endosperm.

Fig. 3. Underdeveloped embryos of fresh (1) and developed embryo of the germinating (2) seeds of Stemonurus apicalis (A) and Hortonia angustifolia (B). EM, embryo; EN, endosperm.
Table 2. Classification of embryo type, embryo shape and dormancy type of studied species

D, developed embryo; UD, underdeveloped embryo; B1, rudimentary; FA1, spatulate axile; FA2, bent axile; FA4, investing axile; LA, linear axile; VS, visual signs present; VSA, visual signs absent MD, morphological dormancy; MPD, morphophysiological dormancy; ND, no dormancy.
Table 3. E:S ratio of some studied species

Embryo morphology
Seeds of most of the study species have FA1: spatulate axile (13 species); LA: linear axile (10 species) and FA2: bent axile (10 species) embryos. In addition, FA4: investing and B1: rudimentary embryo types were also observed (Table 2). Among the spatulate and linear embryos, fully developed and underdeveloped types were observed. However, seeds of Urophyllum ceylanicum were categorized as dwarf seeds as they were very tiny (>1 mm in length) seeds.
Effect of GA3 on germination
As majority of the species germinated well with distilled water, this treatment was only conducted for Glochidion zeylanicum, Urophyllum ceylanicum, Elaeocarpus subvillosus, Madhuca longifolia, Mesua stylosa, Hydnocarpus octandra and unknown sp. as none of their seeds germinated with distilled water within 100 d. Seeds of G. zeylanicum and E. subvillosus germinated >50% within 30 d on tissue papers moistened with GA3 solution, while U. ceylanicum and H. octandra germinated to 50% after 30 d. Although seeds of Mesua stylosa germinated to 40% without any treatment, there was a significant increase in germination (69%) after treatment with 500 ppm GA3.
Diversity of seed dormancy types of studied rainforest species
Dormancy types of the studied species were categorized based on their T 50 value, embryo development during radicle emergence and imbibition studies (Table 2). Embryos of nine study species were underdeveloped, while 33 species had developed embryos. The seeds with underdeveloped embryos have morphological or MPD. Thus, among the studied species, 26 species were identified as ND (62% from the total species), while 16 species (38%) were identified as dormant. Dormant species included one physically dormant species, three morphologically dormant species, six physiologically dormant species and six morphophysiologically dormant species.
Seed dormancy distribution among forest strata
A significant association was discovered between the dormancy type and forest stratification (χ 2 = 11.93, P = 0.003). Non-dormancy was abundant among all the tree species. However, the number of ND species decreased when moving from the upper strata towards the lower strata. Three quarters of the canopy species produced ND seeds. MD and PD were not found among canopy species, while PY and MPD were represented by 12.5% of species in each class. Sixty seven percent of the subcanopy species produced ND seeds, whereas PD and MPD were represented by 25 and 8.3% species, respectively. Fifty two percent of ND species were observed in the tree layer, while MPD, PD and MD were represented by 21, 13 and 13% of species, respectively.
Seed dormancy versus non-dormancy during different seed dispersal seasons
A significant association was found between the seed dormancy type and seed dispersal time (χ 2 = 128.61, P < 0.001). All the seeds (100%) dispersed during the peak rainy season (>500 mm monthly rainfall) during May were ND. The majority of the species (73%) dispersed during other less wet parts of the rainy season (200–500 mm) during April, June, September, October and November were also ND, and 27% of them were dormant. In contrast, 75% of the seeds dispersed during the wet season (100–200 mm) during February and March were dormant, while only 25% of them were ND. See Supplementary Fig. S1 for the generalized climatic profiles for the wet zone of Sri Lanka.
Dormancy profiles for Sri Lankan rainforests versus rainforests in the world biome
Results of the χ 2 test revealed that the dormancy profile constructed for Sri Lankan rainforest tree species during the present study was significantly different (χ 2 = 9.85, P = 0.043) from that created for global rainforest by Baskin and Baskin (Reference Baskin and Baskin2014). The percentage of ND (62%) and morphologically (7%) dormant seed-producing species in the dormancy profile constructed for Sri Lanka was higher than that for the world (49 and 3%, respectively). Consequently, the percentage of PD (14.3%) and PY (2.3%) seed-producing species of the local profile was less than that of reported in the world tropical rainforest biome (25 and 8.5%, respectively).
Discussion
In this study of Sri Lankan lowland rainforest, there was considerable variability in the dominance of different dormancy types across rainforest strata. Most of the species that represent the upper strata of Sri Lankan rainforests produced ND seeds, while most of the species in the lower strata produced seeds with PD or MPD. Similar trends have been identified by Athugala et al. (Reference Athugala, Jayasuriya, Gunaratne and Baskin2021) from montane forest species of Sri Lanka. They suggested that the species from the upper strata produce fast-germinating seeds as they have a higher chance of long distance dispersal than seeds of species in lower strata. Thus, seeds of species occupying lower strata may depend on secondary dispersal that, in turn, would require additional time for dispersal to occur. Therefore, species in lower strata produce seeds that have a high proportion of dormancy. This study found that the same principal applies to the lowland rain forests in Sri Lanka. Species in the upper strata of tropical rainforest are much taller (>30 m) than species in the montane forest upper strata (>20 m). Thus, these species have even higher opportunities to disperse seeds a longer distance with the aid of animals and wind. However, more studies are needed to determine the ecological significance of this phenomenon.
Furthermore, our research showed that the seed dormancy of species seems to be more dependent on the peak seed dispersal period of these species, which was directly linked with rainfall. Species-dispersing seeds in the peak and other wet periods produce ND seeds. Similar results were reported by Garwood (Reference Garwood1983) where Garwood described that the time of germination is controlled by dispersal time. Furthermore, Garwood stated that seeds dispersed in the early rainy season were ND seeds, while seeds dispersed in the late rainy season and in the dry season were dormant. Peak and wet periods provide favourable conditions for seedlings to grow in the forest floor. During these periods, the forest floor is more humid, and thus, pathogenicity and predation pressure is higher for newly dispersed seeds (Dalling et al., Reference Dalling, Davis, Schutte and Elizabeth Arnold2011, Reference Dalling, Davis, Arnold, Sarmiento and Zalamea2020), which can be more easily overcome if seeds have rapid germination and establishment syndromes (Green and Juniper, Reference Green and Juniper2004; Dalling et al., Reference Dalling, Davis, Schutte and Elizabeth Arnold2011, Reference Dalling, Davis, Arnold, Sarmiento and Zalamea2020). Species producing dormant seeds dispersed their seeds during the relatively dry period, which is somewhat less favourable for seedling development and also less favourable for pathogens and predators, and thus, these seeds can stay in the soil seed bank until the peak of the super wet period in the region initiates germination following dormancy release.
Our research also identified, for the first time, the dormancy classes of 27 rainforest species. Among these 27 species, dormancy information is not available for Hortonia angustifolia, Timonius flavescens, Isonandra lanceolata and Stemonurus apicalis even at the genus level. Only genus-level information is available for the remaining 23 species. However, for 12 of the above species, available genus-level information suggested two or more dormancy classes (Baskin and Baskin, Reference Baskin and Baskin2014). The dormancy classes identified for 8 of the remaining 12 species were different from those reported in the literature at the genus level (Baskin and Baskin, Reference Baskin and Baskin2014). Our study confirmed the dormancy class previous identified or speculated at the genus level for 4 of the above 12 species [ND in genus Artocarpus (Beniwal and Singh, Reference Beniwal and Singh1989; Elliott et al., Reference Elliott, Anbusarnsunthorn, Kopachon, Maxwell, Blakesley and Garwood1996), PD in genus Elaeocarpus (Chien et al., Reference Chien, Hong and Lin1998; Iralu and Upadhaya, Reference Iralu and Upadhaya2018), MD in genus Gaertnera (Athugala et al., Reference Athugala, Jayasuriya, Gunaratne and Baskin2021) and PD in genus Glochidian (Athugala et al., Reference Athugala, Jayasuriya, Gunaratne and Baskin2021)]. Fourteen of the study species have been previously studied and dormancy classes were identified or speculated. Six of the above 14 species have previously been studied in different ecosystems [Urophyllum ceylanicum, Turpinia malabarica, Bhesa nitidissima and Eurya ceylanica in tropical mountain forests (Athugala et al., Reference Athugala, Jayasuriya, Gunaratne and Baskin2021), and Messua ferrea (Troup, Reference Troup1921; Joshi et al., Reference Joshi, Phartyal, Khan and Arunkumar2015) and Dillenia indica (Thapliyal et al., Reference Thapliyal, Phartyal, Baskin and Baskin2008) in tropical semi-evergreen forests], while the rest of the species (eight) have been studied in the same ecosystem [Mangifera zeylanica and Dipterocarpus zeylanicus (Holmes, Reference Holmes1954), Shorea affinis (Tompsett, Reference Tompsett, Appanah and Turnbull1998) S. trapezifolia (de Zoysa, Reference de Zoysa1986) Strychnos benthamii (Muthuthanthirige et al., Reference Muthuthanthirige, Wijetunga and Jayasuriya2020) Humboldtia laurifolia (Jayasuriya et al., Reference Jayasuriya, Wijetunga, Baskin and Baskin2010), Gomphia serrata (Ng, Reference Ng1992) and P. mooniana (Jayasuriya et al., Reference Jayasuriya, Wijetunga, Baskin and Baskin2013)]. The previously reported dormancy class (PD) of Bheasa nitidissima, Eurya ceylanica and Gomphia serrata are identified as ND in the present study. However, seed dormancy, of eight species that have been previously studied from the same ecosystem, matched the outcomes of this study. Our study confirmed the class of dormancy for some but not all previously studied species. This information is a valuable addition to understanding the seed conservation of these important endemic and threatened species from tropical lowland rain forest species.
The differences in dormancy classes between the present study and previously published works at the genus level may be a result of: firstly, as Baskin and Baskin (Reference Baskin and Baskin2004) suggested some of these studies did not specifically aim to identify the dormancy class, and thus, the identified or speculated dormancy class through these studies may not be accurate. Secondly, dormancy can vary between species within the same genus (Finch-Savage and Leubner-Metzger, Reference Finch-Savage and Leubner-Metzger2006) and may even vary within the species between populations inhabiting different ecosystems (Tseng et al., Reference Tseng, Burgos, Shivrain, Alcober and Mauromoustakos2013).
Our study also showed that the dormancy profile created for the Sri Lankan rainforest tree species was significantly different from that created for the tropical rainforests worldwide by Baskin and Baskin (Reference Baskin and Baskin2014). This is a similar observation to Athugala et al. (Reference Athugala, Jayasuriya, Gunaratne and Baskin2021) and Lan et al. (Reference Lan, Yin, He, Tan, Liu, Xia and Baskin2018) where they also found that dormancy profiles of Sri Lankan montane forests and seasonal tropical semi-evergreen rainforests in China were different from dormancy profiles constructed for tropical montane forest and seasonal tropical rainforest biomes of the world, respectively. The percentage of ND seed-producing species was higher in Sri Lankan rainforests compared to the world rainforest biome.
In addition, species that produce seeds having MD were often fast germinating with >50% of viable seeds germinating within 30 d. This can be expected as Sri Lankan rainforests are more aseasonal and more humid than most of the other tropical regions (Gunawardene et al., Reference Gunawardene, Daniels, Gunatilleke, Gunatilleke, Karunakaran, Nayak and Vasanthy2007). Fast germination is a sound adaptation for overcoming mortality issues (Dalling et al., Reference Dalling, Davis, Schutte and Elizabeth Arnold2011) associated with humid aseasonal tropical climates (Vázquez-Yanes and Segovia, Reference Vázquez-Yanes, Orozco-Segovia, Medina, Mooney and Vázquez-Yánes1984). Fast-germinating seeds can outgrow high predation and high pathogenicity through fast germination (Daws et al., Reference Daws, Garwood and Pritchard2005). Thus, non-dormancy is the most dominant type of germination behaviour among the tree species in the lowland rainforest of Sri Lanka.
Conservation and restoration implications
This study shows that tests of seed dormancy may require independent validation when extended to other parts of a biome. Simply relying on published sources from other regions or other biome types may lead to seed being inappropriately treated with subsequent loss of germination capacity for translocation or restoration programmes. In addition, the study demonstrated that the speed of germination can vary greatly within the same biome and therefore customizing seed pre-treatments such as priming may be necessary to ensure all seeds are in a readily germinable state.
Supplementary material
To view supplementary material for this article, please visit : https://doi.org/10.1017/S0960258522000162.
Funding
This work was supported by the National Research Council of Sri Lanka (grant no. 17-095).
Conflicts of interest
The author(s) declare none.









