Significant outcomes
-
8.3% of the individuals with T2D initiated psychopharmacological treatment in the 2 years following onset compared to 4.6% among the age- and sex-matched controls.
-
Individuals with newly developed T2D were at increased risk of initiating psychopharmacological treatment and of having psychiatric hospital contact compared to propensity score-matched controls.
-
Risk factors for psychopharmacological treatment/psychiatric hospital contact following development of T2D include older age, somatic comorbidity, and being divorced/widowed.
Limitations
-
Identification of T2D (an HbA1c level ≥ 6.5%) itself might lead to the identification of mental illness (via increased contact with the health care system) and thereby psychopharmacological treatment initiation/psychiatric hospital contact.
-
A proportion of the individuals with T2D will likely have initiated treatment with an antidepressant due to neuropathic pain developed as a complication to T2D.
Introduction
Type 2 diabetes (T2D) is a prevalent disorder (population prevalence of 4.4% in Denmark (Carstensen et al., Reference Carstensen, Rønn and Jørgensen2020)) and is associated with a range of severe complications including micro- and macrovascular disease, neuropathy, cancer, dementia, and infections (Nwaneri et al., Reference Nwaneri, Cooper and Bowen-Jones2013; Harding et al., Reference Harding, Pavkov, Magliano, Shaw and Gregg2019a; Carstensen et al., Reference Carstensen, Rønn and Jørgensen2020). In addition, there is mounting evidence suggesting that psychiatric comorbidity is also highly prevalent in T2D (Semenkovich et al., Reference Semenkovich, Brown, Svrakic and Lustman2015; Harding et al., Reference Harding, Pushpanathan, Whitworth, Nanthakumar, Bucks and Skinner2019b). Specifically, prior studies have shown that individuals with T2D are twice as likely to exhibit symptoms of depression, anxiety, insomnia, and distress compared to the general population (Collins et al., Reference Collins, Corcoran and Perry2009; Khuwaja et al., Reference Khuwaja, Lalani, Dhanani, Azam, Rafique and White2010; Smith et al., Reference Smith, Béland, Clyde, Gariépy, Pagé, Badawi, Rabasa-Lhoret and Schmitz2013; Ducat et al., Reference Ducat, Philipson and Anderson2014; Semenkovich et al., Reference Semenkovich, Brown, Svrakic and Lustman2015; Hackett & Steptoe, Reference Hackett and Steptoe2017; Tan et al., Reference Tan, Van Egmond, Chapman, Cedernaes and Benedict2018) – with up to one out of five developing major depression (Semenkovich et al., Reference Semenkovich, Brown, Svrakic and Lustman2015). Accordingly, prior studies have established that individuals with T2D are more likely to use antidepressants than individuals from the general population (Manderbacka et al., Reference Manderbacka, Sund, Koski, Keskimäki and Elovainio2011), with the point prevalence of antidepressant use in T2D ranging between 7.1% and 14.7% in cross-sectional studies (Cleal et al., Reference Cleal, Panton, Willaing and Holt2018; Kivimäki et al., Reference Kivimäki, Tabák, Lawlor, Batty, Singh-Manoux, Jokela, Virtanen, Salo, Oksanen, Pentti, Witte and Vahtera2010; Ivanova et al., Reference Ivanova, Nitka and Schmitz2010; Mast et al., Reference Mast, Rauh, Groeneveld, Koopman, Beulens, Jansen, Bremmer, Van der Heijden, Elders, Dekker, Nijpels, Hugtenburg and Rutters2017). In addition, the point prevalence of anxiolytic/hypnotic use has been estimated at 6.5% (Mast et al., Reference Mast, Rauh, Groeneveld, Koopman, Beulens, Jansen, Bremmer, Van der Heijden, Elders, Dekker, Nijpels, Hugtenburg and Rutters2017). It remains unclear whether the elevated prevalence of psychiatric symptoms and psychopharmacological treatment stems from psychological distress and pathophysiological alternations caused by T2D (glycemic alterations (Kim et al., Reference Kim, Yu, Shin, Shin and Kim2016), microvascular dysfunction (van Agtmaal et al., Reference Van Agtmaal, Houben, Pouwer, Stehouwer and Schram2017; Geraets et al., Reference Geraets, Van Agtmaal, Stehouwer, Sörensen, Berendschot, Webers, Schaper, Henry, Van Der Kallen, Eussen, Koster, Van Sloten, Köhler, Schram and Houben2020) and inflammation (Calle & Fernandez, Reference Calle and Fernandez2012)) or is due to mental illness preceding T2D (Khuwaja et al., Reference Khuwaja, Lalani, Dhanani, Azam, Rafique and White2010). Another possibility is that T2D is an innocent marker of poor socioeconomic status, somatic comorbidity, obesity, and unhealthy lifestyle, which are all associated with mental illness (Kolb & Martin, Reference Kolb and Martin2017).
Prior studies that have investigated psychopharmacological treatment and need for psychiatric hospital care among individuals with T2D are mainly limited by three things: First, studies aiming at studying these aspects in individuals with newly developed T2D have often not had the exact date of T2D diagnosis at their disposal, and therefore used T2D treatment initiation or hospitalisation as a proxy for the onset of T2D (Mast et al., Reference Mast, Rauh, Groeneveld, Koopman, Beulens, Jansen, Bremmer, Van der Heijden, Elders, Dekker, Nijpels, Hugtenburg and Rutters2017; Cleal et al., Reference Cleal, Panton, Willaing and Holt2018). Second, prior studies have generally not been able to take preexisting mental illness or psychopharmacological treatment into account (Kivimäki et al., Reference Kivimäki, Tabák, Lawlor, Batty, Singh-Manoux, Jokela, Virtanen, Salo, Oksanen, Pentti, Witte and Vahtera2010; Mast et al., Reference Mast, Rauh, Groeneveld, Koopman, Beulens, Jansen, Bremmer, Van der Heijden, Elders, Dekker, Nijpels, Hugtenburg and Rutters2017; Perez et al., Reference Perez, Cabrera, Gutierrez and Valdes2017; Cleal et al., Reference Cleal, Panton, Willaing and Holt2018), that is, whether the need for treatment arose before or after the development of T2D. Third, adjustment for potential confounders of the association between T2D and psychopharmacological treatment requires access to information that has not been available in many prior studies (Kivimäki et al., Reference Kivimäki, Tabák, Lawlor, Batty, Singh-Manoux, Jokela, Virtanen, Salo, Oksanen, Pentti, Witte and Vahtera2010; Mast et al., Reference Mast, Rauh, Groeneveld, Koopman, Beulens, Jansen, Bremmer, Van der Heijden, Elders, Dekker, Nijpels, Hugtenburg and Rutters2017; Perez et al., Reference Perez, Cabrera, Gutierrez and Valdes2017; Cleal et al., Reference Cleal, Panton, Willaing and Holt2018).
As the limitations outlined above may have resulted in inaccurate estimates of incident psychiatric morbidity in individuals with newly developed T2D, we conducted a register-based study aimed at overcoming these limitations. This was done by (i) defining onset of T2D as the first measured HbA1c level ≥6.5% (48 mmol/mol), (ii) excluding individuals having received psychopharmacological treatment or having had a psychiatric hospital contact in the 5 years preceding the onset of T2D, and (iii) conducting a propensity score-matched analysis of the association between newly developed T2D and incident psychopharmacological treatment and psychiatric hospital contact, taking several potential confounders into account.
Material and methods
Design and setting
We conducted a register-based cohort study based on a dataset containing information on all individuals residing in the Northern Denmark and Central Denmark Regions (approximately 1.9 million inhabitants) to investigate the 2-year incidence of psychopharmacological treatment and psychiatric hospital contact among individuals with T2D and age- and sex-matched controls. The registers providing data for the study are described below.
Registers
The Danish Civil Registration System (DCRS) was established in 1968 and contains the unique personal registration numbers, which are assigned to all individuals living in Denmark (at birth or when becoming a legal resident), enabling linkage of data at the individual from all public registers (Pedersen, Reference Pedersen2011). In the current study, we linked data from the following registers: (1) The clinical laboratory information system database (LABKA), which contains laboratory results from all general practitioners and hospitals in the Central and Northern Denmark Regions since the 1990s with full coverage since the early 2000s (Grann et al., Reference Grann, Erichsen, Nielsen, Frøslev and Thomsen2011). (2) The Danish National Patient Register (DNPatR), which contains discharge diagnoses from all admissions to Danish non-psychiatric hospitals since 1977 and from emergency and outpatient hospital settings since 1995 (Lynge et al., Reference Lynge, Sandegaard and Rebolj2011). (3) The Danish Psychiatric Central Research Register (DPCRR), which contains discharge diagnoses from all psychiatric hospital admissions since 1969 and from psychiatric emergency rooms and outpatient units since 1995 (Mors et al., Reference Mors, Perto and Mortensen2011). (4) The Danish National Prescription Register (DNPreR), which contains date on all prescriptions redeemed at Danish pharmacies since 1995 (Kildemoes et al., Reference Kildemoes, Sorensen and Hallas2011).
Study population
LABKA was used to identify all individuals with onset of T2D in the period from 1 January 2000 to 31 October 2015 [defined as the first date with a blood sample with an HbA1c level ≥6.5% (48 mmol/mol)]. Individuals registered with an HbA1c level ≥6.5% (48 mmol/mol) prior to 1 January 2000 were excluded in order to obtain a cohort of individuals with incident T2D. Furthermore, individuals who had redeemed a prescription for a glucose lowering drug (see definition in Supplementary Table S1) or were registered with a diagnosis of T2D (see definition in Supplementary Table S1) prior to the first date with a blood sample with an HbA1c level ≥6.5% (48 mmol/mol) were also excluded to allow for a focus on incident T2D. Finally, individuals who were under the age of 30 at the time of being registered with an HbA1c level ≥6.5% (48 mmol/mol) were excluded to minimise the proportion of individuals with type 1 diabetes in the dataset (Mor et al., Reference Mor, Berencsi, Nielsen, Rungby, Friborg, Brandslund, Christiansen, Vaag, Beck-Nielsen, Sørensen and Thomsen2016). Subsequently, to enable investigation of incident psychopharmacological treatment/psychiatric hospital contact, individuals with psychopharmacological treatment/psychiatric hospital contact (see definition in Supplementary Table S1) in the 5 years preceding the onset of T2D were excluded. The remaining individuals with incident T2D and without preexisting psychopharmacological treatment/psychiatric hospital contact were matched on age, sex, and date [of HbA1c level ≥ 6.5% (48 mmol/mol)] with up to five individuals (controls) without T2D [no HbA1c levels ≥ 6.5% (48 mmol/mol), no redeemed prescriptions for glucose lowering drugs and no registered T2D diagnosis] and without psychopharmacological treatment/psychiatric hospital contact in the 5 years preceding the matched date. Individuals included as matched controls could be included in another matched pair (as an individual with T2D) if they met the criteria for T2D [HbA1c level ≥ 6.5% (48 mmol/mol)] after the matched date.
Propensity score matching
In an attempt to minimise the degree of confounding in the estimation of the association between T2D and psychopharmacological treatment/psychiatric hospital contact, we carried out a propensity score-matched analysis. Specifically, individuals with T2D from the cohort described above were matched 1:1 to one individual from the age- and sex-matched control group on covariates (see section below) associated with T2D and psychopharmacological treatment/psychiatric hospital contact. All covariates that were expected to be associated with exposure and outcome (all of those described below) were used for the propensity score matching. The first step in the matching procedure was to calculate the predicted probability (the propensity score) of each individual in the cohort having T2D on the basis of his or her covariate profile, using logistic regression. Subsequently, the propensity scores were trimmed asymmetrically using the 95th percentile of the propensity scores for individuals without T2D and the 5th percentile for individuals with T2D (Stürmer et al., Reference Stürmer, Rothman, Avorn and Glynn2010). We then matched each individual with T2D to the individual without T2D having the propensity score closest to that of the former using calipers of width equal to 0.2 of the standard deviation (Austin, Reference Austin2011). The matching was checked with a kernel density plot and with the mean standardised difference (see Fig. 1).

Fig. 1. Mean standardised differences before (orange) and after (blue) propensity score matching. aSmoking-related disorder or redemption of a prescription for a drug used in the treatment of chronic obstructive airway disease (COPD) or asthma. bBetablockers, calcium antagonists, rennin–angiotensin system acting agents, lipid modifying agents, diuretics, and antithrombotic agents. cIncluding corticosteroids, analgesics, and anti-inflammatory agents. dCharlson Comorbidity Index.
Covariates
We obtained data for a number of covariates, which were used (i) to calculate the propensity score described above and (ii) as potential predictors of incident psychopharmacological treatment and psychiatric hospital contact among those with T2D. Specifically, information on sex, age, and marital status at the onset of T2D/matched date were extracted from the DCRS. Information on baseline HbA1c, low-density lipoprotein (LDL), and estimated glomerular filtration rate (eGFR) levels were extracted from LABKA. Information on the 17 major disease groups included in the Charlson Comorbidity Index (excluding diabetes and diabetes with chronic complications) were obtained from the DNPatR – and the cohort members were categorised based on the number of diseases registered at baseline (0, 1, and ≥2). In addition to the diseases included in the Charlson Comorbidity Index, information on atrial fibrillation, hypertension, infectious diseases, osteoporosis, alcohol-related disorder, obesity, hyper/hypothyroidism, neurological disorders, and micro- and macrovascular complications were obtained from the DNPatR. As information on smoking behaviour is not available in the Danish registers, we defined a ‘smoking-related disorder’ covariate based on data from the DNPatR (see definition in Supplementary Table S1) and the DNPreR (redemption of a prescription for a drug used in the treatment of obstructive airway disease). Furthermore, information on redeemed prescriptions for the most commonly used medications for somatic illnesses (betablockers, calcium antagonists, rennin–angiotensin system acting agents, lipid modifying agents, diuretics, and antithrombotic agents) in the year preceding the onset of T2D was extracted from the DNPreR. The same was the case for medications with potential relation to T2D and/or mental illness (corticosteroids, analgesics, and anti-inflammatory medication). The definition of all covariates is provided in Supplementary Table S1.
Outcomes
The outcomes were incident psychopharmacological treatment and psychiatric hospital contact (as inpatient or outpatient) during a 2-year follow-up period, respectively. Here, initiation of psychopharmacological treatment can be considered a proxy for mild/moderate mental illness, whereas psychiatric hospital contact is associated with more severe mental illness. The outcomes were not mutually exclusive, that is, an individual could have both outcomes in the analyses. Psychopharmacological treatment was identified using the DNPreR and separate analyses were conducted for antidepressants, antipsychotics, and anxiolytics, respectively (see definitions in Supplementary Table S1). Psychiatric hospital contacts were identified using the DPCRR. In addition to considering psychiatric hospital contact in general – resulting in a diagnosis in the mental disorder chapter of the International Classification of Diseases, 10th Revision (ICD-10) – we also conducted analyses on contacts resulting in diagnoses of depression, anxiety disorder, psychotic disorder, and substance use disorder, specifically (see ICD-10 definitions in Supplementary Table S1).
Statistical analyses
Psychopharmacological treatment and psychiatric hospital contact during follow-up
The cohort members were followed from the onset of T2D [HbA1c ≥ 6.5% (48 mmol/mol)] or the matched date until death, emigration, first redemption of a prescription for psychopharmacological medication (not in the analyses having psychiatric hospital contact as outcome), first psychiatric hospital contact (not in the analyses having redemption of a prescription for psychopharmacological medication as outcome), or 2 years from onset/matched date, whichever came first. If the controls developed T2D [HbA1c ≥ 6.5% (48 mmol/mol)] they were censored at this date. For this follow-up period, the incidence rates of psychopharmacological treatment and psychiatric hospital contact were reported for the individuals with T2D as well as the age and sex matched controls, respectively. In addition, we reported the (cumulative) proportion of individuals that had initiated psychopharmacological treatment or had had a psychiatric hospital contact by the end of the follow-up.
The association between T2D and psychopharmacological treatment and psychiatric hospital contact
For this analysis, the individuals with T2D [HbA1c ≥ 6.5% (48 mmol/mol)] and the propensity score-matched controls were followed from the onset of T2D or the matched date until death, emigration, first redemption of a prescription for psychopharmacological medication (not in the analyses having psychiatric hospital contact as outcome), first psychiatric hospital contact (not in the analyses having redemption of a prescription for psychopharmacological medication as outcome), or 2 years from onset/matched date, whichever came first. If the controls developed T2D [HbA1c ≥ 6.5% (48 mmol/mol)], they were censored at this date. We then carried out a Cox regression analysis with psychopharmacological treatment and psychiatric hospital contact as outcomes (see definitions under ‘Outcomes’ above).
Risk factors for psychopharmacological treatment and psychiatric hospital contact in T2D
Here, we assessed which baseline covariates (see definitions under ‘Covariates’ above) that were associated with subsequent psychopharmacological treatment and psychiatric hospital contact among individuals with T2D using Cox proportional hazards regression. The follow-up was identical to that for the analyses described above. The proportional hazards assumption was tested by plotting the observed survival curves against the estimated survival curves.
Sensitivity analyses
First, we repeated the analyses of incident psychopharmacological treatment by redefining the outcome to require redemption of at least two prescriptions for psychopharmacological drugs within the 2 years following onset of T2D/matched date. This was chosen as redemption of at least two prescriptions which may be a more specific marker of mental illness compared to redemption of only one prescription (McCrea et al., Reference Mccrea, Sammon, Nazareth and Petersen2016; Liu et al., Reference Liu, Agerbo, Ingstrup, Musliner, Meltzer-Brody, Bergink and Munk-Olsen2017). Second, as a large proportion of individuals with newly developed T2D had received psychopharmacological treatment or had had a psychiatric hospital contact in the 5 years leading up to the development of T2D (30.9%), we repeated the analyses of psychopharmacological treatment/psychiatric hospital contact for all individuals with newly developed T2D and controls – that is, without excluding those with psychopharmacological treatment/psychiatric hospital contact in the 5 years leading up to development of T2D. This was done to estimate the overall presence of mental illness – prevalent as well as incident – among those with newly developed T2D. Third and finally, due to the limited number of individuals with psychiatric hospital contact during the 2-year follow-up, we repeated the analyses of the incidence of psychopharmacological treatment and psychiatric hospital contact (and baseline covariates associated with the latter) using 5 years of follow-up. This final sensitivity analysis was based on individuals who developed T2D in the period from 1 January 2000 to 31 October 2012 to include 5 years of follow-up time.
Results
Study population
The definition of the study population is illustrated in Fig. 2. In brief, we identified 120 705 individuals with incident T2D (as defined by the HbA1c values). Among those, 35 983 (29.8%) had redeemed a prescription for a glucose-lowering drug or had received a hospital diagnosis of diabetes prior to the incidence of T2D. For the remaining 84 722 individuals, 2747 were <30 years old (3.2%), leaving 81 975 individuals with newly developed T2D. From these, 25 335 (30.9%) individuals had received psychopharmacological treatment/had had a psychiatric hospital contact in the 5 years leading up to the development of T2D and were excluded from the dataset to allow for investigation of incident psychopharmacological treatment and psychiatric hospital contact over the following 2 years. The 25 335 individuals excluded due to prior psychopharmacological treatment/psychiatric hospital contact did not differ from individuals with T2D without prior psychopharmacological treatment/psychiatric hospital contact with regard to age (median age = 65.0 years vs. median age = 64.0) and baseline HbA1c (median HbA1c = 6.8% vs. median HbA1c = 6.8%). They were however less likely to be male (45.4% vs. 60.7%) and had more somatic comorbidity (Charlson Comorbidity Index score > 0 = 52.3% vs. 36.9%). These steps led to the final cohort of 56 640 individuals. A total of 315 694 age- and sex-matched individuals without diabetes and who had not received psychopharmacological treatment and had not had psychiatric hospital contact in the 5 years prior to the matched date were identified from the general population (in Central and Northern Denmark). From the 315 694 controls, we were able to propensity score match a total of 44 742 individuals to 44 742 cohort members with T2D. Table 1 shows characteristics of all 56 640 patients with newly developed T2D, stratified on experiencing psychopharmacological treatment or psychiatric hospital contact status during follow-up, and the 44 742 propensity score-matched individuals with T2D and their 44 742 controls without T2D.

Fig. 2. Selection of study population. T2D, type 2 diabetes.
Table 1. Characteristics of 56 640 individuals with T2D and no prior psychopharmacological treatment/psychiatric hospital contact, stratified on psychopharmacological treatment or psychiatric hospital contact status during follow-up, and 44 742 propensity-score matched individuals with T2D and 44 742 controls without T2D

T2D, type 2 diabetes.
* Individuals experiencing both psychopharmacological treatment initiation and psychiatric hospital contact count in both columns.
† Having been registered with a smoking-related diagnosis (see Supplementary Table S1) or having redeemed a prescription for COPD medication and/or asthma inhalers.
‡ Including betablockers, calcium antagonists, angiotensin system acting agents, lipid modifying agents, diuretics, and antithrombotic agents.
§ Corticosteroids, analgesics, and anti-inflammatory agents.
Follow-up
The mean follow-up time for the each individual in the cohort (individuals with newly developed T2D and age- and sex-matched controls) was 696 days. The mean follow-up time was 662 days for the individuals with newly developed T2D and 702 days for the age- and sex-matched controls. A total of 4943 (8.7%) of the individuals with newly diagnosed T2D died during follow-up, while 10 553 (3.3%) of the age- and sex-matched controls died during follow-up. A total of 140 (0.2%) of the individuals with newly diagnosed T2D emigrated during the follow-up, while 229 (0.1%) of the age- and sex-matched controls emigrated during follow-up.
Incidence rates of psychopharmacological treatment and psychiatric hospital contact during follow-up
The results of the analyses regarding incidence rates of psychopharmacological treatment and psychiatric hospital contact are shown in Table 2.
Table 2. Incidence rates of psychopharmacological treatment and psychiatric hospital contact in the first 2 years after incident T2D or matched date for the controls

T2D, type 2 diabetes; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin–norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant.
* Admission, outpatient contact, psychiatric emergency room contact.
For the individuals with newly developed T2D the incidence rate of psychopharmacological treatment was 45.7 (95% CI = 44.4–47.0) per 1000 years of follow-up. Among the age- and sex-matched controls, the corresponding incidence rate was 24.0 (95% CI = 23.6–24.4). Among the individuals with newly developed T2D, 8.3% initiated psychopharmacological treatment during follow-up (5.6%, 1.2%, and 2.9% with an antidepressant, antipsychotic, or an anxiolytic agent, respectively – not mutually exclusive). Among the age- and sex-matched controls from the general population, 4.6% initiated psychopharmacological treatment during follow-up (2.6%, 0.6%, and 2.0% with an antidepressant, antipsychotic, or an anxiolytic agent, respectively). A total of 1.0% of the individuals with newly developed T2D had a psychiatric hospital contact in the follow-up period. Among the age- and sex-matched controls, 0.7% had a psychiatric hospital contact during follow-up.
The association between T2D and psychopharmacological treatment and psychiatric hospital contact
A total of 7.7% of the individuals with newly developed T2D initiated psychopharmacological treatment compared to 5.3% of the propensity score-matched controls. The Cox regression analysis showed that Individuals with newly developed T2D were at substantially increased risk of initiating psychopharmacological treatment in general (HR = 1.51, 95% CI = 1.43–1.59), as well as antidepressant (HR = 1.66, 95% CI = 1.56–1.78), antipsychotic (HR = 1.74, 95% CI = 1.49–2.01), and anxiolytic (HR = 1.30, 95% CI = 1.20–1.42) treatment specifically, compared to the propensity score-matched controls (see Table 3). A total of 0.8% of the individuals with newly developed T2D had psychiatric hospital contact compared to 0.7% of the propensity score-matched controls. The Cox regression indicated that individuals with newly developed T2D also had a slightly increased risk of having a psychiatric hospital contact (HR = 1.14, 95% CI = 0.98–1.32).
Table 3. Hazard ratios for psychopharmacological treatment and psychiatric hospital contact in the first 2 years among propensity score-matched incident T2D individuals and controls

T2D, type 2 diabetes; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin–norepinephrine reuptake inhibitor; TCA, tricyclic antidepressant.
* Admission, outpatient contact, psychiatric emergency room contact.
Risk factors for initiation of psychopharmacological treatment and psychiatric hospital contact among patients with T2D
The following characteristics were identified as risk factors for psychopharmacological treatment following onset of T2D: High age (71–80 years vs. ≤50 years; HR = 1.17, 95% CI = 1.05–1.31 and >80 years vs. ≤50 years; HR = 1.46, 95% CI = 1.28–1.66), Charlson Comorbidity Index score of 1 (HR = 1.22, 95% CI = 1.13–1.32), Charlson Comorbidity Index score of 2 (HR = 1.69, 95% CI = 1.52–1.87), macrovascular complications (HR = 1.16, 95% CI = 1.07–1.26), being divorced (HR = 1.22, 95% CI = 1.09–1.35), being widowed (HR = 1.35, 95% CI = 1.23–1.49), having a smoking-related disorder (HR = 1.18, 95% CI = 1.09–1.28), and having an alcohol-related disorder (HR = 1.66, 95% CI = 1.37–2.00). Never being married (HR = 0.71, 95% CI = 0.62–0.81), age 51–60 vs. ≤50 years (HR = 0.86, 95% CI = 0.78–0.95), age 61–70 vs. ≤50 years (HR = 0.89, 95% CI = 0.81–0.98) and use of other commonly prescribed medications (using two medications; HR = 0.76, 95% CI = 0.70–0.82) was associated with reduced likelihood of psychopharmacological treatment (see Table 4).
Table 4. Association between baseline characteristics of patients with T2D and initiation of psychopharmacological treatment/psychiatric hospital contact during the 2-year follow-up
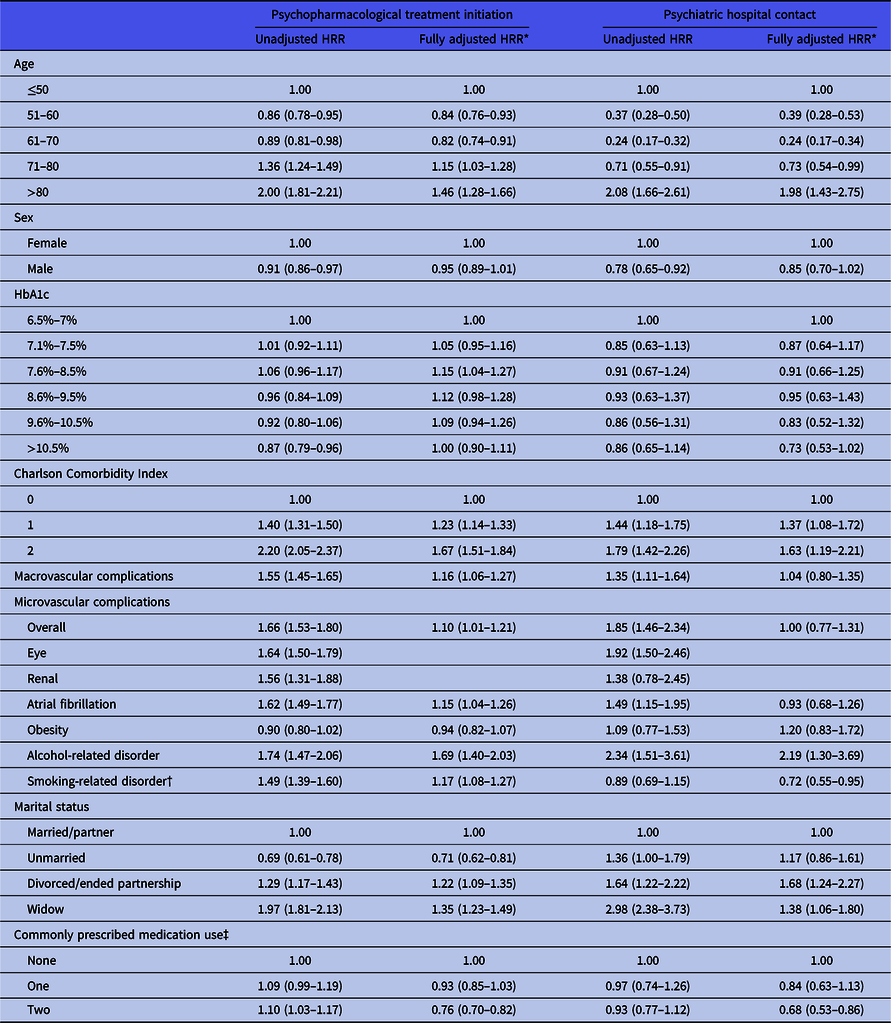
* Adjusted for age, sex, baseline HbA1c, CCI-score, obesity, smoking-related disorders, alcohol-related diagnoses, marital status, commonly prescribed medication use, macro- and microvascular complications.
† Having been registered with a smoking-related diagnosis (see Supplementary Table S1) or having redeemed a prescription for COPD medication and/or asthma inhalers.
‡ Including beta-blockers, calcium channel blockers, rennin–angiotensin system acting agents, lipid modifying agents, diuretics, and antithrombotic agents.
The same characteristics were associated with psychiatric hospital contact (see Table 4), with the following exceptions: Having a macrovascular complication at baseline was not associated with a substantially increased risk of psychiatric hospital contact (HR = 1.04, 95% CI = 0.80–1.35), and only individuals above the age of 80 were at increased risk of psychiatric hospital contact (HR = 1.98, 95% CI = 1.43–2.75), while individuals aged 51–60 vs. ≤50 years (HR = 0.39, 95% CI = 0.28–0.53), 61–70 vs. ≤50 years (HR = 0.24, 95% CI = 0.17–0.34), and 71–80 (HR = 0.73, 95% CI = 0.54–0.99) were less likely to have a psychiatric hospital contact during follow-up compared to individuals under the age of 50 (see Table 4). The proportional hazard assumption was met in these analyses.
Sensitivity analyses
The results of the sensitivity analyses in which having the ‘outcome’ was defined as redemption of at least two prescriptions (rather than only one) for psychopharmacological drugs within the 2 years following onset of T2D/matched date were analogue to those of the main analyses. Specifically, the risk of requiring psychopharmacological treatment associated with T2D was also substantially increased in these analyses, including those stratified on antidepressants, antipsychotics, and anxiolytics (see Supplementary Tables S2 and S3).
When not excluding individuals with psychopharmacological/psychiatric hospital treatment in the 5 years leading up to the development of T2D/matched date, a total of 26.5% of the individuals with newly developed T2D redeemed a psychopharmacological prescription (antidepressants: 19.1%, antipsychotics: 5.9%, and anxiolytics: 10.3%) in the 2 years following the development of T2D. Among the age- and sex-matched controls, the corresponding numbers were 17.1% (antidepressants: 10.9%, antipsychotics: 2.9%, and anxiolytics: 7.7%). With regard to psychiatric hospital contacts, 3.2% of the individuals with newly diagnosed T2D had a psychiatric hospital contact during the follow-up period compared to 2.0% of the controls (see Supplementary Table S4).
When using a 5-year follow-up period, a total of 16.1% of the individuals with newly developed T2D redeemed a psychopharmacological prescription compared to 10.9% among the age- and sex-matched controls. A total of 2.0% and 2.0% had psychiatric hospital contact among individuals with newly diagnosed T2D and controls, respectively (see Supplementary Table S5). Individuals with newly developed T2D were more likely to initiate psychopharmacological treatment compared to the propensity score-matched controls during the 5 years of follow-up, whereas their risk of psychiatric hospital contact was not increased to the same extent (see Supplementary Table S6). With regard to the baseline characteristics associated with subsequent psychopharmacological treatment initiation and psychiatric hospital contact, the results were largely similar to those from the main analyses, with the exception of atrial fibrillation not being associated with psychopharmacological treatment initiation in the 5-year follow-up and the Charlson Comorbidity Index being less strongly associated with psychiatric hospital contact in the 5-year follow-up period (see Supplementary Table S7).
Discussion
In this register-based cohort study, we found that 8.3% of individuals with newly developed T2D initiated psychopharmacological treatment within 2 years compared to 4.6% among age- and sex-matched controls from the general population. Furthermore, in analyses using a propensity score-matched design to minimise confounding, we found that individuals with newly developed T2D were at increased risk of initiating psychopharmacological treatment, compared to controls. Finally, we identified a number of risk factors, which were associated with elevated risk of requiring psychopharmacological treatment or psychiatric hospital contact in the 2 years following the onset of T2D, including older age, somatic comorbidity, and being divorced/widowed
Psychopharmacological treatment and psychiatric hospital contact during follow-up
The findings from this study, namely that 5.6% and 2.9% of the individuals with newly developed T2D initiated antidepressant and anxiolytic treatment, respectively, cannot be compared directly to prior studies in the field (point prevalence of 7.1%–14.7% for antidepressant treatment and 6.5% for anxiolytic treatment (Kivimäki et al., Reference Kivimäki, Tabák, Lawlor, Batty, Singh-Manoux, Jokela, Virtanen, Salo, Oksanen, Pentti, Witte and Vahtera2010; Mast et al., Reference Mast, Rauh, Groeneveld, Koopman, Beulens, Jansen, Bremmer, Van der Heijden, Elders, Dekker, Nijpels, Hugtenburg and Rutters2017; Ivanova et al., Reference Ivanova, Nitka and Schmitz2010; Cleal et al., Reference Cleal, Panton, Willaing and Holt2018)) due to their cross-sectional design. Furthermore, unlike most other studies on this topic, we excluded individuals having received psychopharmacological treatment or having had a psychiatric hospital contact within the 5 years leading up to the onset of T2D, in order to get better estimates of the true incidence of the need for psychopharmacological treatment and specialist psychiatric care (hospital contact) among individuals with newly developed T2D. In the supplementary analyses, in which we did not exclude individuals with prior psychopharmacological/psychiatric hospital treatment, the rates were – as one would expect – substantially higher (see Supplementary Table S4). For instance, 19.1% redeemed a prescription for an antidepressant in the 2 years following the development of T2D.
When comparing the incidence of psychopharmacological treatment and psychiatric hospital contact in T2D to that of age- and sex-matched controls, the rates were substantially higher among those with T2D. This is however not necessarily due to T2D per se, as this condition is associated with a range of characteristics that increase the risk of mental illness – including somatic comorbidities (Adriaanse et al., Reference Adriaanse, Drewes, Van Der Heide, Struijs and Baan2016; Nowakowska et al., Reference Nowakowska, Zghebi, Ashcroft, Buchan, Chew-Graham, Holt, Mallen, Van Marwijk, Peek, Perera-Salazar, Reeves, Rutter, Weng, Qureshi, Mamas and Kontopantelis2019) and unhealthy lifestyle (Schellenberg et al., Reference Schellenberg, Dryden, Vandermeer, Ha and Korownyk2013; Kolb & Martin, Reference Kolb and Martin2017) – that may confound the association between T2D and the outcomes studied here.
The association between T2D and psychopharmacological treatment and psychiatric hospital contact
In order to minimise the degree of confounding in the estimation of the association between newly developed T2D and psychopharmacological treatment/psychiatric hospital contact, we conducted a propensity score-matched analysis, which confirmed a 51% increased risk of psychopharmacological treatment in T2D. While we can by no means claim causality based on these findings, they are compatible with T2D increasing the risk for mental illness. The potential mechanisms underlying such an effect may include, but are not limited to, the psychological distress associated with developing T2D (Chew et al., Reference Chew, Vos, Metzendorf, Scholten and Rutten2017; Aljuaid et al., Reference Aljuaid, Almutairi, Assiri, Almalki and Alswat2018), a potential direct effect of hyperglycemia on the brain (Kim et al., Reference Kim, Yu, Shin, Shin and Kim2016; Giri et al., Reference Giri, Dey, Das, Sarkar, Banerjee and Dash2018), and downstream effects of T2D such as cardiovascular disease (Zheng et al., Reference Zheng, Ley and Hu2018; Gedebjerg et al., Reference Gedebjerg, Almdal, Berencsi, Rungby, Nielsen, Witte, Friborg, Brandslund, Vaag, Beck-Nielsen, Sørensen and Thomsen2018). Also, as demonstrated by Kan and colleagues based on analysis of data from Danish and Swedish twin registers, genetic overlap between T2D and depression may also play a role in this regard (Kan et al., Reference Kan, Pedersen, Christensen, Bornstein, Licinio, Maccabe, Ismail and Rijsdijk2016). The present study was however not designed to investigate the individual contributions of (and potential interaction between) these potential mechanisms.
Interestingly, individuals with newly developed T2D did also have an increased risk of initiating treatment with antipsychotic medication. Whether this reflects that individuals with T2D are also at elevated risk of developing psychotic disorders or that the antipsychotics are prescribed for the treatment of depression, anxiety, or sleep problems cannot be determined based on the data at hand and should ideally be addressed in future studies.
As opposed to the findings for psychopharmacological treatment, the risk of psychiatric hospital contact was only increased by 14% for those with T2D in the propensity-score matched analysis. A possible explanation for this difference – linking the apparently opposing findings – is that those in need of treatment for mental disorders receive relevant psychopharmacological treatment by their general practitioner (Musliner et al., Reference Musliner, Liu, Gasse, Christensen, Wimberley and Munk-Olsen2019) and do therefore not go on to develop conditions so severe that treatment/assessment at psychiatric hospitals is required.
Risk factors for psychopharmacological treatment and psychiatric hospital contact in T2D
In the analysis of which baseline characteristics that were associated with subsequent psychopharmacological treatment and psychiatric hospital contact among individuals with T2D, we found that older age, somatic comorbidity, and being divorced/widowed were risk factors for both psychopharmacological treatment and psychiatric hospital contact in individuals with newly developed T2D. These results are in agreement with risk factor studies from the field of depression (Copeland et al., Reference Copeland, Beekman, Braam, Dewey, Delespaul, Fuhrer, Hooijer, Lawlor, Kivela, Lobo, Magnusson, Mann, Meller, Prince, Reischies, Roelands, Skoog, Turrina, Devries and Wilson2004; Scott et al., Reference Scott, Wells, Angermeyer, Brugha, Bromet, Demyttenaere, De Girolamo, Gureje, Haro, Jin, Karam, Kovess, Lara, Levinson, Ormel, Posada-Villa, Sampson, Takeshima, Zhang and Kessler2010; Schaakxs et al., Reference Schaakxs, Comijs, Van der Mast, Schoevers, Beekman and Penninx2017) and suggest that clinicians should be particularly aware of development of mental disorder (and probably depression in particular) in individuals who are elderly, divorced/widowed and suffer from comorbid somatic illness at the onset of T2D.
Interestingly, individuals with T2D receiving other commonly prescribed medications at baseline were less likely to initiate psychopharmacological treatment. A potential explanation for this finding may be that individuals who are compliant to pharmacological treatment in general may have decreased propensity towards mental illness, that is, that compliance is a proxy for mental health (Chapman & Horne, Reference Chapman and Horne2013). Similarly, these individuals may also be more adherent to glucose lowering medications, which has been associated with reduced risk of mental illness (Wahlqvist et al., Reference Wahlqvist, Lee, Chuang, Hsu, Tsai, Yu and Chang2012).
Limitations
Our findings should be interpreted in the light of the following limitations. First, due to the known comorbidity between T2D and mental illness, the identification of T2D [an HbA1c level ≥ 6.5% (48 mmol/mol)] itself might lead to the identification of mental illness and thereby psychopharmacological treatment initiation/psychiatric hospital contact. If such an ascertainment bias has affected this study, it would result in an overestimation of the strength of the association between T2D and the requirement for psychopharmacological treatment/psychiatric hospital contact. Second, although we primarily consider psychopharmacological treatment as a proxy for development of mental disorder, a proportion of the individuals with T2D will likely have initiated treatment with an antidepressant (serotonin–norepinephrine reuptake inhibitors and tricyclic antidepressants in particular) due to neuropathic pain developed as a complication to T2D (Gilron et al., Reference Gilron, Baron and Jensen2015). However, as we only included individuals with newly developed T2D, only a relatively small proportion will have developed neuropathic pain requiring pharmacological treatment after 2 years. Also, the positive association between T2D and psychopharmacological treatment was observed across all investigated groups of drugs in this category – and not only for those that are typically used in the management of neuropathic pain. Third, the data from this study stems from the Danish national healthcare system, which provides tax-funded healthcare for all citizens. Hence, the results may not necessarily translate to other countries – especially those with healthcare systems based on other models. Therefore, the results should ideally be replicated in other settings to confirm generalisability. Fourth, as we excluded all individuals having received psychopharmacological/psychiatric hospital treatment in the 5 years leading up to the development of T2D, the results of the main analyses are not representative of individuals with newly developed T2D encountered as part of standard clinical practice. The results from the supplementary analyses in which those with prior psychopharmacological treatment/psychiatric hospital contact were not excluded from the cohort are more representative in that regard. Fifth, having an HbA1c level ≥6.5% (48 mmol/mol) was first approved as a criterion for diagnosing T2D in Denmark in 2012. Consequently, it is probably not all individuals with HbA1c levels ≥6.5% (48 mmol/mol) from our cohort who have actually been diagnosed with/treated for T2D. However, as blood glucose levels correlate closely with HbA1c levels (Ito et al., Reference Ito, Maeda, Ishida, Sasaki and Harada2000), we expect that most individuals with HbA1c levels ≥6.5% (48 mmol/mol) would eventually have been diagnosed with T2D. In further support of this, even prior to 2012, the indication for taking the HbA1c test was typically clinical suspicion of T2D.
Conclusion
Individuals with newly developed T2D are at elevated risk of requiring psychopharmacological treatment compared to propensity score-matched controls without T2D. Risk factors for psychopharmacological treatment/psychiatric hospital contact following development of T2D are consistent with those observed for general populations and include older age, somatic comorbidity, and being divorced/widowed.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/neu.2020.39
Acknowledgements
None.
Author contributions
The study was designed in collaboration between all authors. CR conducted the analyses. The results were interpreted by all authors. The manuscript was drafted by CR and SDØ and revised for important intellectual content by NS and RWT. The final version was approved by all authors prior to submission.
Financial support
This study was funded by a grant from the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation (grant number: NNF17SA0031406), and supported by the International Diabetic Neuropathy Consortium (IDNC), which is supported by a Novo Nordisk Foundation Challenge Programme grant (grant number NNF14OC0011633).
Conflict of interest
The authors declare no conflicts of interest. The Department of Clinical Epidemiology, Aarhus University Hospital, receives funding for other studies from companies in the form of research grants to (and administered by) Aarhus University. None of these studies have any relation to the present study.









